Abstract
Bleeding from esophageal and gastric varices remains a significant cause of morbidity and mortality for patients with liver cirrhosis. Currently, therapeutic strategies for gastric variceal bleeding include transjugular intrahepatic portosystemic shunt, cyanoacrylate sclerotherapy and hepatic transplantation. Though relatively safe and efficacious, endoscopic sclerotherapy using cyanoacrylate has known complications including infection, bleeding, and distal embolization. This case report describes a patient who became febrile and tachycardic following sclerotherapy and subsequently had an abnormal chest radiograph that prompted further evaluation for pulmonary embolization of the sclerosant. The focuses of this report are the computed tomographic and radiographic findings associated with 2-octyl-cyanoacrylate/lipiodol pulmonary embolization.
Keywords: 2-octylcyanoacrylate, computed tomography, vascular cast sign, pulmonary embolism, gastric varices
INTRODUCTION
Bleeding from esophageal and gastric varices remains a significant cause of morbidity and mortality for patients with liver cirrhosis. Studies have shown that variceal bleeding can be associated with a mortality rate of up to 50% (1). Currently, therapeutic strategies for variceal bleeding include sclerotherapy, endoscopic band ligation, transjugular intrahepatic portosystemic shunt (TIPS) and hepatic transplantation. Over the past several decades, endoscopic therapy has become the primary treatment modality for esophageal variceal bleeding. In the United States (US), TIPS has remained the primary available intervention for bleeding gastric varices. Outside of the US, tissue adhesive injection sclerotherapy (most commonly using histoacryl) is widely utilized for the management of bleeding gastric varices (1,2,3). In the US, histoacryl is not FDA approved for gastric variceal sclerotherapy. However, several studies have demonstrated efficacy with the cyanoacrylate compound 2-octyl-cyanoacrylate (OCA) (1,3). The OCA is usually mixed with lipiodol (ethiodized poppy seed oil) to decelerate the polymerization of the compound and to aid with visualization during the procedure (1,3). Lipiodol is also used as a final flush to facilitate needle withdrawal following completion of each OCA injection. Though considered to be both efficacious and relatively low risk (1,4), this procedure is known to have the potential for complications including infection, bleeding and distal embolization (1–10). While many reports that describe complications do not denote long-term sequelae, there have been descriptions of cases with serious adverse outcomes including death (1,2,5,6). In particular, patients with pulmonary arteriovenous shunts or patent foramen ovale are at risk for paradoxical embolization (3,5). There have been reports of splenic infarctions and cerebrovascular accidents following cyanoacrylate variceal sclerotherapy (1,2,3,5,7).
This case report describes a patient who became febrile and tachycardic following gastric variceal obliteration with OCA/lipiodol and was subsequently found to have an abnormal chest radiograph which prompted further evaluation for pulmonary embolization. Prior case reports have described similar cases, but noted that the standard workup for pulmonary emboli with a computed tomographic pulmonary angiogram (CTPA) made it difficult to evaluate for OCA/lipiodol embolization (unless viewed on the bone window setting) (3,8). The focuses of this report are the CT and radiographic findings that are expected with OCA/lipiodol pulmonary embolization.
CASE REPORT
A 75-year-old male with past medical history significant for chronic obstructive pulmonary disease, coronary artery disease, and alcoholic liver cirrhosis, status post esophageal variceal banding a year prior to this admission, was admitted to an outside hospital for three episodes of hematemesis and melena. At the outside hospital his vital signs on admission included a heart rate of 95 beats per minute (BPM), a blood pressure of 108/58 millimeters of mercury (mmHg) and a room air oxygen saturation of 100%. He was transfused two units of packed red blood cells and underwent esophagogastroduodenoscopy (EGD), where he was noted to have a small, non-bleeding esophageal varix and multiple gastric varices, one of which with an overlying red spot, consistent with a recent bleed. There was no active bleeding from the gastric varices. No endoscopic intervention was undertaken, and the patient was referred to interventional radiology for a TIPS. However, during the attempted TIPS, the patient became hypotensive and the procedure was aborted.
The patient was subsequently transferred to our hospital for sclerotherapy. Given that OCA is not currently FDA approved for the obliteration of gastric varices, informed consent was obtained after a detailed discussion with the patient regarding its off label use and the risks associated with the procedure. Prior to the EGD, a chest radiograph was obtained (Fig 1) and the patient's vital signs included a temperature of 36.0 degrees Celsius, a heart rate of 92 BPM, and a blood pressure of 123/64 mmHg. EGD was performed and three prominent gastric varices were identified in the fundus. One mililiter (mL) of OCA/lipoidol was injected into each of three gastric varices, for a total of 3mL. Each one mL injection was immediately followed by a lipiodol flush. No immediate complications were noted following the completion of the procedure. The day following the procedure, the patient intermittently became tachycardic with a heart rate of 140 BPM sustained for hours at a time. A twelve lead electrocardiogram obtained confirmed sinus tachycardia. Additionally, he became persistently febrile with a maximum temperature of 39.0 degrees Celsius on post-procedural day three. Moreover, a leukocytosis developed and continued to trend upwards to 12.9 thousand white blood cells per mL on post-procedural day number three. At this time, his hemoglobin and hematocrit measured 9.6 grams per deciliter and 27.6 percent, respectively. The fever and tachycardia, in addition to prior case reports describing infection (1,3,4) as a possible complication of variceal injection, prompted a sepsis workup which included a chest radiograph (Fig 2) and empiric antibiotic coverage. The abnormal findings on the post-procedural chest radiograph raised suspicion for OCA/lipiodol embolization, prompting a non-contrast computed tomogram (CT) of the chest (Fig 3). Upon review of the non-contrast CT of the chest, the vascular hyperdensities seen on plain radiography correlated with hyperdense material lodged within pulmonary vessels. Additionally, an area of wedged shaped pulmonary parenchymal opacification was noted to extend from an involved vessel to the lung periphery, radiographically consistent with pulmonary infarction. Despite these radiographic findings and cessation of empiric antibiotics, the patient's fever, tachycardia and leukocytosis gradually resolved with supportive care and he was discharged to be followed as an outpatient.
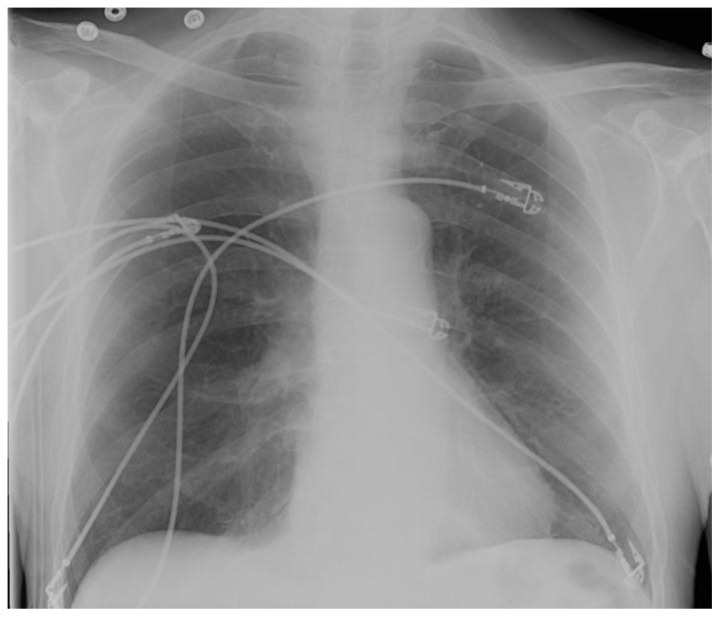
Figure 1.
75 year old male with gastric variceal bleeding. Plain radiograph prior to gastric variceal injection with 2-octylcyanoacrylate demonstrates normal pulmonary vasculature density and gross morphology.
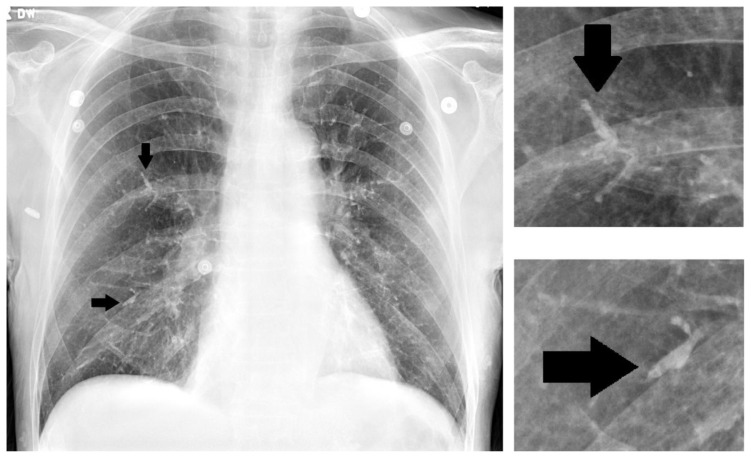
Figure 2.
75 year old male with gastric variceal bleeding. Plain radiograph following 2-octylcyanoacrylate injection. The pulmonary vasculature is significantly more prominent and appears to demonstrate intravascular contrast despite a lack of intravenous contrast administration. Arrowheads indicate examples of particularly prominent vasculature with demonstration of the vascular cast sign. Magnified views of two selected areas of interest are seen on the right.
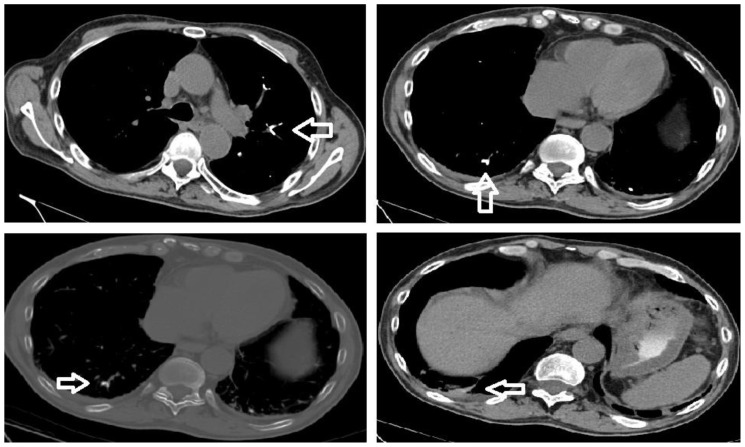
Figure 3.
75 year old male with gastric variceal bleeding. CT study perfomed on Siemens Emotion 16 slice scanner, 141 mAs, kVP 130, slice thickness of 3 mm. Top left and top right: Non-contrast enhanced CT of the chest demonstrating hyperdense material within the pulmonary vasculature, correlating with the vascular cast sign seen on plain radiography (mediastinal window). Bottom left: Non-contrast enhanced CT of the chest demonstrating intraluminal hyperdense material within pulmonary vasculature (bone window). Bottom right: Non-contrast enhanced CT of the chest demonstrating wedged shaped pulmonary parenchymal density extending from an area of suspected 2-octylcyanoacrylate embolism towards the periphery consistent with pulmonary infarction (mediastinal window).
DISCUSSION
This case represents a situation where imaging in the setting of a particular clinical context is essential for the diagnosis and management of an unusual post-procedural complication. A number of case reports have described the possible complications of cyanoacrylate/lipiodol injection sclerotherapy for gastric varices (1–10). Many of the patients treated for varices in this manner are inpatients and therefore have risk factors independent of the sclerotherapy for common hospital acquired complications including infection and venous thromboembolic (VTE) disease. While the clinical context of such a case is very helpful, in this setting, imaging findings can provide pathognomonic information to establish the diagnosis and guide treatment. When presented with such a clinical scenario, the first step should be obtaining a chest radiograph.
The post-procedural chest radiograph should be compared to a prior study if available, with special attention to the pulmonary vasculature, taking into account differences in technique. Specifically, differences in penetration can hinder proper comparison of the pulmonary vasculature. One particular imaging sign, which we term the "vascular cast sign", should raise suspicion for post-procedural cyanoacrylate/lipoidol pulmonary embolization. In the setting of cyanoacrylate/lipiodol embolism, the "vascular cast sign" consists of hyperdense segments of the pulmonary vasculature (radioopaque contrast agent) with respect to the surrounding vasculature that creates a plaster-cast-like appearance of the vessels. The presence of the "vascular cast sign" should raise concern for sclerosant embolization, and a non-contrast CT study can be performed for confirmation.
In the context of high clinical suspicion for pulmonary embolization from a deep venous thrombus, a clinician would request a CTPA, allowing the low-density thrombus to be visualized amongst the higher density contrast-enhanced pulmonary arterial background. However, in the setting of cyanoacrylate/lipiodol embolism, intravenous contrast within the pulmonary arteries will obscure the radioopaque lipiodol, limiting the sensitivity of the CTPA study. While prior studies (3) suggest that analysis of CT imaging on bone window settings is helpful in assessing for cyanoacrylate/lipiodol pulmonary embolization, CT without intravenous contrast analyzed on mediastinal window settings provides excellent contrast to confirm the diagnosis and may be more sensitive in terms of detecting smaller emboli (figures 3b and 3c). A delay in diagnosis could result in further workup, leading to increased costs and overutilization of medications, including anticoagulants or empiric antibiotics. Many reports suggest that OCA/lipiodol embolization to the lung usually results in transient symptoms only. CT imaging can demonstrate whether or not pulmonary parenchymal damage has occurred. As seen on figure 3d, pulmonary infarction can occur in the setting of cyanoacrylate/lipiodol pulmonary embolization. Moreover, in the setting of anatomical abnormalities such as an arteriovenous shunt or a patent foramen ovale, this procedure puts patients at risk for paradoxical embolization of cyanoacrylate/lipiodol (3,5) and further extra-thoracic imaging may be necessary.
In conclusion, though gastric variceal obliteration using cyanoacrylate/lipiodol is a relatively safe and efficacious procedure, complications are known to occur. While the exact rate of embolization is unclear at this time, it is essential for clinicians to be aware of this complication, to vigilantly monitor these patients post procedure and to communicate clinical concerns to the radiologist so that the appropriate imaging study can be obtained in order to ensure a timely diagnosis.
TEACHING POINT
Following a cyanoacrylate/lipiodol endoscopic gastric variceal obliteration procedure, there is the potential for the development of sclerosant pulmonary embolization. The appropriate imaging studies to make the correct diagnosis would be a chest radiograph (to demonstrate the vascular cast sign) and a non-contrast computed tomographic series of the chest (to demonstrate hyperdense material within the pulmonary vasculature).
Table 1.
Summary table for post-procedural cyanoacrylate pulmonary embolism.
| Etiology: | Following endoscopic injection sclerotherapy utilizing cyanoacrylate compounds, distal embolization may result. |
| Incidence: | The exact incidence of this complication is unknown. This procedure is not currently performed routinely in the US and embolization is often clinically silent. Further studies regarding the incidence would be beneficial. |
| Gender Ratio: | There is no known gender predilection. |
| Age Predilection: | There is no known age predilection. |
| Risk Factors: | Persistent portal hypertension with gastric variceal bleeding refractory to TIPS and medical management is the primary indication for this procedure. Therefore, this patient population is at the highest risk for embolization as they would be subject to this procedure. During the procedure, rapid injection of the cyanoacrylate sclerosant increases the risk of distal pulmonary embolization. However, significantly slowed injection increases the risk of sclerosant polymerization within the injection needle. |
| Treatment: | Supportive medical care would be the management for this procedural complication. The key point regarding treatment is that use of anticoagulants or antibiotics would be inappropriate in this setting. |
| Prognosis: | In general, the prognosis appears to be good. Many patients, including the patient in this report, may have transient symptoms or may be completely asymptomatic. However, prognosis may be significantly worsened in certain scenarios. In particular, patients with a massive sclerosant pulmonary embolism burden in the setting of low cardiopulmonary reserve due to concomitant medical illness may have poor outcomes. Additionally, those patients with right to left shunts are at increased risk for systemic embolization of sclerosant which may have disastrous results. |
| Imaging Findings: | Plain radiographs may demonstrate hyperdense segments of pulmonary vasculature (“vascular cast sign”). Non-contrast enhanced CT will demonstrate hyperdense material within pulmonary vasculature. There may also be associated findings consistent with pulmonary infarction. Of note, it is important to remember that these findings are dependent on the use of contrast material with the sclerosant (which is a standard practice when performing this procedure). |
Table 2.
Differential table for cyanoacrylate pulmonary embolism
| Etiology | Radiograph | CT |
|---|---|---|
| Cyanoacrylate Pulmonary Embolism | Hyperdense material along the trajectory of the pulmonary vasculature producing the appearance of a cast being made of the vessels (vascular cast sign) | Non-contrast enhanced CT will demonstrate hyperdense material within segments of the pulmonary vasculature. This will be apparent on both mediastinal and bone windows. Use of intravenous contrast will obscure the embolic material and will make the diagnosis difficult if not impossible. Additionally, there may be radiographic evidence of pulmonary infarction including atelectasis or peripheral wedge shaped pulmonary parenchymal opacities. |
| Thromboembolic Pulmonary Embolism | The radiograph may be normal. Atelectasis may be evident. Rarely, classic signs such as peripheral wedge shaped pulmonary parenchymal opacities or relative hyporemia may be noted. | Non-contrast enhanced CT may demonstrate peripheral wedge shaped pulmonary parenchymal opacities or atelectasis. Contrast enhanced CT (CT pulmonary angiogram) will demonstrate intravascular filling defects. |
| Pneumonia | Various findings may be seen which represent pneumonia. These may present in a parenchymal or airspace distribution (or both). The specific radiographic findings of various types of pneumonia are beyond the scope of this case report. Intravascular hyperdensities will be absent. | Various findings may be seen which represent pneumonia. These may present in a parenchymal or airspace distribution (or both). The specific radiographic findings of various types of pneumonia are beyond the scope of this case report. Intravascular hyperdensities will be absent. |
| Atelectasis | Complete lobar collapse can be seen for various reasons. Additionally, subsegmental atelectasis may present as linear hyperdensities. These densities will not follow the course of pulmonary vessels. | Complete lobar collapse can be seen for various reasons. Additionally, subsegmental atelectasis may present as linear hyperdensities. These densities will not follow the course of pulmonary vessels. |
ACKNOWLEDGEMENTS
Special thanks to Dr. Firas Al-Kawas, the gastrointesintal attending involved in this case who helped with the editing of this manuscript. Additionally, special thanks to Dr. Joseph Rahill who helped with taking care of this patient and with editing the manuscript.
ABBREVIATIONS
- BPM
beats per minute
- CT
computed tomography
- CTPA
computed tomography pulmonary angiogram
- EGD
esophagogastroduodenoscopy
- FDA
Food and Drug Administation
- mL
mililiter
- mm
milimeter
- mmHg
milimeters of mercury
- OCA
2-octyl-cyanoacrylate
- SIRS
systemic inflammatory response syndrome
- TIPS
transjugular intrahepatic portosystemic shunt
- US
United States
- VTE
venous thromboembolic
REFERENCES
- 1.Helmy A, Hayes PC. Review article: current endoscopic therapeutic options in the management of variceal bleeding. Aliment Pharmacol Ther. 2001;15(5):575–94. doi: 10.1046/j.1365-2036.2001.00950.x. [DOI] [PubMed] [Google Scholar]
- 2.Tan YM, Goh K, Kamarulzaman A, et al. Multiple systemic embolisms with septicemia after gastric variceal obliteration with cyanoacrylate. Gastrointestinal Endoscopy. 2002;55(2):276–278. doi: 10.1067/mge.2001.118651. [DOI] [PubMed] [Google Scholar]
- 3.Rickman OB, Utz JP, Aughenbaugh GL, Gostout CJ. Pulmonary Embolization of 2-Octyl Cyanoacrylate After Endoscopic Injection Therapy for Gastric Variceal Bleeding. Mayo Clin Proc. 2004 Nov;79(11):1455–1458. doi: 10.4065/79.11.1455. [DOI] [PubMed] [Google Scholar]
- 4.Türler A, Wolff M, Dorlars D, Hirner A. Embolic and septic complications after sclerotherapy of fundic varices with cyanoacrylate. Gastrointestinal Endoscopy. 2001;53(2):228–230. doi: 10.1067/mge.2001.111561. [DOI] [PubMed] [Google Scholar]
- 5.Takasugi JE, Shaw C. Inadvertent bucrylate pulmonary embolization: a case report. J Thorac Imag. 1989;4(4):71–73. doi: 10.1097/00005382-198910000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Naga M, Foda A. An unusual complication of histoacryl injection. Endoscopy. 1997;29(2):140. doi: 10.1055/s-2007-1004099. [DOI] [PubMed] [Google Scholar]
- 7.Shim CS, Cho YD, Kim JO, et al. A case of portal and splenic vein thrombosis after Histoacryl injection therapy in gastric varices. Endoscopy. 1996;28(5):461. doi: 10.1055/s-2007-1005514. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto A, Takimoto K, Inokuchi I. Prevention of Systemic Embolization Associated With Treatment of Gastric Fundal Varices. Mayo Clin Proc. 2005 May;80(5):703–705. doi: 10.4065/80.5.705. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SS, Kim HH, Park SH, et al. N-butyl-2-cyanoacrylate pulmonary embolism after endoscopic injection sclerotherapy for gastric variceal bleeding. J Comput Assist Tomogr. 2001;25(1):16–22. doi: 10.1097/00004728-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Marco de LE, Fidalgo I, García-Barón PL, Sádaba P, Delgado ML, López-Calderón M. Radiopaque pulmonary arteries on chest radiography. J Thorac Imaging. 2004;19(4):264–6. doi: 10.1097/01.rti.0000141351.93631.2b. [DOI] [PubMed] [Google Scholar]