Abstract
Prohibitin 1 (PHB1), a pleiotropic protein in the cell, has been implicated in the regulation of proliferation, apoptosis, transcription, mitochondrial protein folding, and as a cell-surface receptor. This diverse array of functions of PHB1 is attributed to the cell type studied and its subcellular localization. This review discusses recent data that indicate a diverse role of PHB1 in disease pathogenesis and suggest that targeting PHB1 may be a potential therapeutic option for treatment of diseases including cancer, inflammatory bowel disease, insulin resistance/type 2 diabetes, and obesity. These diseases are associated with increased oxidative stress and mitochondrial dysfunction and therefore, the role of PHB1 in both responses will also be discussed.
Keywords: prohibitin, therapeutic, cancer, inflammation, obesity, diabetes
Introduction
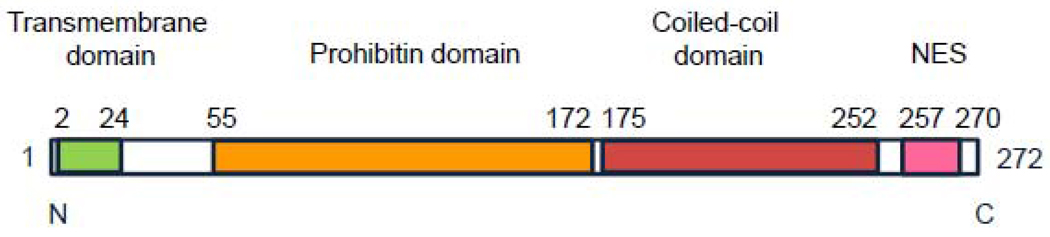
Prohibitin 1 (PHB1) is a 32 kDa protein that belongs to a family of proteins that share an evolutionarily conserved stomatin/prohibitin/flotillin/HflK/C (SPFH) domain. It is closely related to prohibitin 2 (PHB2), also called B-cell receptor-associated protein 37 (BAP 37) and repressor of estrogen receptor activity (REA). Prohibitin exhibits a remarkable degree of sequence conservation across species; the protein sequences of mouse and rat prohibitin are virtually identical, and these differ from the human protein sequence by a single amino acid [1]. An N-terminal transmembrane domain consists of a hydrophobic membrane-anchoring alpha helix (Figure 1). The prohibitin domain, characteristic of the SPFH family of proteins, spans amino acid residues 55–172. This domain has been suggested to be important for lipid raft associations and protein-protein interactions [2]. A coiled-coil structure of alpha helices is present at the C-terminal end of the protein, consisting of amino acids residues 175–252 [2]. A nuclear export sequence consisting of a leucine/isoleucine-rich motif is present at the C-terminal end, spanning residues 257 to 270 [3]. At the subcellular level, PHB1 has been localized to the cell membrane and mitochondrial inner membrane complexed to PHB2 [4, 5], as well as the cytoplasm, or the nucleus depending on the cell type and situation [3, 6]. In most cell types studied, its predominant localization is in the mitochondria. Its subcellular localization influences its multiple functions within the cell.
Figure 1.
The functional domains of PHB1.
As implied by the name, PHB1 was originally thought to have a central role in the inhibition of cell-cycle progression. Genetic deletion of prohibitin is embryonically lethal in mice and flies [7] and the Drosophila orthologue of PHB1 is essential for normal development [8]. Although the best-characterized function of the prohibitins is as chaperones involved in the stabilization of mitochondrial proteins [5], it has also been implicated in diverse cellular processes including the regulation of proliferation, apoptosis, and gene transcription [3, 6, 9]. Recent data have indicated a diverse role of PHB1 in disease pathogenesis. This review will focus on the potential involvement of PHB1 in cancer, inflammatory bowel disease, insulin resistance/type 2 diabetes, and obesity. Many of these diseases have increased oxidative stress and mitochondrial dysfunction as common factors, which will be discussed. The therapeutic potential of targeting PHB1 during disease will also be discussed.
Oxidative stress
In most eukaryotic cells, mitochondria are responsible for providing energy by generating adenosine triphosphate (ATP) through oxidative phosphorylation and controlling levels of oxidative stress. During normal function of the mitochondrial respiratory chain, reactive oxygen species (ROS), which are partially reduced oxygen species such as superoxide radical (O2−.), hydrogen peroxide (H2O2), and hydroxyl radical (·OH) are generated at low levels. Nitric oxide (NO) is also generated during normal function of the respiratory chain or by induction of nitric oxide synthase. Subsequently, peroxynitrate (NOO−) is formed by a reaction between superoxide and nitric oxide. Although most electrons are successfully transferred down the electron transport chain, up to 10% of the reducing equivalents from NADH leak to form superoxide radical and hydrogen peroxide, which diffuse from mitochondria. Approximately 1% of oxygen uptake under normal physiological conditions is converted to superoxide during respiration [10]. An elaborate system of neutralizing antioxidant enzymes, including superoxide dismutase, catalase, glutathione peroxidase, and antioxidant thiols (glutathione and thioredoxin) are responsible for consuming superoxide anion and hydrogen peroxide, thereby preventing their cytotoxic effects under normal conditions. An imbalance between excessive production of ROS (oxidants) and impaired protective mechanisms acting to eliminate ROS (antioxidant defense system) can cause tissue damage, which is defined as oxidative stress. [11]. An imbalance of oxidants to antioxidants influences the redox state of the cells, tissue, and whole organism. Although oxidants may not always be harmful depending on duration, intensity and location of the redox imbalance, continued oxidative stress leads to damage of DNA, lipids, and proteins and can modulate signal transduction pathways influencing inflammation, apoptosis, proliferation, repair machinery, and the production of antioxidants. ROS are potent immunoregulatory and tissue-destructive molecules associated with the pathophysiology of several diseases including Parkinson's disease/neurodegeneration, cancer, diabetes mellitus, obesity and virtually all inflammatory diseases including inflammatory bowel diseases, all of which are also associated with mitochondrial dysfunction which is discussed below [12].
Emerging data suggest that PHB1 plays a role in combating oxidative stress in multiple cells types. PHB1 levels are decreased during oxidative stress in intestinal epithelial cells [13] and in ex vivo lung tissue during hyperoxia [14]. PHB1 knockdown in endothelial cells increased production of ROS in mitochondria via decreased activity of complex I and partial blockade of the electron transport chain [15]. This increase in ROS caused cellular senescence, subsequently decreasing cell motility and migration, thereby reducing endothelial cell angiogenic capacity. In another study by Liu et al. [16], overexpression of PHB1 in cultured cardiomyocytes protected mitochondria from injury and apoptosis induced by hydrogen peroxide and maintained mitochondrial membrane permeability. Tsutsumi et al. [17] showed that PHB1 interacts with and regulates assembly of subunits I and IV of cytochrome c oxidase in cultured hepatocytes. Infection with hepatitis C virus core protein impaired the interaction of PHB1 with cytochrome c oxidase and subsequently caused an increase in ROS production during respiration. These results may explain the reduction of cytochrome c oxidase activity and increased ROS production in hepatitis C virus infected liver. Studies in intestinal epithelial cells using in vitro and in vivo models of colitis suggest that PHB1 protects these cells from oxidative stress that is associated with the inflammatory process [13, 18, 19]. These studies are discussed below.
Mitochondrial dysfunction
Mitochondrial dysfunction can be characterized by impaired mitochondrial function, loss of mitochondrial respiratory chain complexes, mitochondrial DNA (mtDNA) mutations or a decrease in mtDNA levels, and alterations in mitochondrial membrane potential. ROS can cause mutations in mtDNA and accumulation of such mutations can inactivate respiratory chain activity and subsequently cause further increases in mtDNA mutations [20]. This cyclical progression is thought to contribute to the exponential increases in oxidative damage and reduced cellular functions noted in aging and diseases associated with mitochondrial dysfunction such as cancer, insulin resistance and type 2 diabetes, obesity, inflammatory bowel diseases, Parkinson’s disease and neurodegeneration [21–23].
Given that the predominant subcellular localization of PHB1 is in the mitochondria in most cell types studied to date, emerging data suggest that PHB1 plays a role in maintaining normal mitochondrial function and morphology (reviewed in [24]). In yeast, loss of function of PHB1 leads to an altered mitochondrial morphology, loss of normal reticular morphology and disorganized mitochondrial distribution [25]. Similar results are evident in mammalian cells. Complexes of PHB1 and PHB2 in the inner membrane of mitochondria control cristae morphogenesis and the processing of the dynamin-like GTPase optic atrophy 1 (OPA1) (reviewed in [26]). OPA1 is required for mitochondrial fusion and cristae maintenance. Loss of PHB2 and subsequent disruption of PHB1/PHB2 complexes in mouse embryonic fibroblasts (MEFs) or HeLa cells causes accumulation of fragmented mitochondria and loss of OPA1 expression [27]. These results were corroborated in cancer cell lines expressing PHB1-directed shRNAs [28]. Furthermore, knockdown of PHB1 in HeLa cells caused disorganization of mitochondrial nucleoids and a 30% reduction of mtDNA copy number, indicative of mitochondrial dysfunction [29]. It remains less clear whether PHB1 expression maintains the mitochondrial membrane potential; knockdown of PHB1 in endothelial cells [15] and knockdown of PHB2 and subsequent disruption of PHB1/PHB2 complexes in MEFs [27] depolarized mitochondrial membrane potential, whereas in HeLa cells and cancer cell lines mitochondrial membrane potential was maintained despite knockdown of PHB1 [28, 29]. Overexpression of PHB1 protected cardiomyocytes from hypoxia-induced decrease in mitochondrial membrane potential [30].
Despite opposing results in regards to mitochondrial membrane potential, it is clear that PHB1 plays a critical role in maintaining normal mitochondrial function and morphology, but the mechanism has yet to be determined. Studies in yeast have suggested that the major function of PHB1 is that of a chaperone for imported proteins in mitochondria, protecting newly imported proteins from degradation by the m-AAA protease [26]. It has been shown that PHB1 interacts with complex I and subunits of cytochrome c oxidase of the respiratory chain and regulates their assembly [15, 17]. It is therefore conceivable that loss of PHB1 in mitochondria could lead to accelerated proteolysis of membrane proteins and impair function of the mitochondrial respiratory chain. One obvious effect of respiratory chain dysfunction is increased oxidant production leading to oxidative stress, which can cause alterations in mitochondrial morphology and membrane potential.
Cancer
PHB1 plays a critical role in the regulation of cell cycle progression and can inhibit DNA replication in multiple cell types [25, 31–35]. PHB1 levels are increased in cancers of the cervix [36], esophagus [37], stomach [38–40], breast [41], lung [42], bladder [43], thyroid [44], ovary [45], and prostrate [46], whereas levels are decreased in gliomas [47]. Consensus binding sites for the oncoprotein Myc are present in the PHB1 promoter and likely contributes to the increased PHB1 levels in many tumors [32]. Serum levels of PHB1 are increased in cancer patients compared to normal controls [48, 49]. Although somatic mutations in the PHB1 gene were observed in a few sporadic breast cancers, none were identified in the ovary, liver or lung cancers examined [41, 50]. The role of PHB1 in cancer cell proliferation and/or tumor suppression remains controversial especially since PHB1 expression is increased in many transformed cells and tumors; opposing results have been reported depending upon the type of tissue involved and data that suggest a pro-tumorigenic versus an anti-tumorigenic role are discussed below. Figure 2 depicts PHB1 involvement in influencing tumorigenesis.
Figure 2.
Simplified model of the opposing roles of PHB1 in tumorigenesis. Subcellular localization of PHB1 may dictate its role in tumorigenesis and cell fate. A) Activated Ras directly interacts with plasma membrane-bound PHB1, causing the activation of C-Raf. The Ras-Raf pathway is involved in cell growth and transformation [62]. B) PHB1 interacts with the tumor suppressor Rb in the nucleus, thereby suppressing E2F-mediated transcription [6, 56]. C) Nuclear PHB1 interacts with p53, resulting in maximal p53 transcriptional activity and p53 check point control of the cell cycle [54, 55].
The first studies indicating that PHB1 plays a role in tumor suppression in breast cancer actually showed that this effect was attributable to the 3′ untranslated region of the PHB1 gene which encodes a functional RNA that blocks transition between the G1 and S phases of the cell cycle, thereby arresting cell proliferation [51–53]. However, further studies in breast and prostate cancer cell lines indicate that a portion of PHB1 is localized in the nucleus and interacts with and regulates the tumor suppressors p53 and Rb in steroid-responsive cells. PHB1 co-localizes with p53 in breast carcinoma cell lines and its binding to p53 increases p53 transcriptional activity via increased DNA binding [54]. It was also shown that PHB1 is degraded during Skp2B overexpression in breast cancer lines and this subsequently causes attenuation of p53 activity in vivo and in vitro [55]. These results suggest that PHB1 is required for maximal p53 transcriptional activity and may play a critical role in p53 checkpoint control of the cell cycle. PHB1 has also been shown to interact with the retinoblastoma tumor suppressor protein (Rb) and its family members, p107 and p130, in the nucleus [56]. The Rb family suppresses growth via binding to and inhibiting the E2F family of transcription factors. PHB1 was shown to repress E2F-mediated transcription function via its interaction with Rb and histone deacetylase activity [6] as well as the recruitment of the ATP-dependent nucleosome remodeling complexes containing Brg-1 and Brm [57]. These findings suggest that PHB1 may suppress tumor growth by regulating transcription.
Further support exists for the anti-tumorigenic role of PHB1 in prostate cancer [58], gastric cancer [59], and liver cancer [60]. Overexpression of PHB1 arrested prostate tumor growth while knockdown using siRNA accelerated tumor growth, even in castrated mice lacking androgen signaling [58]. Liu et al. reported that PHB1 levels were reduced in gastric adenocarcinoma [59], which opposes previous findings showing an increase in PHB1 [38–40]. Liu et al. reported that PHB1 is a target of microRNA-27a, which is upregulated in gastric cancer and reduces PHB1 levels in vivo. They suggest that down-regulation of PHB1 by miR-27a may explain why suppression of miR-27a can inhibit gastric cancer cell growth. A recent study by Ko et al. [60], support a tumor suppressor role of PHB1 in hepatocytes. Mice with hepatocyte-specific deletion of PHB1 exhibited liver injury, fibrosis, oxidative stress and hepatocellular carcinoma developing by 8 months of age. Mitochondrial abnormalities are evident by 3 weeks of age including no cristae formation.
Despite the evidence that PHB1 has anti-tumorigenic properties, there are a number of studies as well indicating a pro-tumorigenic role of PHB1. PHB1 was shown to be necessary for activation of C-Raf by the oncogene Ras in HeLa cells [61]. Ras was shown to directly interact with PHB1 leading to C-Raf activation and was required for Ras-induced cell migration. The authors speculate that PHB1 may play a role of a chaperone or membrane anchor for C-Raf to interact with Ras [62]. These results suggest that PHB1 may have a prominent role in the progression of malignant transformation. Furthermore, in a recent study using cultured cancer cell lines, PHB1-directed shRNAs reduced proliferation and the ability to exhibit anchorage-independent growth [28]. In chemoresistant ovarian cancer cells, PHB1 expression was associated with drug-resistance [63] and silencing of PHB1 expression increased the sensitivity of the cells to apoptosis [45].
Such opposing results regarding the role of PHB1 in tumorigenesis may be explained by its subcellular localization. Indeed, the subcellular localization of PHB1 has been shown to affect cell fate, specifically apoptosis [3]. Although many studies have reported that levels of PHB1 are increased in many types of tumors, only a handful of reports describe its subcellular localization. Patel et al. [63], found that PHB1 levels were increased on the cell surface of chemoresistant cells compared to chemosensitive cells. Similarly, studies showing PHB1 interaction with C-Raf induced by Ras oncogene found PHB1 in the caveolin-1-rich fractions of the plasma membrane of HeLa cells [61]. These studies suggest that increased expression of PHB1 on the plasma membrane may facilitate tumorigenesis. However, studies showing PHB1 interaction with the tumor suppressors p53 and Rb also describe a significant portion of PHB1 presiding in the nucleus. In this regard, increased levels of PHB1 as seen in many types of cancer may act in an anti-tumorigenic role, especially if the levels of PHB1 are increased in the nucleus. Further studies need to elucidate the intracellular localization of PHB1 during the progression of normal tissue to carcinoma. Targeting specific subsets of cellular PHB1 may have therapeutic potential; reducing plasma membrane PHB1 and/or increasing nuclear PHB1 may modulate tumor progression. Also, PHB1 can translocate from the nucleus to the cytoplasm upon induction of apoptosis [3]. C-Raf bound to PHB is recruited to the plasma membrane by RAS [62]. It remains unknown whether PHB1 can translocate to or from the mitochondria or from the cytoplasm into the nucleus. Re-locating the portion of PHB1 from the cell surface to the nucleus may also affect tumor outcome by localizing the protein to the organelle where it may elicit its tumor suppressing functions.
Inflammatory bowel disease
Oxidative stress associated with inflammatory diseases originates predominately from immune cells mounting an immune response to bacteria, fungi, or other antigens. Neutrophils and macrophages, when stimulated by bacterial products or antigen, undergo a respiratory burst via the activation of membrane-bound NAPDH oxidase, releasing super oxide and hydrogen peroxide. Neutrophils and macrophages also release the enzyme myeloperoxidase, constituting 5% of their total protein load, which metabolizes hydrogen peroxide and chloride ion to form hypochlorous acid, commonly known as bleach [64]. Hypochlorous acid is a potent oxidizing agent which is thought to be 100–1000 times more toxic than superoxide radical or hydrogen peroxide and is critical in the oxygen-dependent microbicidal activity of phagocytes. Excessive release of oxidants and generation of myeloperoxidase-derived oxidants causes tissue damage characteristic of inflammatory diseases.
The most common inflammatory bowel diseases (IBD), Crohn’s disease and ulcerative colitis, are associated with increased ROS and decreased antioxidant enzymes in the intestinal mucosa [65–68]. A number of key antioxidants such as glutathione and thioredoxin levels are decreased during inflammation [69, 70]. Animals with genetic manipulation of enzymes involved in oxidative defense pathways exhibit increased susceptibility to the development of colitis and transgenic mice with overexpression of certain antioxidant enzymes are protected from the development of colitis [71–73]. In IBD, ROS have been demonstrated to play a pathophysiologic role in barrier dysfunction, apoptosis, and wound healing [74–76]. Importantly, the levels of ROS have been correlated to clinical and endoscopic assessment of severity of disease [77]. In addition, oxidative stress-induced p53 inactivation and DNA damage in the setting of chronic inflammation has been associated with the risk of IBD-associated colon cancer [78]. Since PHB1 has been shown to modulate p53 activation [54, 55], it is of interest to determine whether PHB1 plays a role in the transition of dysplasia to cancer in the colon.
Not surprisingly, there has been considerable interest in developing antioxidant-based therapeutic strategies for the treatment of IBD. Commonly used drugs, in particular sulfasalazine and its active moiety 5-aminosalicylic acid, are potent ROS scavengers (reviewed by Miles and Grisham[79]). Only two past studies have been published using therapies specifically to combat oxidative stress; high positive response rates (> 80% remission) were observed when patients with severe Crohn's disease (n=30) or ulcerative colitis (n=4) were treated with free or liposomal-encapsulated bovine Cu/Zn-superoxide dismutase [80, 81]. However, the therapeutic applicability of natural antioxidant enzymes has restrictions in terms of limited cell permeability, short circulating half-life, immunogenicity and cost of production. In addition, these therapies target single pathways and given the complexity of the oxidant response, targeting single pathways may not be efficient in combating oxidative stress in IBD. Thus, there is an unmet need for designing potent antioxidant based treatment strategies in IBD.
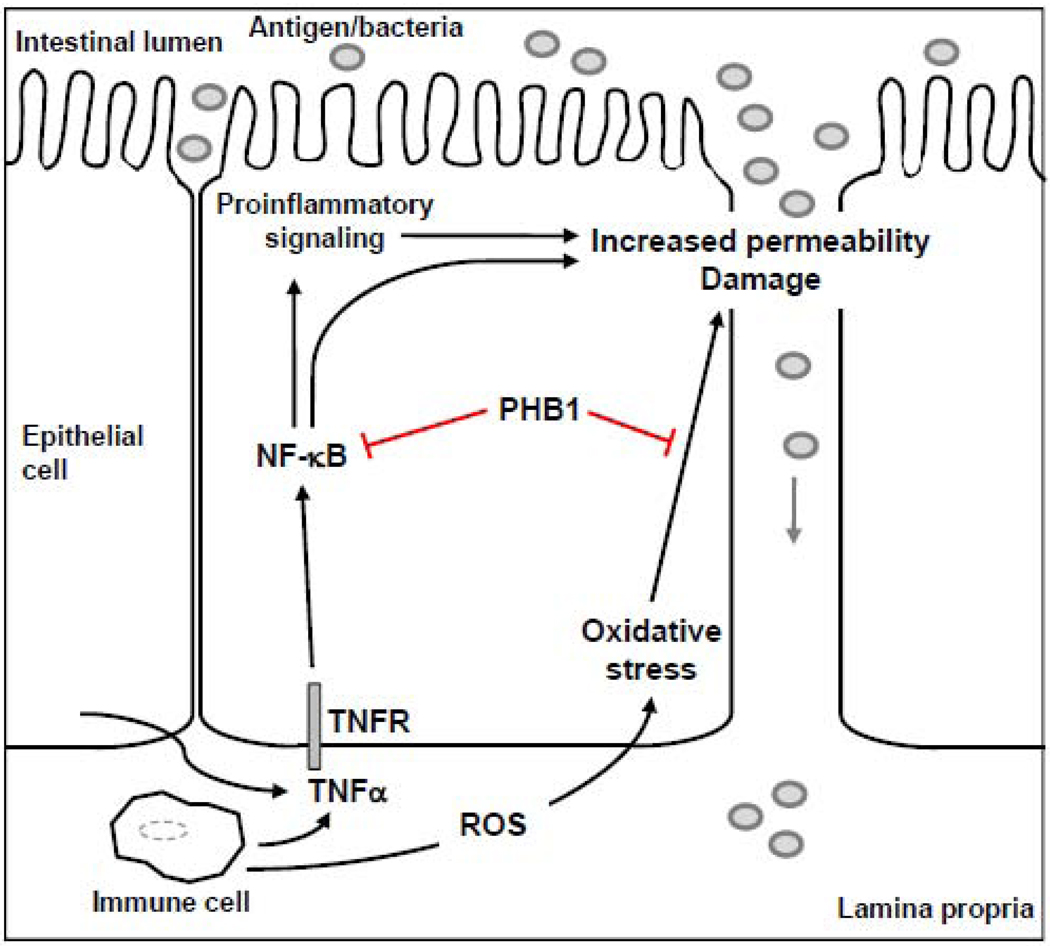
Studies in intestinal epithelial cells using in vitro models of oxidative stress/inflammation and in vivo models of colitis suggest that PHB1 protects these cells from inflammation-associated oxidative stress and may modulate the inflammatory process (Figure 3). Cell-surface associated PHB1 in intestinal epithelial cells binds to Vi polysaccharide of Salmonella typhi and inhibits the inflammatory response to S. typhi infection [4]. PHB1 mRNA and protein levels are decreased in inflamed mucosa during IBD [13, 82] and in experimental models of colitis [13]. Tumor necrosis factor alpha (TNFα), a key cytokine that plays a central role in IBD, decreases PHB1 mucosal levels in vivo and in cultured intestinal epithelial cells [83]. These results suggest that TNFα may contribute to the reduced levels of prohibitin in the mucosa of IBD patients. Exogenous expression of PHB1 in intestinal epithelial cells reduced the activation of the nuclear factor-kappa B (NF-κB) pathway, which plays a central role in inflammatory responses and also regulates transcription of multiple cytokines [83]. Collectively, these results suggest that PHB1 may be crucial in modulating intestinal inflammatory processes.
Figure 3.
Cartoon representation of PHB1 in intestinal epithelial cells. In IBD, a chronic and exaggerated immune response ensues during which activated immune cells and intestinal epithelial cells release ROS and immunoregulatory cytokines including TNFα. ROS causes oxidative stress, leading to increased permeability and damage. Altered epithelial barrier function allows translocation of antigen/bacteria from the intestinal lumen to the lamina propria. TNFα activates the transcription factor NF-κB which can stimulate proinflammatory signaling and increase permeability. In intestinal epithelial cells PHB1 levels are decreased during inflammation. Restoration of PHB1 levels in these cells indicate that it inhibits oxidative stress and activation of NF- κB. NF-κB, nuclear factor-kappa B; TNFα, tumor necrosis factor alpha; TNFR, tumor necrosis factor receptor; ROS, reactive oxygen species.
Restoration of PHB1 levels in intestinal epithelial cells during inflammation was achieved using transgenic overexpression in mice [19], adenovirus infection and nanoparticle delivery [18]. Transgenic mice overexpressing PHB1 under the control of the villin promoter showed restored levels of PHB1 in the intestinal epithelium during colitis induced by dextran sodium sulfate and Salmonella typhimurium. Compared to wild-type littermates, PHB1 transgenic mice exhibited decreased oxidative stress and colitis and sustained activation of the antioxidant nuclear factor erythroid 2-related factor 2 (Nrf2), a transcriptional regulator of antioxidant responses [19]. As a proof-of-principle to demonstrate the therapeutic efficacy of PHB1 for human IBD, adenovirus-directed administration by enema and nanoparticle-based colonic delivery of biologically active PHB1 were tested in mice during experimental colitis [18]. Both methods of delivery resulted in increased levels of PHB1 in the surface epithelial cells of the colon and reduced severity of dextran sodium sulfate-induced colitis as measured by body weight loss, clinical score, myeloperoxidase activity, pro-inflammatory cytokine expression, and histological score. Mice treated with PHB1 delivered by adenovirus or nanoparticle also showed reduced protein carbonyl content, a marker of oxidative stress [18]. Collectively, these results suggest that PHB1 acts as a regulator of antioxidant response in intestinal epithelial cells and that restoration of PHB1 levels represents a potential therapeutic approach in IBD.
Insulin resistance/type 2 diabetes
It is well established that insulin resistance increases the chance of developing type 2 diabetes. Mitochondrial dysfunction is thought to contribute to the development of insulin resistance and type 2 diabetes. Alcoholism is a type 2 diabetes risk factor [84]. In pancreatic β-cells, ethanol increases oxidative stress, subsequently causing cell injury, mitochondrial dysfunction, apoptosis and the inhibition of insulin secretion [85–87]. In a study by Lee et al. [88], PHB1 overexpression prevented pancreatic β-cell apoptosis induced by ethanol treatment. These results were further recapitulated by incubation of cultured pancreatic β-cells with exogenous PHB1. Knockdown of PHB1 using siRNA enhanced ethanol-induced apoptosis in these cells. Upon ethanol treatment, PHB1 protein level increased and localized to the mitochondria after export from the nucleus. These results suggest that PHB1 protects β-cells from oxidative stress induced by ethanol and that PHB1 could be a potential target for diabetes treatment.
An opposing study by Vessal et al, [89] showed that PHB1 added to fibroblasts or adipocytes inhibits insulin-stimulated glucose and fatty acid oxidation. These results suggest that PHB1 would inhibit glucose-induced insulin secretion which is dependent on glucose oxidation. Perhaps the effect of PHB1 is cell type specific and therefore, the effect of PHB1 on glucose oxidation warrants further investigation.
Recent studies by Mishra and Ande et al. have explored the O-linked β-N-acetylglucosamine (O-GlcNAc) posttranslational modification of mitochondrial proteins, including PHB1, in response to insulin and high glucose [44, 90]. An increase in O-GlcNAcylation of various proteins correlates with the development of insulin resistance [91]. O-GlcNAcylation is likely important for normal mitochondrial function and PHB1 is O-GlcNAc modified under basal conditions [90]. O-GlcNAc modification was shown to modulate tyrosine phosphorylation of PHB1 in response to insulin. Furthermore, O-GlcNAcylation and tyrosine phosphorylation of PHB1 was increased by exposing cells to high glucose [44]. These studies suggest an association between posttranslational modifications (O-GlcNAcylation and phosphorylation) of PHB1 and the cellular response to insulin and glucose. Further studies are needed to determine whether changes in O-GlcNAcylation and phosphorylation of PHB1 are associated with mitochondrial dysfunction and insulin resistance/type 2 diabetes.
Obesity
Deficient PHB1 activity in the liver contributes to the progression of non-alcoholic steatohepatitis and obesity although the mechanism remains unknown [9]. Kolonin et al. [92] reported the first study identifying PHB1 as a marker of adipose vasculature. They found that plasma membrane-bound PHB1 acts as a vascular receptor in subcutaneous and peritoneal white fat for a proapoptotic peptide resulting in weight loss and prevention of obesity in mice. Internalization of the peptide by PHB1 caused fat resorption, apoptosis of white fat vasculature, and improved glucose tolerance and serum insulin levels. Hossen et al. [93] recently showed that a similar adipose-specific peptide delivered using a nanocarrier was internalized by cell-surface PHB1 and blocking PHB1 using an anti-prohibitin antibody prevented this uptake. Drugs targeted to PHB1, which is also expressed in the vasculature of human white fat, may be a therapeutic option for the treatment of obesity.
Concluding remarks
The potential of PHB1 to interact with tumor suppressor proteins and transcription factors, inhibit oxidative stress and mitochondrial dysfunction, act as a cell-surface receptor for the vasculature of adipose tissue, and modulate cell proliferation and apoptosis, suggest that PHB1 harbors great potential for the development of therapeutic agents for obesity, diabetes, cancer and inflammatory diseases. Depending upon the disease state, therapeutic potential exists in altering the levels of PHB1 expressed in affected cells, such as in diabetes or inflammation, altering the subcellular localization of PHB1 expression, such as in cancer, or targeting cell surface PHB1, such as in obesity (Table 1). The multiple functions of PHB1 are only beginning to be elucidated, but it is clear this protein provides new avenues for therapeutic-based research.
Table 1.
The role and therapeutic potential of prohibitin in disease.
| PHB1 function |
Cell type | Subcellular localization |
Disease | Potential therapeutic strategy |
Reference |
|---|---|---|---|---|---|
|
Combat oxidative stress |
Pancreatic β- cells |
Mitochondria/ nucleus |
Type 2 diabetes |
Increase PHB1 levels |
[88] |
| Intestinal epithelial cells |
Mitochondria | IBD | Increase PHB1 levels |
[13, 19] | |
| Cardiomyocytes | Mitochondria | Myocardium injury |
Increase PHB1 levels |
[16] | |
| Hepatocytes | Mitochondria | Hepatitis C Virus |
Restore PHB1 function |
[17] | |
|
Activate p53 and modulate Rb tumor suppressors |
Cancer cell lines (T47D, MCF7, SW13, C33A, Ramos) |
Nucleus | Cancer | Increase nuclear PHB1 levels |
[54–56] |
|
c-Raf activation by Ras oncogene |
HeLa | Plasma membrane |
Cancer | Decrease cell surface PHB1 |
[61] |
|
Vascular Receptor |
Adipose vasculature |
Plasma membrane |
Obesity | Target PHB1 | [92, 93] |
Acknowledgements
This work was supported by The Broad Foundation grant IBD-0226R (S.V.S) and National Institutes of Health grants R01-DK06411 (S.V.S.) and K01-DK085222 (A.L.T.).
Abbreviations
- IBD
inflammatory bowel disease
- mtDNA
mitochondrial DNA
- OPA1
optic atrophy 1
- O-GlcNAc
O-linked β-N-acetylglucosamine
- PHB1
prohibitin
- Rb
retinoblastoma
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist.
References
- 1.Altus MS, Wood CM, Stewart DA, Roskams AJ, Friedman V, Henderson T, Owens GA, Danner DB, Jupe ER, Dell'Orco RT, et al. Regions of evolutionary conservation between the rat and human prohibitin-encoding genes. Gene. 1995;158:291–294. doi: 10.1016/0378-1119(95)00164-2. [DOI] [PubMed] [Google Scholar]
- 2.Winter A, Kamarainen O, Hofmann A. Molecular modeling of prohibitin domains. Proteins. 2007;68:353–362. doi: 10.1002/prot.21355. [DOI] [PubMed] [Google Scholar]
- 3.Rastogi S, Joshi B, Fusaro G, Chellappan S. Camptothecin induces nuclear export of prohibitin preferentially in transformed cells through a CRM-1-dependent mechanism. J. Biol. Chem. 2006;281:2951–2959. doi: 10.1074/jbc.M508669200. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc. Natl. Acad. Sci. U S A. 2004;101:17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nijtmans LG, de Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, van der Spek H, Grivell LA. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. Embo. J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. 2002;21:8388–8396. doi: 10.1038/sj.onc.1205944. [DOI] [PubMed] [Google Scholar]
- 7.McClung JK, Jupe ER, Liu XT, Dell'Orco RT. Prohibitin: potential role in senescence, development, and tumor suppression. Exp. Gerontol. 1995;30:99–124. doi: 10.1016/0531-5565(94)00069-7. [DOI] [PubMed] [Google Scholar]
- 8.Eveleth DD, Jr, Marsh JL. Sequence and expression of the Cc gene, a member of the dopa decarboxylase gene cluster of Drosophila: possible translational regulation. Nucleic. Acids. Res. 1986;14:6169–6183. doi: 10.1093/nar/14.15.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Quiles V, Santamaria E, Segura V, Sesma L, Prieto J, Corrales FJ. Prohibitin deficiency blocks proliferation and induces apoptosis in human hepatoma cells: molecular mechanisms and functional implications. Proteomics. 2010;10:1609–1620. doi: 10.1002/pmic.200900757. [DOI] [PubMed] [Google Scholar]
- 10.Du G, Mouithys-Mickalad A, Sluse FE. Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free. Radic. Biol. Med. 1998;25:1066–1074. doi: 10.1016/s0891-5849(98)00148-8. [DOI] [PubMed] [Google Scholar]
- 11.Jones DP. Redefining oxidative stress. Antioxid. Redox. Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 12.Durackova Z. Some current insights into oxidative stress. Physiol. Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 13.Theiss AL, Idell RD, Srinivasan S, Klapproth JM, Jones DP, Merlin D, Sitaraman SV. Prohibitin protects against oxidative stress in intestinal epithelial cells. Faseb J. 2007;21:197–206. doi: 10.1096/fj.06-6801com. [DOI] [PubMed] [Google Scholar]
- 14.Henschke P, Vorum H, Honore B, Rice GE. Protein profiling the effects of in vitro hyperoxic exposure on fetal rabbit lung. Proteomics. 2006;6:1957–1962. doi: 10.1002/pmic.200500245. [DOI] [PubMed] [Google Scholar]
- 15.Schleicher M, Shepherd BR, Suarez Y, Fernandez-Hernando C, Yu J, Pan Y, Acevedo LM, Shadel GS, Sessa WC. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J. Cell. Biol. 2008;180:101–112. doi: 10.1083/jcb.200706072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Ren Z, Zhan R, Wang X, Wang X, Zhang Z, Leng X, Yang Z, Qian L. Prohibitin protects against oxidative stress-induced cell injury in cultured neonatal cardiomyocyte. Cell. Stress. Chaperones. 2009;14:311–319. doi: 10.1007/s12192-008-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsutsumi T, Matsuda M, Aizaki H, Moriya K, Miyoshi H, Fujie H, Shintani Y, Yotsuyanagi H, Miyamura T, Suzuki T, Koike K. Proteomics analysis of mitochondrial proteins reveals overexpression of a mitochondrial protein chaperon, prohibitin, in cells expressing hepatitis C virus core protein. Hepatology. 2009;50:378–386. doi: 10.1002/hep.22998. [DOI] [PubMed] [Google Scholar]
- 18.Theiss AL, Laroui H, Obertone TS, Chowdhury I, Thompson WE, Merlin D, Sitaraman SV. Nanoparticle-based therapeutic delivery of prohibitin to the colonic epithelial cells ameliorates acute murine colitis. Inflamm. Bowel. Dis. 2010 doi: 10.1002/ibd.21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology. 2009;137:199–208. doi: 10.1053/j.gastro.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CY, Lee CF, Wei YH. Role of reactive oxygen species-elicited apoptosis in the pathophysiology of mitochondrial and neurodegenerative diseases associated with mitochondrial DNA mutations. J. Formos. Med. Assoc. 2009;108:599–611. doi: 10.1016/s0929-6646(09)60380-6. [DOI] [PubMed] [Google Scholar]
- 21.Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 1992;2:324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- 22.Mawrin C, Kirches E, Krause G, Schneider-Stock R, Bogerts B, Vorwerk CK, Dietzmann K. Region-specific analysis of mitochondrial DNA deletions in neurodegenerative disorders in humans. Neurosci. Lett. 2004;357:111–114. doi: 10.1016/j.neulet.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 23.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell. Physiol. 2007;292:C670–C686. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- 24.Artal-Sanz M, Tavernarakis N. Prohibitin and mitochondrial biology. Trends Endocrinol. Metab. 2009;20:394–401. doi: 10.1016/j.tem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Berger KH, Yaffe MP. Prohibitin family members interact genetically with mitochondrial inheritance components in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998;18:4043–4052. doi: 10.1128/mcb.18.7.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merkwirth C, Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim. Biophys. Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Lower B, Wunderlich FT, von Kleist-Retzow JC, Waisman A, Westermann B, Langer T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes. Dev. 2008;22:476–488. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sievers C, Billig G, Gottschalk K, Rudel T. Prohibitins are required for cancer cell proliferation and adhesion. PLoS One. 2010;5:e12735. doi: 10.1371/journal.pone.0012735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasashima K, Sumitani M, Satoh M, Endo H. Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp. Cell. Res. 2008;314:988–996. doi: 10.1016/j.yexcr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Muraguchi T, Kawawa A, Kubota S. Prohibitin protects against hypoxia-induced H9c2 cardiomyocyte cell death. Biomed. Res. 2010;31:113–122. doi: 10.2220/biomedres.31.113. [DOI] [PubMed] [Google Scholar]
- 31.Coates PJ, Jamieson DJ, Smart K, Prescott AR, Hall PA. The prohibitin family of mitochondrial proteins regulate replicative lifespan. Curr. Biol. 1997;7:607–610. doi: 10.1016/s0960-9822(06)00261-2. [DOI] [PubMed] [Google Scholar]
- 32.Coates PJ, Nenutil R, McGregor A, Picksley SM, Crouch DH, Hall PA, Wright EG. Mammalian prohibitin proteins respond to mitochondrial stress and decrease during cellular senescence. Exp. Cell. Res. 2001;265:262–273. doi: 10.1006/excr.2001.5166. [DOI] [PubMed] [Google Scholar]
- 33.Dell'Orco RT, McClung JK, Jupe ER, Liu XT. Prohibitin and the senescent phenotype. Exp. Gerontol. 1996;31:245–252. doi: 10.1016/0531-5565(95)02009-8. [DOI] [PubMed] [Google Scholar]
- 34.Nuell MJ, Stewart DA, Walker L, Friedman V, Wood CM, Owens GA, Smith JR, Schneider EL, Dell' Orco R, Lumpkin CK, et al. Prohibitin, an evolutionarily conserved intracellular protein that blocks DNA synthesis in normal fibroblasts and HeLa cells. Mol. Cell. Biol. 1991;11:1372–1381. doi: 10.1128/mcb.11.3.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizwani W, Alexandrow M, Chellappan S. Prohibitin physically interacts with MCM proteins and inhibits mammalian DNA replication. Cell Cycle. 2009;8:1621–1629. doi: 10.4161/cc.8.10.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai HW, Chow NH, Lin CP, Chan SH, Chou CY, Ho CL. The significance of prohibitin and c-Met/hepatocyte growth factor receptor in the progression of cervical adenocarcinoma. Hum. Pathol. 2006;37:198–204. doi: 10.1016/j.humpath.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Ren HZ, Wang JS, Wang P, Pan GQ, Wen JF, Fu H, Shan XZ. Increased expression of prohibitin and its relationship with poor prognosis in esophageal squamous cell carcinoma. Pathol. Oncol. Res. 2010;16:515–522. doi: 10.1007/s12253-009-9242-1. [DOI] [PubMed] [Google Scholar]
- 38.Ryu JW, Kim HJ, Lee YS, Myong NH, Hwang CH, Lee GS, Yom HC. The proteomics approach to find biomarkers in gastric cancer. J. Korean. Med. Sci. 2003;18:505–509. doi: 10.3346/jkms.2003.18.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He QY, Cheung YH, Leung SY, Yuen ST, Chu KM, Chiu JF. Diverse proteomic alterations in gastric adenocarcinoma. Proteomics. 2004;4:3276–3287. doi: 10.1002/pmic.200300916. [DOI] [PubMed] [Google Scholar]
- 40.Kang X, Zhang L, Sun J, Ni Z, Ma Y, Chen X, Sheng X, Chen T. Prohibitin: a potential biomarker for tissue-based detection of gastric cancer. J. Gastroenterol. 2008;43:618–625. doi: 10.1007/s00535-008-2208-3. [DOI] [PubMed] [Google Scholar]
- 41.Sato T, Sakamoto T, Takita K, Saito H, Okui K, Nakamura Y. The human prohibitin (PHB) gene family and its somatic mutations in human tumors. Genomics. 1993;17:762–764. doi: 10.1006/geno.1993.1402. [DOI] [PubMed] [Google Scholar]
- 42.Nan Y, Yang S, Tian Y, Zhang W, Zhou B, Bu L, Huo S. Analysis of the expression protein profiles of lung squamous carcinoma cell using shot-gun proteomics strategy. Med. Oncol. 2009;26:215–221. doi: 10.1007/s12032-008-9109-4. [DOI] [PubMed] [Google Scholar]
- 43.Wu TF, Wu H, Wang YW, Chang TY, Chan SH, Lin YP, Liu HS, Chow NH. Prohibitin in the pathogenesis of transitional cell bladder cancer. Anticancer. Res. 2007;27:895–900. [PubMed] [Google Scholar]
- 44.Gu Y, Ande SR, Mishra S. Altered O-GlcNAc modification and phosphorylation of mitochondrial proteins in myoblast cells exposed to high glucose. Arch. Biochem. Biophys. 2010 doi: 10.1016/j.abb.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Gregory-Bass RC, Olatinwo M, Xu W, Matthews R, Stiles JK, Thomas K, Liu D, Tsang B, Thompson WE. Prohibitin silencing reverses stabilization of mitochondrial integrity and chemoresistance in ovarian cancer cells by increasing their sensitivity to apoptosis. Int. J. Cancer. 2008;122:1923–1930. doi: 10.1002/ijc.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ummanni R, Junker H, Zimmermann U, Venz S, Teller S, Giebel J, Scharf C, Woenckhaus C, Dombrowski F, Walther R. Prohibitin identified by proteomic analysis of prostate biopsies distinguishes hyperplasia and cancer. Cancer. Lett. 2008;266:171–185. doi: 10.1016/j.canlet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 47.Chumbalkar VC, Subhashini C, Dhople VM, Sundaram CS, Jagannadham MV, Kumar KN, Srinivas PN, Mythili R, Rao MK, Kulkarni MJ, Hegde S, Hegde AS, Samual C, Santosh V, Singh L, Sirdeshmukh R. Differential protein expression in human gliomas and molecular insights. Proteomics. 2005;5:1167–1177. doi: 10.1002/pmic.200401202. [DOI] [PubMed] [Google Scholar]
- 48.Mengwasser J, Piau A, Schlag P, Sleeman JP. Differential immunization identifies PHB1/PHB2 as blood-borne tumor antigens. Oncogene. 2004;23:7430–7435. doi: 10.1038/sj.onc.1207987. [DOI] [PubMed] [Google Scholar]
- 49.Mishra S, Moulik S, Murphy LJ. Prohibitin binds to C3 and enhances complement activation. Mol. Immunol. 2007;44:1897–1902. doi: 10.1016/j.molimm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 50.Sato T, Saito H, Swensen J, Olifant A, Wood C, Danner D, Sakamoto T, Takita K, Kasumi F, Miki Y, et al. The human prohibitin gene located on chromosome 17q21 is mutated in sporadic breast cancer. Cancer. Res. 1992;52:1643–1646. [PubMed] [Google Scholar]
- 51.Manjeshwar S, Branam DE, Lerner MR, Brackett DJ, Jupe ER. Tumor suppression by the prohibitin gene 3'untranslated region RNA in human breast cancer. Cancer. Res. 2003;63:5251–5256. [PubMed] [Google Scholar]
- 52.Manjeshwar S, Lerner MR, Zang XP, Branam DE, Pento JT, Lane MM, Lightfoot SA, Brackett DJ, Jupe ER. Expression of prohibitin 3' untranslated region suppressor RNA alters morphology and inhibits motility of breast cancer cells. J. Mol. Histol. 2004;35:639–646. doi: 10.1007/s10735-004-2185-7. [DOI] [PubMed] [Google Scholar]
- 53.Jupe ER, Badgett AA, Neas BR, Craft MA, Mitchell DS, Resta R, Mulvihill JJ, Aston CE, Thompson LF. Single nucleotide polymorphism in prohibitin 39 untranslated region and breast-cancer susceptibility. Lancet. 2001;357:1588–1589. doi: 10.1016/s0140-6736(00)04747-4. [DOI] [PubMed] [Google Scholar]
- 54.Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J. Biol. Chem. 2003;278:47853–47861. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- 55.Chander H, Halpern M, Resnick-Silverman L, Manfredi JJ, Germain D. Skp2B attenuates p53 function by inhibiting prohibitin. EMBO Rep. 2009;11:220–225. doi: 10.1038/embor.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Nath N, Adlam M, Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. 1999;18:3501–3510. doi: 10.1038/sj.onc.1202684. [DOI] [PubMed] [Google Scholar]
- 57.Wang S, Zhang B, Faller DV. Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. Embo J. 2002;21:3019–3028. doi: 10.1093/emboj/cdf302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dart DA, Spencer-Dene B, Gamble SC, Waxman J, Bevan CL. Manipulating prohibitin levels provides evidence for an in vivo role in androgen regulation of prostate tumours. Endocr. Relat. Cancer. 2009;16:1157–1169. doi: 10.1677/ERC-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu T, Tang H, Lang Y, Liu M, Li X. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer. Lett. 2009;273:233–242. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Ko KS, Tomasi ML, Iglesias-Ara A, French BA, French SW, Ramani K, Lozano JJ, Oh P, He L, Stiles BL, Li TW, Yang H, Martinez-Chantar ML, Mato JM, Lu SC. Liver-specific deletion of prohibitin 1 results in spontaneous liver injury, fibrosis, and hepatocellular carcinoma in mice. Hepatology. 2010 doi: 10.1002/hep.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajalingam K, Wunder C, Brinkmann V, Churin Y, Hekman M, Sievers C, Rapp UR, Rudel T. Prohibitin is required for Ras-induced Raf-MEK-ERK activation and epithelial cell migration. Nat. Cell. Biol. 2005;7:837–843. doi: 10.1038/ncb1283. [DOI] [PubMed] [Google Scholar]
- 62.Rajalingam K, Rudel T. Ras-Raf signaling needs prohibitin. Cell Cycle. 2005;4:1503–1505. doi: 10.4161/cc.4.11.2142. [DOI] [PubMed] [Google Scholar]
- 63.Patel N, Chatterjee SK, Vrbanac V, Chung I, Mu CJ, Olsen RR, Waghorne C, Zetter BR. Rescue of paclitaxel sensitivity by repression of Prohibitin1 in drug-resistant cancer cells. Proc. Natl. Acad. Sci. U S A. 2010;107:2503–2508. doi: 10.1073/pnas.0910649107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid. Redox. Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 65.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, Fisher SE, Mullin GE. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig. Dis. Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 66.Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995;36:718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shiratora Y, Aoki S, Takada H, Kiriyama H, Ohto K, Hai K, Teraoka H, Matano S, Matsumoto K, Kamii K. Oxygen-derived free radical generating capacity of polymorphonuclear cells in patients with ulcerative colitis. Digestion. 1989;44:163–171. doi: 10.1159/000199906. [DOI] [PubMed] [Google Scholar]
- 68.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Droge W. Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 1998;42:485–492. doi: 10.1136/gut.42.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamaki H, Nakamura H, Nishio A, Nakase H, Ueno S, Uza N, Kido M, Inoue S, Mikami S, Asada M, Kiriya K, Kitamura H, Ohashi S, Fukui T, Kawasaki K, Matsuura M, Ishii Y, Okazaki K, Yodoi J, Chiba T. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131:1110–1121. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 71.Segui J, Gironella M, Sans M, Granell S, Gil F, Gimeno M, Coronel P, Pique JM, Panes J. Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J. Leukoc. Biol. 2004;76:537–544. doi: 10.1189/jlb.0304196. [DOI] [PubMed] [Google Scholar]
- 72.Ardite E, Sans M, Panes J, Romero FJ, Pique JM, Fernandez-Checa JC. Replenishment of glutathione levels improves mucosal function in experimental acute colitis. Lab. Invest. 2000;80:735–744. doi: 10.1038/labinvest.3780077. [DOI] [PubMed] [Google Scholar]
- 73.Kruidenier L, van Meeteren ME, Kuiper I, Jaarsma D, Lamers CB, Zijlstra FJ, Verspaget HW. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free. Radic. Biol. Med. 2003;34:753–765. doi: 10.1016/s0891-5849(02)01426-0. [DOI] [PubMed] [Google Scholar]
- 74.Musch MW, Walsh-Reitz MM, Chang EB. Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am. J. Physiol. Gastrointest. Liver. Physiol. 2006;290:G222–G231. doi: 10.1152/ajpgi.00301.2005. [DOI] [PubMed] [Google Scholar]
- 75.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J. Biol. Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 76.von Ritter C, Grisham MB, Hollwarth M, Inauen W, Granger DN. Neutrophil-derived oxidants mediate formyl-methionyl-leucyl-phenylalanine-induced increases in mucosal permeability in rats. Gastroenterology. 1989;97:778–780. doi: 10.1016/0016-5085(89)90654-9. [DOI] [PubMed] [Google Scholar]
- 77.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003;201:28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 78.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch. Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 79.Miles AM, Grisham MB. Antioxidant properties of aminosalicylates. Methods Enzymol. 1994;234:555–572. doi: 10.1016/0076-6879(94)34128-1. [DOI] [PubMed] [Google Scholar]
- 80.Emerit J, Pelletier S, Likforman J, Pasquier C, Thuillier A. Phase II trial of copper zinc superoxide dismutase (CuZn SOD) in the treatment of Crohn's disease. Free. Radic. Res. Commun. 1991;12–13(Pt 2):563–569. doi: 10.3109/10715769109145831. [DOI] [PubMed] [Google Scholar]
- 81.Niwa Y, Somiya K, Michelson AM, Puget K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free. Radic. Res. Commun. 1985;1:137–153. doi: 10.3109/10715768509056547. [DOI] [PubMed] [Google Scholar]
- 82.Hsieh SY, Shih TC, Yeh CY, Lin CJ, Chou YY, Lee YS. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics. 2006;6:5322–5331. doi: 10.1002/pmic.200500541. [DOI] [PubMed] [Google Scholar]
- 83.Theiss AL, Jenkins AK, Okoro NI, Klapproth JM, Merlin D, Sitaraman SV. Prohibitin inhibits tumor necrosis factor alpha-induced nuclear factor-kappa B nuclear translocation via the novel mechanism of decreasing importin alpha3 expression. Mol. Biol. Cell. 2009;20:4412–4423. doi: 10.1091/mbc.E09-05-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kao WH, Puddey IB, Boland LL, Watson RL, Brancati FL. Alcohol consumption and the risk of type 2 diabetes mellitus: atherosclerosis risk in communities study. Am. J. Epidemiol. 2001;154:748–757. doi: 10.1093/aje/154.8.748. [DOI] [PubMed] [Google Scholar]
- 85.de la, Monte SM, Neely TR, Cannon J, Wands JR. Ethanol impairs insulin-stimulated mitochondrial function in cerebellar granule neurons. Cell. Mol. Life. Sci. 2001;58:1950–1960. doi: 10.1007/PL00000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patel DG, Singh SP. Effect of ethanol and its metabolites on glucose mediated insulin release from isolated islets of rats. Metabolism. 1979;28:85–89. doi: 10.1016/0026-0495(79)90173-2. [DOI] [PubMed] [Google Scholar]
- 87.Suh SK, Hood BL, Kim BJ, Conrads TP, Veenstra TD, Song BJ. Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver. Proteomics. 2004;4:3401–3412. doi: 10.1002/pmic.200400971. [DOI] [PubMed] [Google Scholar]
- 88.Lee JH, Nguyen KH, Mishra S, Nyomba BL. Prohibitin is expressed in pancreatic beta-cells and protects against oxidative and proapoptotic effects of ethanol. Febs J. 2009;277:488–500. doi: 10.1111/j.1742-4658.2009.07505.x. [DOI] [PubMed] [Google Scholar]
- 89.Vessal M, Mishra S, Moulik S, Murphy LJ. Prohibitin attenuates insulin-stimulated glucose and fatty acid oxidation in adipose tissue by inhibition of pyruvate carboxylase. Febs J. 2006;273:568–576. doi: 10.1111/j.1742-4658.2005.05090.x. [DOI] [PubMed] [Google Scholar]
- 90.Ande SR, Moulik S, Mishra S. Interaction between O-GlcNAc modification and tyrosine phosphorylation of prohibitin: implication for a novel binary switch. PLoS One. 2009;4:e4586. doi: 10.1371/journal.pone.0004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am. J. Physiol. Endocrinol. Metab. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 93.Hossen MN, Kajimoto K, Akita H, Hyodo M, Ishitsuka T, Harashima H. Ligand-based targeted delivery of a peptide modified nanocarrier to endothelial cells in adipose tissue. J. Control Release. 2010;147:261–268. doi: 10.1016/j.jconrel.2010.07.100. [DOI] [PubMed] [Google Scholar]