Abstract
Junctional adhesion molecule-C (JAM-C) is an adhesion molecule expressed at junctions between adjacent endothelial and epithelial cells and implicated in multiple inflammatory and vascular responses. In addition, we recently reported on the expression of JAM-C in Schwann cells (SCs) and its importance for the integrity and function of peripheral nerves. To investigate the role of JAM-C in neuronal functions further, mice with a specific deletion of JAM-C in SCs (JAM-C SC KO) were generated. Compared to wild-type (WT) controls, JAM-C SC KO mice showed electrophysiological defects, muscular weakness, and hypersensitivity to mechanical stimuli. In addressing the underlying cause of these defects, nerves from JAM-C SC KO mice were found to have morphological defects in the paranodal region, exhibiting increased nodal length as compared to WTs. The study also reports on previously undetected expressions of JAM-C, namely on perineural cells, and in line with nociception defects of the JAM-C SC KO animals, on finely myelinated sensory nerve fibers. Collectively, the generation and characterization of JAM-C SC KO mice has provided unequivocal evidence for the involvement of SC JAM-C in the fine organization of peripheral nerves and in modulating multiple neuronal responses.—Colom, B., Poitelon, Y., Huang, W., Woodfin, A., Averill, S., Del Carro, U., Zambroni, D., Brain, S. D., Perretti, M., Ahluwalia, A., Priestley, J. V., Chavakis, T., Imhof, B. A., Feltri, M. L., Nourshargh, S. Schwann cell-specific JAM-C-deficient mice reveal novel expression and functions for JAM-C in peripheral nerves.
Keywords: adhesion molecules, tight junctions, peripheral nerves
Junctional adhesion molecule-C (JAM-C) is a type I transmembrane protein and member of an immunoglobulin subfamily currently composed of JAM-A, JAM-B, JAM-C, JAM-4, JAM-L, ESAM (endothelial cell selective adhesion molecule) and CAR (coxsackie virus and adenovirus receptor). The molecule consists of 2 extracellular immunoglobulin domains and a short cytoplasmic tail, which supports extracellular and intracellular interactions with surface ligands and cytoskeletal/signaling partners, respectively (1, 2). The principal JAM-C ligands to date are JAM-C, JAM-B, and the integrin αMβ2, although their relative contributions to the diverse functions of JAM-C (see below) remain unclear (3–6). JAM-C was originally detected on endothelial cells (ECs; ref. 7) and on human activated T cells (6) and has since been reported to be expressed on other subsets of human leukocytes (4, 8) and platelets (3), vascular smooth muscle cells (9), and a wide range of nonvascular cells, such as spermatids (10), epithelial cells (11), and fibroblasts (12). In addition, we have reported recently on the expression of JAM-C in peripheral nerves, where it is localized on Schwann cells (SCs) at sites characteristic of junctional regions of noncompact myelin, such as the paranodal regions, Schmidt-Lanterman incisures (SLIs), and the mesaxonal regions (13).
In line with its broad expression pattern, JAM-C has been associated with numerous biological functions. Most notably, JAM-C has been studied in the context of vascular and inflammatory responses, where direct evidence indicates its involvement in leukocyte transmigration (14), vascular permeability (15), and angiogenesis (16). Although much of these early works were performed using in vitro assays, more recently the role of JAM-C in inflammation and vascular biology has been investigated in vivo within numerous disease models. These include murine models of arthritis, acute pancreatitis, peritonitis, ischemia/reperfusion injury, atherosclerosis, and pulmonary inflammation (17–22), with some of these studies involving the use of genetically modified animals. Characterization of JAM-C-knockout (KO) mice has also identified additional functions for JAM-C, such as roles in cell polarity, immunity, and inflammation (10, 19, 23). As a result of its multiple and wide-ranging biological roles, mice with complete deletion of JAM-C exhibit a severe phenotype that includes growth retardation, development of megaoesophagus, defects in hematopoiesis (17, 24), and defective motor functions and abnormalities in neural morphology and electrophysiology (13). Collectively, the findings of the latter study indicated an important role for JAM-C in maintaining the integrity and function of peripheral nerves and suggested an association between defective expression of JAM-C and pathogenesis of inherited and/or acquired peripheral neuropathies. However, as the severe and complex phenotype of the complete JAM-C-KO mice makes the study of the functional role of JAM-C in nerves difficult, to extend our previous works we now report on the generation and investigations of a novel mouse colony in which the expression of JAM-C is selectively deleted in SCs. Nerves from these animals exhibited mild morphological and functional defects and behavioral tests revealed muscle weakness and hypersensitivity to mechanical nociceptive stimuli. The findings of the present study also report on previously unknown expressions of JAM-C in peripheral nerves, specifically in finely myelinated sensory fibers and at cell–cell junctions of perineural cells. Overall, through the generation and characterization of a novel conditional-KO mouse colony with SC-specific deletion of JAM-C, the findings of this study provide greater insight into the expression and function of JAM-C in peripheral nerves.
MATERIALS AND METHODS
Antibodies
Antibodies used in this study are listed in Table 1. For double or triple staining, some antibodies were directly conjugated with Alexa dyes using the Alexa-Fluor monoclonal antibody labeling kits (Invitrogen, Carlsbad, CA, USA). Otherwise, appropriate Alexa fluorescently labeled secondary antibodies were used (Invitrogen).
Table 1.
Antibodies used for immunofluorescence staining and Western blotting
| Antigen | Species | Use | Clone | Source |
|---|---|---|---|---|
| JAM-C | Rabbit | IF, WB | pAb, 322501 | B.A.I. (16) |
| JAM-C | Rat | IF, WB | mAb, H33 | B.A.I. (7) |
| JAM-C | Rat | IF, WB | mAb, H36 | B.A.I. (7) |
| JAM-A | Rat | IF | mAb, BV11 | Elisabetta Dejana (Istituto Fondazione Italiana per la Ricerca sul Cancro di Oncologia Molecolare, Milan, Italy; ref. 50) |
| Claudin-19 | Rabbit | IF, WB | pAb | Alan S. Yu (University of Southern California, Los Angeles, CA, USA; ref. 51) |
| Caspr | Rabbit | WB | pAb | Elior Peles (Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot, Israel; ref. 52) |
| VE-Cadherin | Rat | IF | mAb, BV14 | eBiosciences (San Diego, CA, USA) |
| E-Cadherin | Rat | IF, WB | mAb, ECCD-2 | Invitrogen (Carlsbad, CA, USA) |
| Claudin-1 | Rabbit | WB | pAb | Invitrogen |
| Mupp-1 | Rabbit | WB | pAb | Invitrogen |
| α-SMA | Mouse | IF | mAb, 1A4 | Sigma-Aldrich (St. Louis, MO, USA) |
| Laminin α2 | Rat | IF | mAb, 4H8-2 | Sigma-Aldrich |
| β-Tubulin | Mouse | WB | mAb, TUB 2.1 | Sigma-Aldrich |
| PanNav | Mouse | IF | mAb, K58/35 | Sigma-Aldrich |
| Neurofilament-M | Rabbit | WB | pAb | Millipore (Bedford, MA, USA) |
| L1 | Rat | IF | mAb, 324 | Millipore |
| Myelin P0 | Rabbit | WB | pAb | Abcam (Cambridge, UK) |
| Collagen IV | Rabbit | IF | pAb | Abcam |
| CGRP | Rabbit | IF | pAb | Biomol (Exeter, UK) |
| Kv1.1 | Rabbit | IF | pAb | Alomone Labs (Jerusalem, Israel) |
IF, immunofluorescence staining; mAb, monoclonal antibody; pAb, polyclonal antibody; WB, Western blotting.
Animals
JAM-C SC KO mice (P0Cre;JAM-Cf/f) were generated by crossing JAM-C floxed mice (JAM-Cf/f; ref. 25) with P0Cre transgenic mice (26) and maintained on a mixed 129Sv:C57BL/6 background. JAM-Cf/f littermates were used as controls for JAM-C SC KO mice. Transient receptor potential cation channel V1 (TRPV1)−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). JAM-C complete deficient mice (JAM-C−/−, on a 129Sv:C57BL/6 background) were obtained as gift from Dr. Ralf Adams (Max-Planck-Institute for Molecular Biomedicine, Münster, Germany) and generated as previously detailed (10). WT C57BL/6 mice were obtained from Harlan-Olac (Bicester, UK). Animal experiments were conducted in accordance with the UK legislation.
Western blotting
Tissues were homogenized in RIPA buffer using the Precellys24 beat-beading system (Bertin Technologies, France). Samples were resolved on 10% polyacrilamide SDS-PAGE gels and electrotransferred onto PVDF membranes (Millipore, Bedford, MA, USA). Membranes were incubated overnight with primary antibodies and 1 h with appropriate HRP-conjugated secondary antibodies and developed using Supersignal West Pico chemoluminescent substrate (Thermo Scientific, Waltham, MA, USA). Photodensitometric analysis was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence staining and confocal microscopy
Tissues (ear, cremaster muscle, and nerves) were dissected and immunostained as described previously (13) and analyzed using a Zeiss LSM5 PASCAL confocal laser-scanning microscope (Zeiss, Welwyn Garden City, UK) equipped with Ar (λex: 488 nm) and HeNe (λex: 543 and 633 nm) lasers. Multiple Z-stack images at a resolution of 1024 × 1024 were acquired with oil immersion Plan-Apochromat 63 × 1.4 NA or A-Plan 40 × 0.65 NA objectives and reconstructed in 3D using the image processing software IMARIS (Bitplane, Zurich, Switzerland). JAM-C and VE-cadherin levels in perineural cells and E-cadherin and claudin-19 in paranodes and SLIs were quantified in 3D reconstructed images using IMARIS software as described previously (22).
Hematological parameters
Circulating leukocytes and arterial blood pressure were quantified as previously reported (19).
Behavioral experiments
Muscular strength was measured using a grip strength meter (Linton Instrumentation, Norfolk, UK). Mice were allowed to grip onto a wire and then were pulled by the tail in the opposite direction. The forelimb grip strength preceding the loss of grip was measured by a digital scale according to the manufacturer's unit of force (grams). Experiments were performed in a blinded fashion, and sessions consisting of 3 trials with a 15-min recovery time were repeated on 3 different days. Touch sensation in the hindpaws was assessed using the Von Frey hair test. Animals were placed in clear plexiglass boxes with plastic mesh flooring and allowed to acclimatize for ∼15 min until exploratory behavior ceased. Graded Von Frey hairs were applied to midplantar surface of the hindpaws, and the withdrawal responses were measured. Mice were considered to respond to the stimulus when hairs produced ≥3 withdrawals in 5 trials. The experiments were performed in a blinded fashion and repeated 3 times on different days.
Carrageenan-induced paw edema
Mice received a subplantar injection of λ-carrageenan (500 μg in 50 μl; Sigma, St. Louis, MO, USA) or PBS as a control. The paw volume was measured at different time points after stimuli injection with a hydroplethysmometer equipped with mouse paw adaptors (Ugo Basile, Varese, Italy).
Neuropeptide depletion
Neuropeptide depletion from sensory neurons was induced in anesthetized mice by a single subcutaneous injection of capsaicin (50 mg/kg, Sigma) in 10% ethanol, 10% Tween-80, and PBS (27). Mice were allowed to recover for 3 d before being used for experiments. Depletion of neuropeptides was confirmed by immunofluorescence staining of mouse ears (Supplemental Fig. S4B).
In vivo skin plasma extravasation assay
Evans blue (5% in saline; 5 μl/g body weight) was injected into the tail vein of anesthetized mice, and 5 and 20 min later, mustard oil (5% in mineral oil) or vehicle was topically applied onto the ears. Mice were sacrificed 30 min after the initial dose. To measure plasma extravasation, the accumulated Evans blue was eluted and quantified from the dissected ears, as reported previously (22).
Electrophysiology
Mice were anesthetized with tribromoethanol (0.02 ml/g body weight, i.p.) and kept warm under a heating lamp. Sciatic nerve motor conduction velocity was obtained as reported before (28).
Nerve morphology
Analysis of nerve semithin and ultrathin sections was conducted as described previously (29); sections were examined by light microscopy or electron microscopy (EM). Determination of g ratio on semithin sections was performed by digitally tracing the inner and outer layers of myelinated fibers using Leica Qwin software (Leica Microsystems, Wetzlar, Germany). The g ratio was calculated by dividing the diameter of the inner circumference of the axon (without myelin) by the diameter of the outer circumference of the total fiber (including myelin).
Statistics
Data analysis was performed using the statistical software GraphPad Prism 4 (GraphPad, San Diego, CA, USA). Results are expressed as means ± se, unless stated otherwise. Statistical significance was assessed by Student's t test, 1-way ANOVA with Student-Newman-Keuls multiple comparison test, or 2-way ANOVA with Bonferroni post hoc test as appropriate. Analysis of the withdrawal response and scatter plots was assessed by Fisher's exact test and Mann-Whitney test, respectively. Values of P < 0.05 were considered significant.
RESULTS
JAM-C is expressed in a broad range of peripheral nerves
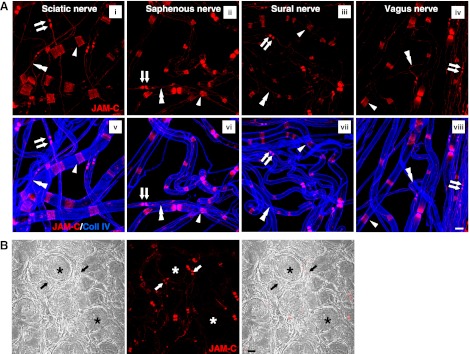
In a recent study, we reported on the novel expression of JAM-C in peripheral nerves, specifically localized to SCs at junctions between adjoining myelin end loops (13). As our previous work largely analyzed the expression profile of JAM-C in sciatic nerves, to extend these findings we have now investigated and compared the expression of JAM-C in fibers isolated from several different types of nerves, namely the vagus (supplying parasympathetic motor and sensory fibers) and the saphenous and sural (supplying largely sensory nerve fibers), as compared with the sciatic nerve (supplying a mixture of motor and sensory fibers). For this purpose, whole-mount teased fibers isolated from fixed nerve samples were immunostained for JAM-C and collagen IV (a component of the SC basement membrane) and analyzed by confocal microscopy. The results show that in all samples, nerve fibers expressed JAM-C in SCs, at sites characteristic of junctional regions of noncompact myelin (Fig. 1A), in line with our previous findings (13). Junctional structures of SCs have been reported to occur in 3 different locations of noncompact myelin, i.e., the paranodal regions on either side of the nodes of Ranvier, the SLIs, and the inner and outer mesaxons (30), and our results show that JAM-C is expressed at these sites in myelinated fibers of all nerves studied (Fig. 1A). We also analyzed expression of JAM-C in the dorsal root ganglia (DRG). Here JAM-C was not detected in the neuronal cell bodies but in line with the above was detected in paranodes of fibers stemming from them within the DRG (Fig. 1B). Notably, JAM-C was not detected on teased nonmyelinating SCs in sciatic nerve (identified through the use of the marker L1; Supplemental Fig. S1), confirming the specific expression of this adhesion molecule in myelinating SCs.
Figure 1.
JAM-C expression in different nerves. A) Confocal images showing immunostaining of JAM-C and collagen IV in whole-mounted teased fibers from sciatic (i, v), saphenous (ii, vi), sural (iii, vii), and vagus (iv, viii) nerves. In all nerves studied, JAM-C was expressed at paranodal regions (arrows) on both side of the nodes of Ranvier, SLIs (arrowheads) and mesaxons (double arrowheads). B) Dorsal root ganglia sections showed lack of JAM-C in the neuronal cell bodies (asterisks), but positive expression was seen in paranodes of the fibers originating directly from them (arrows). Scale bars = 10 μm.
Expression of JAM-C in sensory and motor fibers
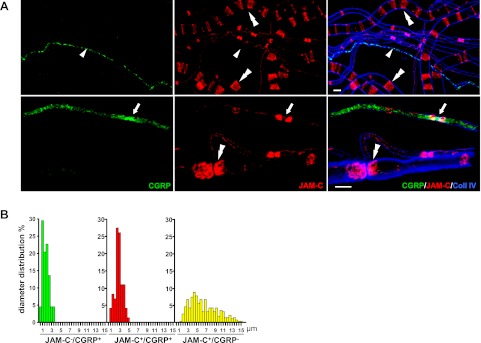
We next sought to investigate the expression of JAM-C in more detail in different types of nerve fibers. For this purpose, we chose to investigate the profile and characteristics of fibers in sciatic nerves, a nerve trunk that contains a mixture of motor and sensory fibers, thus enabling direct comparison of JAM-C expression in multiple types of fibers. Sensory fibers were identified through expression of the neuropeptide calcitonin gene related peptide (CGRP). Immunofluorescence staining of teased sciatic nerve fibers showed that 62.4% of the CGRP+ fibers expressed JAM-C, confirming that sensory fibers can be JAM-C+ (Fig. 2A). To characterize the different type of fibers expressing JAM-C, sciatic nerve teased fibers were classified into 3 populations according to their diameter and the expression profile of JAM-C/CGRP (Fig. 2B). The JAM-C−/CGRP+ population of fibers showed an average diameter of 2.72 ± 0.11 μm and highly likely comprise the slow-conducting small unmyelinated sensory C fibers. JAM-C+/CGRP+ fibers showed an average diameter of 3.81 ± 0.10 μm, highly characteristic of the medium-size finely myelinated Aδ sensory fibers. Finally, the JAM-C+/CGRP− fibers exhibited a wider diameter distribution with an average diameter of 7.58 ± 0.18 μm, which would mostly include large motor fibers, as well as possibly a population of Aδ sensory fibers, which are negative for CGRP, and the large Aβ sensory fibers. Together, these results indicate that JAM-C is expressed in both motor and sensory myelinated fibers, which suggests that it may be involved in the regulation of nociception and/or neurogenic inflammation, issues that were addressed in subsequent studies detailed below.
Figure 2.
JAM-C is expressed in medium- but not small-diameter murine sensory fibers. A) Expression profiles of JAM-C and CGRP discriminate 3 subpopulations of fibers in mouse sciatic nerves: JAM-C−/CGRP+, unmyelinated small sensory C fibers (arrowheads); JAM-C+/CGRP+, thinly myelinated Aδ sensory fibers (arrows); and JAM-C+/CGRP−, myelinated fibers, including large motor fibers, a subset of Aδ fibers, and Aβ fibers (double arrowheads). B) Diameter distribution of the 3 fiber subpopulations described in panel A (JAM-C−/CGRP+, n=44; JAM-C+/CGRP+, n=73; JAM-C+/CGRP−, n=286 fibers). Scale bars: 10 μm.
Generation of mice with selective deletion of JAM-C in SCs
To generate a clean model for investigation of JAM-C in peripheral nerves, we developed mutant mice with selective deletion of JAM-C in SCs. For this purpose, homozygous mice for floxed JAM-C (25) were crossed with transgenic mice hemizygous for protein 0 (P0)-Cre, which activates Cre-mediated DNA recombination of flanked lox-p regions specifically in SCs of peripheral nerves (26), to finally produce the JAM-C SC KO mice.
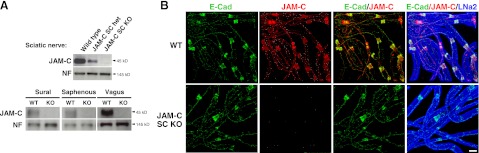
Western blot (WB) of isolated sciatic, vagus, sural, and saphenous nerves confirmed the lack of JAM-C expression in peripheral nerves from JAM-C SC KO mice as compared to wild-type (WT) control or heterozygous littermates (Fig. 3A). The expression and localization of JAM-C in whole-mount teased fibers isolated from WT and JAM-C SC KO mouse sciatic nerves was also investigated by immunofluorescent staining and confocal microscopy. Samples were immunostained for JAM-C, E-cadherin (a marker for adherens junctions) and laminin α2 (a component of SC basement membrane). Fibers from WT mice showed the characteristic junctional expression of JAM-C, colocalized with E-cadherin, in SCs (Fig. 3B). The JAM-C SC KO fibers showed no expression of JAM-C but normal localization and expression of E-cadherin and laminin α2.
Figure 3.
Characterization of SC-specific JAM-C-KO mice. A) WB analysis showed deletion of JAM-C as compared to neurofilament-M (NF) in sciatic, sural, saphenous, and vagus nerves from JAM-C SC KO mice as compared to littermate WT or heterozygous controls (5 μg of homogenate protein was loaded). B) Analysis of sciatic nerve teased fibers by confocal microscopy confirmed the deletion of JAM-C from SCs in JAM-C SC KO as compared to WT mice. Expression of E-cadherin and laminin-α2 showed no differences between strains. Scale bar = 10 μm.
The mutant mice were born at the expected mendelian ratio and, interestingly, in contrast to the severe phenotype noted in the complete JAM-C-KO mice (10, 17, 19), the JAM-C SC KO mice were fertile, appeared healthy, and did not develop megaoesophagus (Supplemental Fig. S2A, B). Furthermore, these mice did not show any significant difference in their growth, hematological parameters, or blood pressure, as compared to littermate controls (Supplemental Fig. S2C, D).
SC-specific deletion of JAM-C reveals novel expression of JAM-C in perineural cells
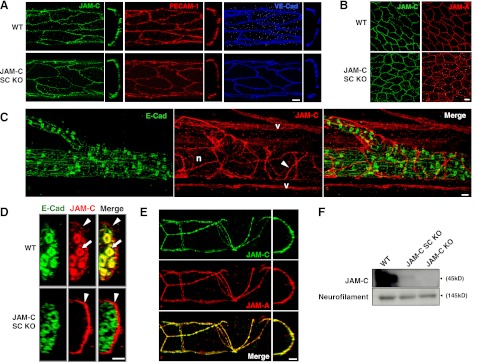
To determine whether the deletion of JAM-C in the JAM-C SC KO mice was exclusively limited to SCs, the expression of JAM-C in other cell types known to express this molecule (i.e., endothelial and epithelial cells) was investigated by immunofluorescent staining and analysis by confocal microscopy of whole-mount cremaster muscles. This skeletal muscle is well innervated, is highly vascularized, and is encased in an epithelial layer, thus enabling the expression of JAM-C to be investigated in nerves, ECs, and epithelial cells in the same tissue. Tissues from WT and JAM-C SC KO mice showed comparable expression profiles of JAM-C in blood vessels, where JAM-C was largely expressed at junctions between adjacent ECs colocalized with other EC junctional molecules; i.e., PECAM-1 and VE-cadherin (Fig. 4A). Cremaster muscles from WT and mutant mice also showed similar expression of JAM-C in epithelial cells, colocalized with JAM-A (Fig. 4B).
Figure 4.
Expression of JAM-C in endothelial, epithelial, and perineural cells from JAM-C SC KO mice. A) Longitudinal (left panels) and cross-sectional (right panels) confocal images of cremasteric venules showing normal expression of JAM-C at junctions of ECs (stained for PECAM-1 and VE-cadherin) from JAM-C SC KO as compared to WT mice. B) Confocal images of epithelial cells (stained for JAM-A) from JAM-C SC KO as compared to WT mice. C) Images of whole-mount cremaster muscles from JAM-C SC KO mice demonstrated deletion of JAM-C from SC (stained for E-cadherin) in peripheral tissues. Unexpectedly, JAM-C (arrowheads) was found to be expressed surrounding peripheral nerve bundles (n). Specific staining of JAM-C is shown by the positive staining of microvascular ECs (v). D) Cross-section analysis of confocal images from cremasteric nerve bundles showed expression of JAM-C at both perineural (arrowheads) and SCs (arrows) in WT animals, whereas JAM-C SC KO mice only expressed JAM-C in perineural cells (arrowheads). E) Expression of JAM-C and JAM-A at junctions of perineural cells from JAM-C SC KO mice. F) WB of sciatic nerves from WT, JAM-C SC KO, and JAM-C complete-KO mice (50 μg of homogenate protein was loaded). Neurofilament-M was used as a loading control. Scale bars = 10 μm.
Of interest, and surprisingly, we noted that in tissues from JAM-C SC KO mice, JAM-C was expressed at sites associated with nerve bundles (Fig. 4C). Double staining of tissues for JAM-C and E-cadherin showed that this JAM-C expression was not linked with SCs and was, in fact, localized to junctions between adjacent perineural cells within the perineural sheath that surrounds peripheral nerves (Fig. 4C, D). Using tissues from WT mice, we previously reported on the expression of JAM-A on perineural cells but not SCs (13). However, as WT tissues express high levels of JAM-C on SCs, the relatively lower expression of JAM-C on perineural cells cannot readily be distinguished from that on SCs and was, therefore, not noted in our previous investigations. The difference in expression levels of JAM-C in these two structures is clearly demonstrated in Fig. 4D, where, at saturation image-acquisition settings in WT tissues, the high JAM-C fluorescence signal detected in E-cadherin+ SCs surrounding individual axons (indicated by arrows) makes the detection of the low- JAM-C-expressing perineural cells surrounding nerve bundles (arrowheads) difficult. In the present study, the availability of SC-specific JAM-C-KO mice enabled us to observe and, for the first time, report on the expression of JAM-C at junctions between adjacent perineural cells. Indeed, costaining of JAM-C SC KO cremaster muscle tissues for JAM-C and JAM-A illustrated the direct colocalization of these molecules in the perineural sheath surrounding peripheral nerves (Fig. 4E). Of note, detailed quantitative analysis showed no significant differences between strains in the levels of perineural cell JAM-C (Supplemental Fig. S3). Finally, the small contribution of perineural vs. SC JAM-C to the total JAM-C content in isolated sciatic nerves was also illustrated by WB (Fig. 4F), comparing the bands for JAM-C in nerves from WT (expressing JAM-C in both SCs and perineural cells) and JAM-C SC KO mice (expressing JAM-C only in perineural cells). Notably, no band for JAM-C was detected in sciatic nerves isolated from JAM-C complete-KO mice (lacking JAM-C in both SCs and perineural cells). Together, the findings show that deletion of JAM-C in JAM-C SC KO mice is specific to SCs and that the availability of these mice identified the previously undetected expression of JAM-C in another component of nerves, within the perineural sheath at junctions between adjacent perineural cells.
JAM-C SC KO mice exhibit neural function and behavioral defects
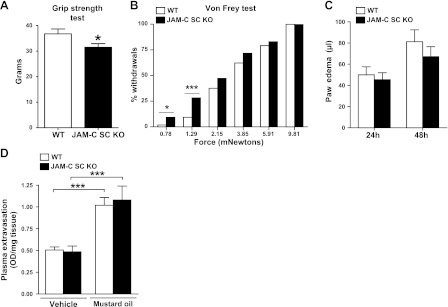
To investigate potential defects in neuronal functions of the JAM-C SC KO mice, we initially evaluated their electrophysiological properties. Sciatic nerves from JAM-C SC KO mice exhibited a small but significant reduced conduction velocity after stimulation compared to nerves from littermate controls (31.7±1.7 and 35.9±0.7 m/s, respectively; P<0.05 for n=8 nerves; ∼12% slower). This mild alteration in nerve function was accompanied by a poorer performance of the JAM-C SC KO mice in a behavioral test of muscular strength (Fig. 5A). Because JAM-C is expressed in sensory fibers (Fig. 2), we also investigated the potential role of SC JAM-C in models of sensory nerve function. Specifically, we investigated the phenotype of the JAM-C SC KO mice in models of acute pain and neurogenic inflammation. With respect to the former, we analyzed the hindpaw withdrawal response of JAM-C SC KO mice to static mechanical stimuli using calibrated Von Frey filaments, a well-characterized method for measuring nociceptive reflex behavior. In this assay, the JAM-C SC KO mice showed a significant increase in the number of paw withdrawals in response to low-intensity stimuli as compared to WT littermates (Fig. 5B), indicating mechanical hypersensitivity in the KOs.
Figure 5.
JAM-C SC KO mice exhibit mild neural functional and behavioral defects. A, B) JAM-C SC KO mice showed reduced grip strength (A) and higher withdrawal response to Von Frey hairs (B) as compared to WT controls (n=9 mice/group), indicating muscular weakness and mechanical hypersensitivity, respectively. C, D) JAM-C SC KO mice showed no significant differences as compared to WT in two models of neurogenic inflammation: 24- and 48-h carrageenan-induced paw edema formation (C; n=8 mice/group) and mustard oil-induced ear skin plasma extravasation (D; n=3–5 animals/group). *P < 0.05; ***P<0.001.
To investigate the role of SC JAM-C in models of neurogenic inflammation, we assessed mouse responses to the inflammatory stimuli carrageenan and mustard oil, stimuli known to elicit sensory nerve-mediated reactions (31, 32). In initial studies, induction of paw edema by subplantar injection of carrageenan was shown to have a C-fiber component (Supplemental Fig. S4), as it was dependent on capsaicin-sensitive unmyelinated sensory neurons and on the functional presence of TRPV1. TRPV1 is a receptor mainly expressed in C fibers and involved in the release of a number of inflammatory mediators (33). Both in this model and in a model of skin plasma extravasation as induced by topical application of mustard oil to the ears, the JAM-C SC KO mice exhibited identical responses to those detected in littermate controls (Fig. 5C, D).
Collectively, the data indicate that the specific deletion of JAM-C from SCs in peripheral nerves causes mild motor and sensory function defects but no defects in neurogenic inflammation. These results are in line with the specific expression of JAM-C in different types of nerve fibers; i.e., expression in motor fibers and Aδ but not sensory C fibers (Fig. 2).
JAM-C SC KO nerves exhibit mild morphological defects
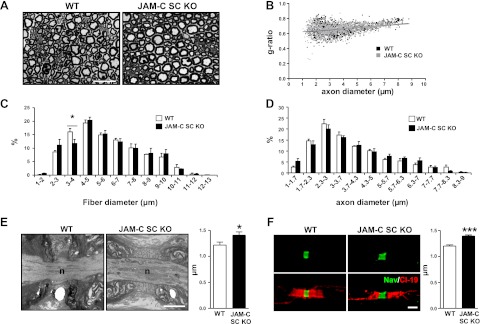
To investigate gross morphology of nerve fibers from JAM-C SC KO mice, semithin cross sections of sciatic nerves were analyzed by light microscopy (Fig. 6A). Quantification of the acquired images showed no significant differences between groups in the averaged g ratios (0.66±0.04 and 0.66±0.03 for the WT and JAM-C SC KO mice, respectively, with a total of 1161 and 1263 fibers analyzed from 4 animals/group) or in the scatter plot of g ratios of individual fibers vs. axon diameters (Fig. 6B). However, JAM-C SC KO mice exhibited a significant decrease in the percentage of 3- to 4-μm-diameter fibers (Fig. 6C), while the distribution of the axonal diameter was not altered between strains (Fig. 6D), suggesting that axon myelination defects in specific subpopulations of fibers may occur within the KO sciatic nerves.
Figure 6.
Neural morphology analysis of JAM-C SC KO mice. A) Representative images of transverse semithin sections of WT and JAM-C SC KO sciatic nerve fibers. B) Scatter plot of g ratio according to axonal diameter of sciatic nerves from WT and JAM-C SC KO mice. C, D) Distribution of sciatic nerve fiber (C) and axon (D) diameters from WT and JAM-C SC KO mice. E, F) Average length of the node of Ranvier was quantified from WT and JAM-C SC KO mouse sciatic nerves by EM (E; n=19 and 30 nodes, respectively, from 3 mice/group) and by confocal microscopy (F) with teased sciatic nerve fibers immunostained for Nav channel and claudin-19 (n=113 and 109 nodes, respectively, from 3 mice/group). Scale bars = 20 μm (A); 2 μm (E, F). *P < 0.05; ***P < 0.001.
We next addressed the possibility that nerves from the mutant mice may have disrupted expression profile of key proteins involved in maintaining the structural integrity and hence normal function of peripheral nerve fibers. In this context, WB analysis of sciatic nerves from JAM-C SC KO mice showed normal levels of key adhesion molecules present in the SC myelin compartment, such as E-cadherin, claudin-1, claudin-19, and Mupp-1, as compared to WT nerves (Supplemental Fig. S5). Levels of the structural proteins neurofilament-M and peripheral myelin-P0 were also normal in the KO mice. Confocal microscopy analysis of immunofluorescence-stained isolated sciatic nerve fibers also showed normal distribution and localization of E-cadherin, claudin-19, caspr, and Kv1.1 channel in mutant sciatic nerve fibers (Fig. 3B and Supplemental Figs. S6A and S7). In addition, exhaustive analysis of E-cadherin and claudin-19 expression specifically in paranodes and SLIs showed no differences in the levels of these proteins within these specialized structures in WT and KO nerves (Supplemental Fig. S6A, B). Also, we found no differences between WT and JAM-C SC KO nerves in the number of SLIs per millimeter of sciatic nerve fiber [13.7±1.1 vs. 14.1±0.7 SLIs/mm, for WT (1059 SLIs analyzed) and KO nerves (897 SLIs analyzed), respectively, n=3 mice/group] or in the internodal length [708±15 vs. 739±42 μm for KO (n=67 fibers) and WT (n=58) fibers, respectively, with n=3 mice/group].
Ultrastructural EM analysis of transverse sections of sciatic and vagus nerves showed no significant defects in the myelin structure of JAM-C SC KO fibers or in the “kissing points” of tight junctions on the mesaxons as compared to WT nerve fibers (data not shown). However, EM analysis of sciatic nerve longitudinal sections revealed subtle defects in the alignment of sequential paranodal terminal loops in JAM-C SC KO fibers as compared to WT. Specifically, as compared to WTs, in KO fibers a higher proportion of terminal loops exhibited an apparent looser adhesion to adjacent loops and the axon (5.2 and 13.0% of abnormal loops in WT and SC KO, respectively, with 210 and 455 loops analyzed from n=3 mice/group, P<0.01). Furthermore, this apparent abnormality in paranodal terminal loops was associated with a significant increase in the average length of the nodes from JAM-C SC KO nerves as compared to WT, as shown by EM analysis (Fig. 6E). The morphology of the nodes of Ranvier was also investigated in sciatic nerve teased fibers immunofluorescently stained for anti-Nav channel and analyzed by confocal microscopy. Analysis of images showed that, although Nav channels remained tightly clustered at the nodes (Fig. 6F), they occupied a longer portion of the axolemma, confirming a significant increase in nodal length (Fig. 6F). Collectively, the results indicate that selective deletion of JAM-C from SCs results in subtle defects in the paranode/node structure, a phenomenon that may account for the neural dysfunctions noted in JAM-C SC KO nerves and mice.
DISCUSSION
JAM-C is a cell adhesion molecule that to date has been implicated in cell polarity and multiple inflammatory and vascular responses (1, 2). Recent findings from our group have also shown that JAM-C is expressed in myelinated peripheral nerves, localized to SCs, and plays a critical role in their integrity and function (13). As the latter remains the only published work to date reporting on the role of JAM-C in SCs, to further investigate the functions of JAM-C in nerves here we report on the generation of a novel mouse colony in which JAM-C is deleted selectively in SCs, JAM-C SC KO. The characterization and investigations of these mice provided clear cut evidence for the involvement of SC JAM-C in the fine organization of peripheral nerves and in modulating multiple neuronal responses. In addition, the present study characterizes the expression of JAM-C on myelinated sensory fibers, and reports on the previously undetected expression of JAM-C at cell-cell junctions of perineural cells.
Axons exhibit elaborate interactions with myelinating glial cells; i.e., SCs in the peripheral nervous system (PNS) and oligodendrocytes in the central nervous system (CNS). These interactions involve the existence of multiple polarized domains composed of numerous cell adhesion/scaffolding molecules and ion channels that collectively ensure rapid saltatory nerve conduction (30, 34). Specifically, glial cells wrap around axons in such a way so as to provide electrical insulation of segments of the axon that are separated by distinct, noninsulated regions known as nodes of Ranvier (30, 34). These sites are rich in voltage-gated sodium channels, causing the action potential to be propagated rapidly. To support efficient saltatory conduction, tight interactions must exist between the axon and the glial cells, both at regions that flank the nodes of Ranvier (paranodal junctions) and between adjacent membrane layers of individual glial cells. Compact myelin seals the intermembranous spaces, whereas junctions within noncompact myelin, which are found in the outer and inner mesaxons, the SLIs, and the paranodal regions, collectively seal the borders of the wrapped glial cell and contribute to maintaining the integrity of the myelin sheath. Detailed knowledge of the molecules involved in forming junctions at these regions is still lacking (30), but it is known that the complexes include adherens junctions (composed of E-cadherin; ref. 35) and gap junctions (composed of connexin 32; ref. 36). Furthermore, EM studies have indicated the existence of tight junctions (37, 38), and to date, a number of claudin molecules have been identified at these regions in both the CNS and PNS (39–41), though recent studies have reported on some distinct differences in expression of these molecules in human and rodent SCs (42). In the present study, we analyzed the expression profile of JAM-C in multiple large nerves composed of different types of nerve fibers. Immunofluorescent staining and confocal microscopy of isolated sciatic, vagus, saphenous, and sural nerves, as well as DRG sections, collectively demonstrated the expression of JAM-C in motor and sensory nerve fibers. Closer analysis of expression of JAM-C in sensory nerve fibers (detected through immunostaining for the neuropeptide CGRP) showed its presence on medium-size finely myelinated Aδ sensory fibers (mean diameter ∼4 μm) and, as anticipated, not on small sensory fibers (diameter ∼2.5 μm), representing unmyelinated C-fibers. In all JAM-C+ fibers, JAM-C was expressed at paranodal regions, SLIs, and the mesaxonal bands. The present findings, in conjunction with our previous work (13), make a significant contribution to the field by demonstrating the existence of JAM-C in SCs at regions of noncompact myelin in motor and sensory nerves of the PNS, an expression profile that appears to hold true for both human and mouse nerves. As JAM-C is an adhesion molecule commonly localized at tight junctions, our collective results have indicated that JAM-C is expressed at tight junctions between the membrane folds of individual SCs. Based on this expression profile, it would be plausible to hypothesize a role for JAM-C in maintaining the integrity, architecture, and function of myelinated peripheral nerves, as discussed below.
Analysis of KO animals is the most direct mode of investigating the functional role of a molecule, especially within in vivo models. In line with this approach, previous work from our laboratory investigated the role of JAM-C in neural functions using JAM-C-KO mice (13). These studies provided evidence for the involvement of JAM-C in maintaining the integrity of the myelin sheath, as indicated by structural and functional defects in JAM-C-deficient peripheral nerves, as well as behavioral motor abnormalities in these animals (13). However, as JAM-C exhibits a broad cellular expression profile, and JAM-C-KO animals have a severe phenotype (e.g., male infertility, a high mortality rate, growth impairment, and immune defects; refs. 10, 17, 19, 23), we considered it essential to extend our previous works by investigating the functional role of SC JAM-C through the development of conditional mice with selective deletion of JAM-C in SCs. This approach would rule out the potential interference of JAM-C deletion in other cell types on neuronal functional readouts. For this reason, JAM-C SC KO mice were generated by Cre-mediated recombination using P0 as the specific promoter for targeting SCs (26).
Initial characterization of JAM-C SC KO mice confirmed specific ablation of JAM-C in nerves and its lack of expression at junctional sites in SCs. Furthermore no evidence of compensatory mechanisms was noted, as the protein level and/or localization of key molecules implicated in maintaining the integrity and architecture of myelinated peripheral nerves (e.g., E-cadherin, claudin-1 and -19, Mupp-1, caspr and myelin-P0) appeared to be normal. Of importance, contrary to the severe phenotype of the full JAM-C-KO mice (10, 17, 19, 23), the JAM-C SC KO male mice were fertile, and the crossing with JAM-Cwt/f females produced litters at expected mendelian ratios. Mutant animals also appeared healthy and did not show any differences in their growth, size, weight, or hematological parameters, as compared to littermate controls. Collectively, the results indicate that SC JAM-C does not play a significant role in notable characteristics of the full JAM-C-KO mice, e.g., growth, development of megaoesophagus, male sterility, and susceptibility to infections.
As part of the characterization studies of the JAM-C SC KO mice, we noted normal expression of JAM-C at junctions between adjacent endothelial and epithelial cells but also identified, for the first time, the expression of JAM-C at contacts between perineural cells. These cells are the building blocks of the perineural sheath that encase nerve bundles, and we have reported previously on the expression of JAM-A on these cells (13). The expression of JAM-C on perineural cells was not noted in our earlier works or when initially performing WB analysis of nerve samples due to its relatively low expression as compared to the levels expressed on SCs, an issue that was overcome with the availability of nerve samples from JAM-C SC KO mice. The novel identification of JAM-C at regions between adjacent perineural cells, in addition to the expression of JAM-A, is highly supportive of the existence of bona fide intercellular junctions defining the contact points between neighboring perineural cells, as suggested by early electron microscopists (43). The existence of JAMs in the perineural sheath suggests a role for these molecules in maintaining the barrier function of this structure, as well as possibly contributing to its integrity, normal cellular functions (e.g., generation of basement membrane) and polarity. Deletion of JAM-C from this protective sheath in the full JAM-C-KO mice may have contributed to some of the neuronal defects observed in these mice (13), strengthening the argument for a need for investigations of neural functions in the JAM-C SC KO mice.
Functional analysis showed that compared to WTs, JAM-C SC KO mice exhibited mild muscular weakness as well as reduced sciatic nerve conduction velocity. Overall, the noted defects were milder than those quantified in JAM-C complete-KO mice (13), which suggests that the absence of JAM-C in other cells (e.g., perineural cells) may have contributed to the observed neuronal defects of the full-KO animals. Of relevance, mice deficient in SC-derived desert hedgehog exhibit defective nerve-tissue barrier due to abnormal junctions between adjacent perineural cells, resulting in enhanced permeability of this barrier to proteins and neutrophils (44). Defective barrier function of the perineural sheath may compromise the integrity and hence function of the “vulnerable” exposed encased nerves, rendering nerves more susceptible to trauma.
Since JAM-C was detected on myelinated sensory nerves, we also tested the JAM-C SC KO mice in models of nociceptive reflex behavior and neurogenic inflammation. Specifically, by measuring the withdrawal response to mechanical stimuli using calibrated Von Frey hairs, JAM-C SC KO mice exhibited an exaggerated response to lower stimuli as compared to littermate controls, indicating mechanical hypersensitivity in the mutants, but responded normally in two inflammation models. These results are in line with our findings indicating expression of JAM-C on myelinated Aδ sensory fibers, known to be involved in nociception (45), and not on unmyelinated C fibers, implicated in development of neurogenic edema and leukocyte infiltration. Overall, the present results demonstrate that through its expression in both motor and sensory myelinated peripheral nerve fibers, JAM-C mediates muscle control and processing of pain signals.
Finally, in investigating the mechanisms through which SC JAM-C may be supporting normal neuronal functions, we hypothesized that its presence was critical in maintaining the structure and development of normal polarized domains within peripheral nerves. However, exhaustive analysis of the morphology of nerve fibers from JAM-C SC KO mice failed to show any detectable defects of tight junctions within SCs that are important for intermembraneous sealing of the glial cells and hence maintaining the integrity of the myelin sheath. However, nerves from JAM-C SC KO mice showed mild defects in the alignment of their paranodal terminal loops and increased length of the nodes of Ranvier, as compared to WTs. Such abnormalities may account for the defective neural functions observed in the mutants, as increased nodal length has been reported in several genetically modified mouse strains that exhibit defects in the paranodal region (46), including caspr-null mice, which also exhibit neural dysfunction (47). Hence, it appears that absence of SC JAM-C results in loose adhesion of terminal paranodal loops to adjacent loops, in line with our previous findings using the complete JAM-C-KO mice (13), and that this defect causes increased nodal length that can underlie defective nerve conduction.
In summary, our studies to date have presented evidence for the expression of JAM-C at junctional sites in the perineural sheath that provides a barrier and protective coverage of nerve bundles, as well as JAM-C expression at autotypic junctions between the paranodal loops of SCs in both motor and myelinated sensory nerve fibers. Furthermore, through the generation and use of conditional-KO mice with specific deletion of JAM-C in SCs, we provide conclusive evidence for the involvement of SC JAM-C in regulation of neural functions and acute mechanical pain, rendering these mice a valuable tool for further investigations of the functions of JAM-C in peripheral nerves and in the pathophysiology of peripheral neuropathies. Finally, as numerous mutations are causally linked with different forms of inherited neuropathies, our findings suggest a need for detailed investigations into associations of potential JAM-C mutations with neural disease. To our knowledge, to date no such investigations have taken place with respect to peripheral neuropathies, though, of interest, genome-wide association studies have identified JAM-C as a candidate gene for bipolar disorder (48, 49), which suggests a broader role for JAM-C in neurological functions and disorders.
Supplementary Material
Acknowledgments
This work was funded by the Wellcome Trust (081172/Z/06/Z to S.N.). The study was also supported by the Swiss National Science Foundation (310030-120184 to B.A.I.), the Deutsche Forschungsgemeinschaft and the Center for Regenerative Therapies Dresden (to T.C.), the U.S. National Institute of Neurological Disorders and Stroke (R01NS045630 to M.L.F.), and Telethon Italia (GGP08021 to M.L.F.). The authors thank Stefania Saccucci for superb technical assistance.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CGRP
- calcitonin gene related peptide
- CNS
- central nervous system
- DRG
- dorsal root ganglia
- EC
- endothelial cell
- EM
- electron microscopy
- JAM-C
- junctional adhesion molecule-C
- PNS
- peripheral nervous system
- SC
- Schwann cell
- SLI
- Schmidt-Lanterman incisure
- TRPV1
- transient receptor potential cation channel V1
REFERENCES
- 1. Bradfield P. F., Nourshargh S., Aurrand-Lions M., Imhof B. A. (2007) JAM family and related proteins in leukocyte migration. Arterioscl. Thromb. Vasc. Biol. 27, 2104–2112 [DOI] [PubMed] [Google Scholar]
- 2. Weber C., Fraemohs L., Dejana E. (2007) The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. 7, 467–477 [DOI] [PubMed] [Google Scholar]
- 3. Santoso S., Sachs U. J., Kroll H., Linder M., Ruf A., Preissner K. T., Chavakis T. (2002) The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 196, 679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang T. W., Chiu H. H., Gurney A., Sidle A., Tumas D. B., Schow P., Foster J., Klassen T., Dennis K., DeMarco R. A., Pham T., Frantz G., Fong S. (2002) Vascular endothelial-junctional adhesion molecule (VE-JAM)/JAM 2 interacts with T, NK, and dendritic cells through JAM 3. J. Immunol. 168, 1618–1626 [DOI] [PubMed] [Google Scholar]
- 5. Lamagna C., Meda P., Mandicourt G., Brown J., Gilbert R. J., Jones E. Y., Kiefer F., Ruga P., Imhof B. A., Aurrand-Lions M. (2005) Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: function in junctional complexes and leukocyte adhesion. Mol. Biol. Cell 16, 4992–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arrate M. P., Rodriguez J. M., Tran T. M., Brock T. A., Cunningham S. A. (2001) Cloning of human junctional adhesion molecule 3 (JAM3) and its identification as the JAM2 counter-receptor. J. Biol. Chem. 276, 45826–45832 [DOI] [PubMed] [Google Scholar]
- 7. Aurrand-Lions M., Duncan L., Ballestrem C., Imhof B. A. (2001) JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J. Biol. Chem. 276, 2733–2741 [DOI] [PubMed] [Google Scholar]
- 8. Ody C., Jungblut-Ruault S., Cossali D., Barnet M., Aurrand-Lions M., Imhof B. A., Matthes T. (2007) Junctional adhesion molecule C (JAM-C) distinguishes CD27+ germinal center B lymphocytes from non-germinal center cells and constitutes a new diagnostic tool for B-cell malignancies. Leukemia 21, 1285–1293 [DOI] [PubMed] [Google Scholar]
- 9. Keiper T., Al-Fakhri N., Chavakis E., Athanasopoulos A. N., Isermann B., Herzog S., Saffrich R., Hersemeyer K., Bohle R. M., Haendeler J., Preissner K. T., Santoso S., Chavakis T. (2005) The role of junctional adhesion molecule-C (JAM-C) in oxidized LDL-mediated leukocyte recruitment. FASEB J. 19, 2078–2080 [DOI] [PubMed] [Google Scholar]
- 10. Gliki G., Ebnet K., Aurrand-Lions M., Imhof B. A., Adams R. H. (2004) Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature 431, 320–324 [DOI] [PubMed] [Google Scholar]
- 11. Betanzos A., Schnoor M., Severson E. A., Liang T. W., Parkos C. A. (2009) Evidence for cross-reactivity of JAM-C antibodies: implications for cellular localization studies. Biol. Cell 101, 441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morris A. P., Tawil A., Berkova Z., Wible L., Smith C. W., Cunningham S. A. (2006) Junctional adhesion molecules (JAMs) are differentially expressed in fibroblasts and co-localize with ZO-1 to adherens-like junctions. Cell. Commun. Adhes. 13, 233–247 [DOI] [PubMed] [Google Scholar]
- 13. Scheiermann C., Meda P., Aurrand-Lions M., Madani R., Yiangou Y., Coffey P., Salt T. E., Ducrest-Gay D., Caille D., Howell O., Reynolds R., Lobrinus A., Adams R. H., Yu A. S., Anand P., Imhof B. A., Nourshargh S. (2007) Expression and function of junctional adhesion molecule-C in myelinated peripheral nerves. Science 318, 1472–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson-Leger C. A., Aurrand-Lions M., Beltraminelli N., Fasel N., Imhof B. A. (2002) Junctional adhesion molecule-2 (JAM-2) promotes lymphocyte transendothelial migration. Blood 100, 2479–2486 [DOI] [PubMed] [Google Scholar]
- 15. Orlova V. V., Economopoulou M., Lupu F., Santoso S., Chavakis T. (2006) Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J. Exp. Med. 203, 2703–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamagna C., Hodivala-Dilke K. M., Imhof B. A., Aurrand-Lions M. (2005) Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 65, 5703–5710 [DOI] [PubMed] [Google Scholar]
- 17. Imhof B. A., Zimmerli C., Gliki G., Ducrest-Gay D., Juillard P., Hammel P., Adams R., Aurrand-Lions M. (2007) Pulmonary dysfunction and impaired granulocyte homeostasis result in poor survival of Jam-C-deficient mice. J. Pathol. 212, 198–208 [DOI] [PubMed] [Google Scholar]
- 18. Palmer G., Busso N., Aurrand-Lions M., Talabot-Ayer D., Chobaz-Peclat V., Zimmerli C., Hammel P., Imhof B. A., Gabay C. (2007) Expression and function of junctional adhesion molecule-C in human and experimental arthritis. Arthritis Res. Ther. 9, R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheiermann C., Colom B., Meda P., Patel N. S., Voisin M. B., Marrelli A., Woodfin A., Pitzalis C., Thiemermann C., Aurrand-Lions M., Imhof B. A., Nourshargh S. (2009) Junctional adhesion molecule-C mediates leukocyte infiltration in response to ischemia reperfusion injury. Arterioscl. Thromb. Vasc. Biol. 29, 1509–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vonlaufen A., Aurrand-Lions M., Pastor C. M., Lamagna C., Hadengue A., Imhof B. A., Frossard J. L. (2006) The role of junctional adhesion molecule C (JAM-C) in acute pancreatitis. J. Pathol. 209, 540–548 [DOI] [PubMed] [Google Scholar]
- 21. Shagdarsuren E., Djalali-Talab Y., Aurrand-Lions M., Bidzhekov K., Liehn E. A., Imhof B. A., Weber C., Zernecke A. (2009) Importance of junctional adhesion molecule-C for neointimal hyperplasia and monocyte recruitment in atherosclerosis-prone mice-brief report. Arterioscl. Thromb. Vasc. Biol. 29, 1161–1163 [DOI] [PubMed] [Google Scholar]
- 22. Woodfin A., Voisin M. B., Beyrau M., Colom B., Caille D., Diapouli F. M., Nash G. B., Chavakis T., Albelda S. M., Rainger G. E., Meda P., Imhof B. A., Nourshargh S. (2011) The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 12, 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmerli C., Lee B. P., Palmer G., Gabay C., Adams R., Aurrand-Lions M., Imhof B. A. (2009) Adaptive immune response in JAM-C-deficient mice: normal initiation but reduced IgG memory. J. Immunol. 182, 4728–4736 [DOI] [PubMed] [Google Scholar]
- 24. Praetor A., McBride J. M., Chiu H., Rangell L., Cabote L., Lee W. P., Cupp J., Danilenko D. M., Fong S. (2009) Genetic deletion of JAM-C reveals a role in myeloid progenitor generation. Blood 113, 1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langer H. F., Orlova V. V., Xie C., Kaul S., Schneider D., Lonsdorf A. S., Fahrleitner M., Choi E. Y., Dutoit V., Pellegrini M., Grossklaus S., Nawroth P. P., Baretton G., Santoso S., Hwang S. T., Arnold B., Chavakis T. (2011) A novel function of junctional adhesion molecule-C in mediating melanoma cell metastasis. Cancer Res. 71, 4096–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feltri M. L., D'Antonio M., Previtali S., Fasolini M., Messing A., Wrabetz L. (1999) P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann. N. Y. Acad. Sci. 883, 116–123 [PubMed] [Google Scholar]
- 27. Jancso G., Kiraly E., Jancso-Gabor A. (1977) Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 270, 741–743 [DOI] [PubMed] [Google Scholar]
- 28. Occhi S., Zambroni D., Del Carro U., Amadio S., Sirkowski E. E., Scherer S. S., Campbell K. P., Moore S. A., Chen Z. L., Strickland S., Di Muzio A., Uncini A., Wrabetz L., Feltri M. L. (2005) Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. J. Neurosci. 25, 9418–9427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quattrini A., Previtali S., Feltri M. L., Canal N., Nemni R., Wrabetz L. (1996) Beta 4 integrin and other Schwann cell markers in axonal neuropathy. Glia 17, 294–306 [DOI] [PubMed] [Google Scholar]
- 30. Salzer J. L., Brophy P. J., Peles E. (2008) Molecular domains of myelinated axons in the peripheral nervous system. Glia 56, 1532–1540 [DOI] [PubMed] [Google Scholar]
- 31. Cao T., Pinter E., Al-Rashed S., Gerard N., Hoult J. R., Brain S. D. (2000) Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J. Immunol. 164, 5424–5429 [DOI] [PubMed] [Google Scholar]
- 32. Inoue H., Asaka T., Nagata N., Koshihara Y. (1997) Mechanism of mustard oil-induced skin inflammation in mice. Eur. J. Pharmacol. 333, 231–240 [DOI] [PubMed] [Google Scholar]
- 33. Tominaga M., Caterina M. J., Malmberg A. B., Rosen T. A., Gilbert H., Skinner K., Raumann B. E., Basbaum A. I., Julius D. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 [DOI] [PubMed] [Google Scholar]
- 34. Sherman D. L., Brophy P. J. (2005) Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6, 683–690 [DOI] [PubMed] [Google Scholar]
- 35. Fannon A. M., Sherman D. L., Ilyina-Gragerova G., Brophy P. J., Friedrich V. L., Jr., Colman D. R. (1995) Novel E-cadherin-mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J. Cell Biol. 129, 189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scherer S. S., Deschenes S. M., Xu Y. T., Grinspan J. B., Fischbeck K. H., Paul D. L. (1995) Connexin32 is a myelin-related protein in the PNS and CNS. J. Neurosci. 15, 8281–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sandri C., Van Buren J. M., Akert K. (1977) Membrane morphology of the vertebrate nervous system. A study with freeze-etch technique. Prog. Brain Res. 46, 1–384 [PubMed] [Google Scholar]
- 38. Schnapp B., Mugnaini E. (1976) Freeze-fracture properties of central myelin in the bullfrog. Neuroscience 1, 459–467 [DOI] [PubMed] [Google Scholar]
- 39. Gow A., Southwood C. M., Li J. S., Pariali M., Riordan G. P., Brodie S. E., Danias J., Bronstein J. M., Kachar B., Lazzarini R. A. (1999) CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell 99, 649–659 [DOI] [PubMed] [Google Scholar]
- 40. Miyamoto T., Morita K., Takemoto D., Takeuchi K., Kitano Y., Miyakawa T., Nakayama K., Okamura Y., Sasaki H., Miyachi Y., Furuse M., Tsukita S. (2005) Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. J. Cell Biol. 169, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poliak S., Matlis S., Ullmer C., Scherer S. S., Peles E. (2002) Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J. Cell Biol. 159, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alanne M. H., Pummi K., Heape A. M., Grenman R., Peltonen J., Peltonen S. (2009) Tight junction proteins in human Schwann cell autotypic junctions. J. Histochem. Cytochem. 57, 523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomas P. K. (1963) The connective tissue of peripheral nerve: an electron microscope study. J. Anat. 97, 35–44 [PMC free article] [PubMed] [Google Scholar]
- 44. Parmantier E., Lynn B., Lawson D., Turmaine M., Namini S. S., Chakrabarti L., McMahon A. P., Jessen K. R., Mirsky R. (1999) Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron 23, 713–724 [DOI] [PubMed] [Google Scholar]
- 45. Priestley J. V., Michael G. J., Averill S., Liu M., Willmott N. (2002) Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can. J. Physiol. Pharmacol. 80, 495–505 [DOI] [PubMed] [Google Scholar]
- 46. Poliak S., Peles E. (2003) The local differentiation of myelinated axons at nodes of Ranvier. Nat. Rev. Neurosci. 4, 968–980 [DOI] [PubMed] [Google Scholar]
- 47. Bhat M. A., Rios J. C., Lu Y., Garcia-Fresco G. P., Ching W., St Martin M., Li J., Einheber S., Chesler M., Rosenbluth J., Salzer J. L., Bellen H. J. (2001) Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron 30, 369–383 [DOI] [PubMed] [Google Scholar]
- 48. Baum A. E., Akula N., Cabanero M., Cardona I., Corona W., Klemens B., Schulze T. G., Cichon S., Rietschel M., Nothen M. M., Georgi A., Schumacher J., Schwarz M., Abou Jamra R., Hofels S., Propping P., Satagopan J., Detera-Wadleigh S. D., Hardy J., McMahon F. J. (2008) A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiat. 13, 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fullerton J. M., Donald J. A., Mitchell P. B., Schofield P. R. (2010) Two-dimensional genome scan identifies multiple genetic interactions in bipolar affective disorder. Biol. Psychiat. 67, 478–486 [DOI] [PubMed] [Google Scholar]
- 50. Martin-Padura I., Lostaglio S., Schneemann M., Williams L., Romano M., Fruscella P., Panzeri C., Stoppacciaro A., Ruco L., Villa A., Simmons D., Dejana E. (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 142, 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Angelow S., El-Husseini R., Kanzawa S. A., Yu A. S. (2007) Renal localization and function of the tight junction protein, claudin-19. Am. J. Physiol. Renal Physiol. 293, F166–F177 [DOI] [PubMed] [Google Scholar]
- 52. Peles E., Nativ M., Lustig M., Grumet M., Schilling J., Martinez R., Plowman G. D., Schlessinger J. (1997) Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 16, 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.