Abstract
Rosai-Dorfman disease (RDD) involves abnormal proliferations of oddly behaving histocytes that are not derived from the Langerhan’s Cell linage. These collections tend to occur within lymph nodes, with occasional extra nodal presentation. While RDD is a rare entity itself, extra nodal cases are even more so, with even fewer reporting cardiac involvement, and previously only in adults. This report describes the disease in a pediatric patient who had the unique feature of an extra nodal cardiac mass. The patient, who was known to have sickle cell disease, was initially erroneously thought to have acute chest syndrome. Sudden changes in the patient’s status, including development of 3rd degree heart block, demanded investigation with additional imaging. Chest CT revealed a mass arising from the cardiac interatrial septum and encircling the entire thoracic aorta. Imaging features of Rosai-Dorfman disease are nonspecific, complicating the diagnosis. We present this case with discussion of this extremely uncommon entity. We describe the diagnostic methods, the differential diagnosis, and the treatment options.
Keywords: Rosai-Dorfman Disease, Heart
CASE REPORT
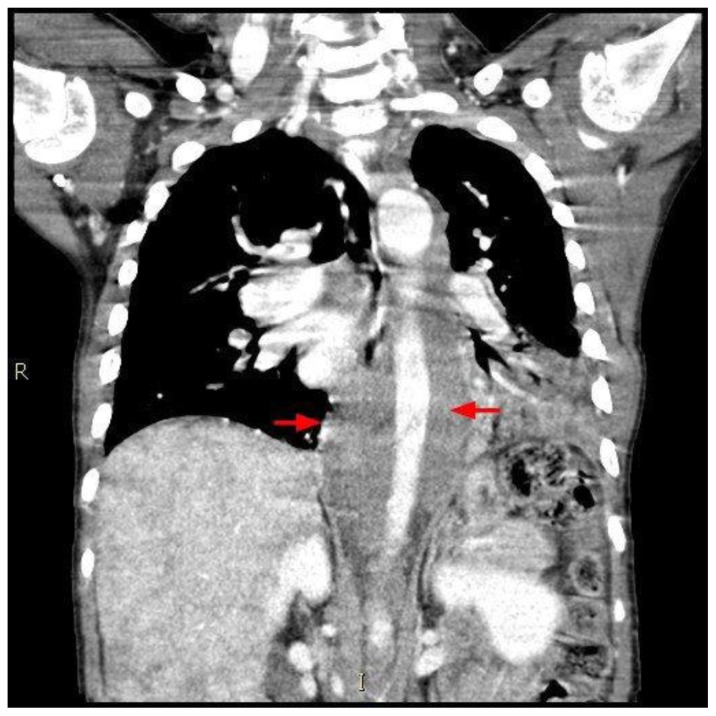
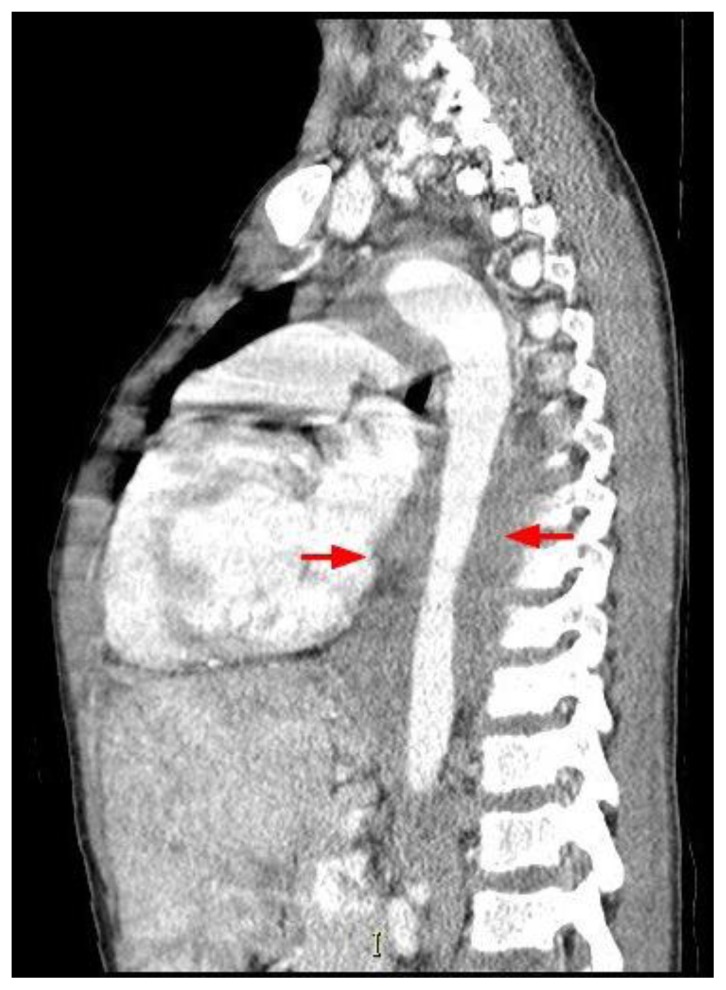
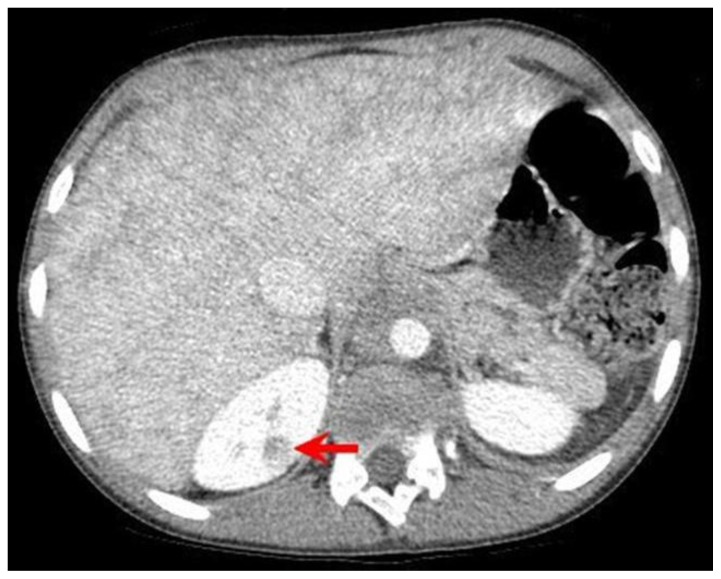
A 12 year old male presented to a local emergency room with dyspnea for 4 days and left sided chest pain accompanied by fever. The patient’s medical history includes sickle cell disease, asthma, pneumonia and several previous episodes of acute chest syndrome. Additionally He has a surgical history of prophylactic cholecystectomy and asplenia due to auto-infarction related to his sickle cell disease. An initial diagnosis of acute chest syndrome (ACS) was made and the patient was transferred to a tertiary care center, to receive higher pediatric care. On arrival, the child’s initial chest radiograph showed an enlarged cardiac silhouette and retrocardiac opacity (figure 1). Shortly after arrival the he developed a 3rd degree heart block. This prompted additional investigation, during which a chest CT revealed a soft tissue density representing a mass originating from the interatrial septum, extending into both atria and abutting the atrioventricular groove (figure 2). This mass was noted to encircle the aortic root (figure 3) and the aortic arch and to compress the left lower lobe bronchus and distal esophagus (figure 4). An additional mass was noted in the posterior mediastinum encasing the descending aorta and extending caudally with inferior margins at the level of the superior renal poles (figures 5, 6). CT also revealed a complex cystic lesion in the right kidney (figure 7).
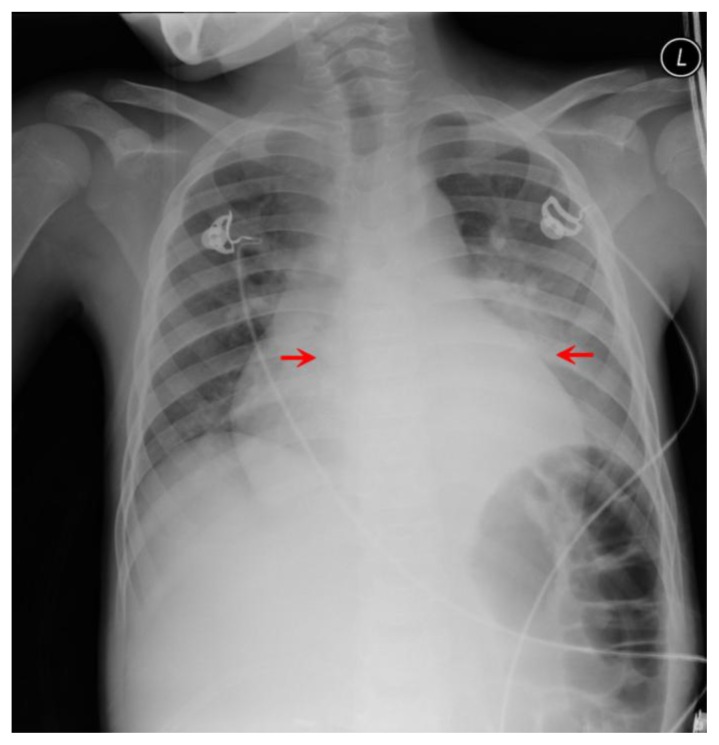
Figure 1.
12-year-old male with Rosai-Dorfman disease. Frontal radiograph of the chest reveals a magnified cardiac silhouette and retrocardiac opacity (red arrows).
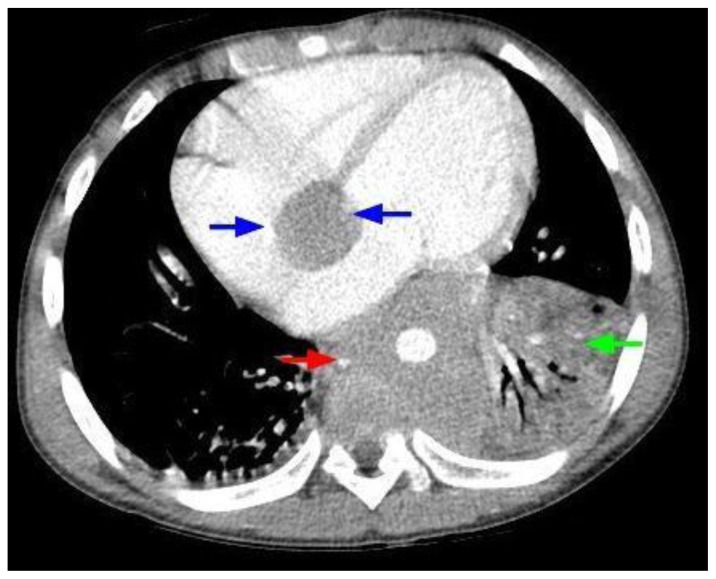
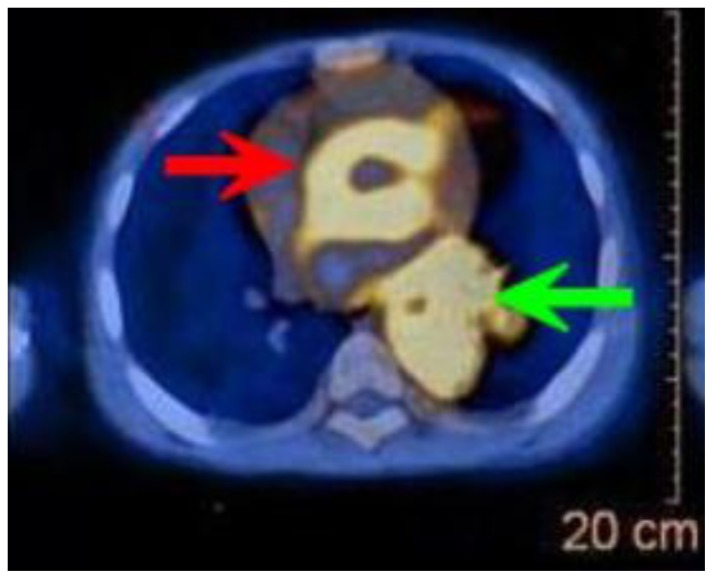
Figure 2.
12-year-old male with Rosai-Dorfman disease. Axial chest CT obtained following the intravenous administration of 100 ml of Omnipaque 350. The CT setting was 100 kVp with modulated mAs. The image reveals a cardiac mass originating from the interatrial septum and abutting the atrioventricular groove (blue arrows). There is soft tissue density encasing the descending aorta (red arrow). There is left lower lobe atelectasis (green arrow) due to compression of the left lower lobe bronchus by the mass.
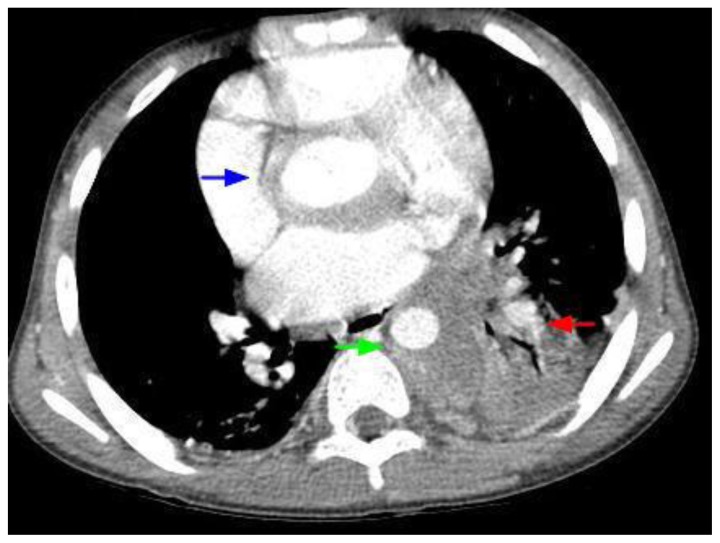
Figure 3.
12-year-old male with Rosai-Dorfman disease. Axial chest CT obtained following the intravenous administration of 100 ml of Omnipaque 350. The CT setting was 100 kVp with modulated mAs. The image reveals a mass encasing the aortic root (blue arrow). There is left lower lobe atelectasis (red arrow) secondary to compression of the left lower lobe bronchus. The mass is noted to encircle the descending aorta (green arrow) and compress the esophagus.
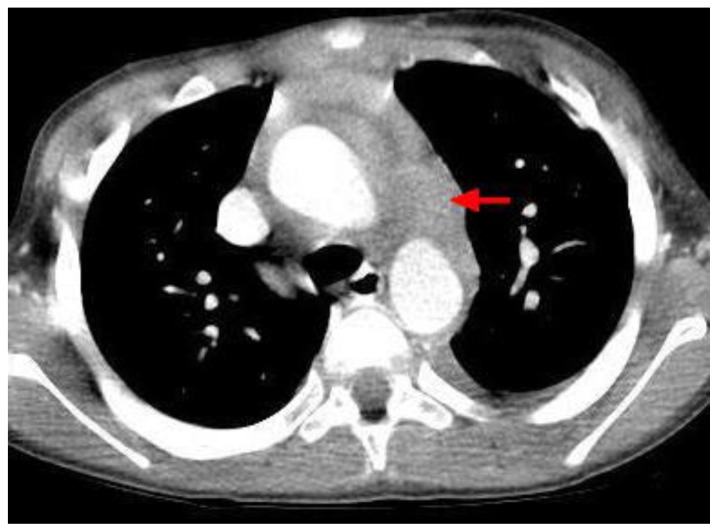
Figure 4.
12-year-old male with Rosai-Dorfman disease. Axial chest CT obtained following the intravenous administration of 100 ml of Omnipaque 350. The CT setting was 100 kVp with modulated mAs. The image reveals a mass encircling the aortic arch (red arrow) and the ascending and descending aorta.
Figure 5.
12-year-old male with Rosai-Dorfman disease. Coronal chest CT obtained following the intravenous administration of 100 ml of Omnipaque 350. The CT setting was 100 kVp with modulated mAs. The image reveals a soft tissue mass encasing the descending aorta (red arrows). The mass extend to the level of the upper abdomen. Note the mottled appearance of the liver.
Figure 6.
12-year-old male with Rosai-Dorfman disease. Sagittal chest CT obtained following the intravenous administration of 100 ml of Omnipaque 350. The CT setting was 100 kVp with modulated mAs. The image reveals a soft tissue mass encasing the descending aorta (red arrows). The mass extend to the level of the upper abdomen. Note the mottled appearance of the liver.
Figure 7.
12-year-old male with Rosai-Dorfman disease. Axial chest CT obtained following the intravenous administration of 100 ml of Omnipaque 350. The CT setting was 100 kVp with modulated mAs. The image reveals a complex cyst with enhancing septa in the upper pole of the right kidney (red arrow). Note the mottled appearance of the liver.
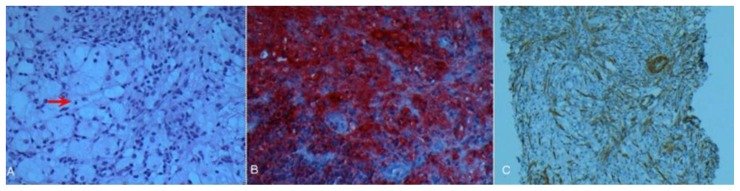
With these non specific imaging findings it became prudent to obtain a tissue sample for better characterization. Needle biopsy of the mass was successfully completed under CT guidance. The tissues were analyzed with tissue specific stains for S-100, CD1a, CD3 and IgG4. Tissue staining patterns were especially strong for S-100 and the histology demonstrated the unique finding of emperipolesis (Active penetration of one cell by another, both of which remain intact), thus the diagnosis of Rosai-Dorfman disease (RDD) was made (figure 8).
Figure 8.
12-year-old male with Rosai-Dorfman disease. (a) Section demonstrating large bland histiocytic cells with pale to foamy cytoplasm displaying emperipolesis (red arrow), admixed with mature lymphocytes, plasma cells and occasional polymorphs. Mild delicate myofibroblastic proliferation is noted in a reactive pattern (H&E × 100). (b) Histiocytic cells are intensely immunoreactive for S-100 protein (S-100 × 200). (c) Immunostain for smooth muscle actin highlights the delicate myofibroblastic proliferation in a reactive pattern with relatively scant collagen deposition (SMA × 100).
PET-CT was ordered to further characterize the mass and look for possible occult areas of involvement. Flurodeoxyglucose (FDG) uptake was noted in the regions that correlated with the mass on CT. Areas corresponding with the mediastinal mass had a maximal SUV of 9.8 (figure 9) with the atrial septum mass showing an increased SUV of 6.1. No occult lesions were noted.
Figure 9.
12-year-old male with Rosai-Dorfman disease. Axial view of a PET/CT obtained following the intravenous administration of 8.7 mCi of F-18-FDG. The enhanced CT portion of the study was obtained following the intravenous administration of 100 ml of Omnipaque 350. The CT setting was 100 kVp with modulated mAs. There is intense hypermetabolism in the mass that surrounds the ascending and the descending aorta. The maximal SUV measured in the mass was 9.8, significantly higher than the blood pool activity.
An implanted pacemaker was placed to address the arrhythmia. However, the patient later experienced a new onset of seizures. MRI imaging was performed to investigate the cause of the seizures and to determine if there were intracranial RDD manifestations. Although not diagnostic of the seizure etiology, MRI studies suggested no intracranial RDD lesions. The patient’s condition continues to be monitored, and is without further progression at this time. Due to the general indolent course and frequent spontaneous remission as well as a lack of established treatment, no specific treatment regimen for this patient’s RDD has been initiated. The patient continues to do well in follow up.
DISCUSSION
Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy (SHML), is a rare and complex disease with a variable presentation and course. Specific incidence and prevalence information is not known for this very rare disease with only about 1000 reported cases worldwide. However, the broader category of histiocytosis type diseases has an incidence of about 3 per million. RDD is one of the rarer anomalies of the hematopoietic system, and presents with diagnostic and classification problems common to histiocytic/dendritic derived diseases. These include the scarcity of histiocyte specific markers, poor determination of monoclonality and overlap with many reactive and infectious processes [1]. Classically RDD presents with cervical lymphadenopathy and secondary cutaneous involvement, however other presentations including extranodal tissue involvement occur [2, 3]. SHML was recognized as a separate entity by Rosai and Dorfman in 1969 [2], however the pathogenesis of this disease remains elusive even today. Even though there is no clear causative agent in most cases, a link between human herpes virus 6 (HHV-6) and Epstein-Barr virus (EBV) has been suggested, though not conclusively [1, 3]. Although not detected in earlier studies, RDD shows a significant male gender predilection of approximately 4:1 [4, 5, 6]. Of interest is a purely cutaneous variant which occurs much more frequently in older females [5]. The disease most commonly presents in the first three decades of life [1, 7]. However, RDD can present at any age with cases of patients nearing their 8th decade being reported [8]. Literature varies as to whether or not there is any racial disparity in disease occurrence. In the early 1990s, increased frequency in African populations was proposed [6, 9], as well as in people from the West Indies [4, 6]. Recent reports show the disease to be equally common in those of African and Caucasian descent and less frequent in Asian populations [1]. Recently, association of RDD in concurrence with sickle cell anemia (HbSS) has been reported, although no causal relationship was postulated [6]. Disease progression is generally indolent and spontaneously remits and/or resolves in many cases [3]. This is especially true in cases of purely nodal involvement. Despite generally being considered benign, death has been reported, most frequently from disease involving extra nodal sites in critical organs [3]. Mortality occurs primarily due to compression and disruption of normal tissue function more often than from the disease itself.
Diagnosis of RDD is generally dependent on pathology specimens and immunohistological staining. Even in nodal involvement, the lymph node architecture is usually preserved, with the sinuses expanded by proliferating and often distinctly appearing histiocytes [1], the exact appearance of which can vary from case to case. The key point of light microscopic differentiation from reactive or other proliferative processes is the presence of emperipolesis [1]. This engulfment by histiocytes of lymphocytes, monocytes and even erythrocytes, without subsequent destruction of the cells, is classic for RDD and rarely seen in other disease processes. Regulation and mechanisms of emperipolesis are still being elucidated and may hold promise for future cancer treatment [10]. Immunohistological studies are often employed for diagnosis. RDD usually expresses pan-macrophage antigens; however the strong expression of S-100 is the most helpful marker in diagnosis [1].
Imaging is important in the diagnosis and management of RDD despite generally non-specific findings. Plain film and CT imaging are extremely useful in detecting and characterizing masses, but are not specific for RDD with a wide differential diagnosis including lymphoma and inflammatory and infectious processes [1]. The appearance on CT is one of homogenous masses, frequently with diffusely or locally enlarged lymph nodes [11]. With extranodal disease, CT and MRI findings are also nonspecific and typically reveal polygonal masses, mucosal thickening, or soft-tissue opacification [4]. CT of the head shows RDD lesions to be hyperdense in comparison to grey matter as well as strongly enhancing with contrast [12], while CT of the abdomen yields lesions that are isodense to pancreatic tissue [13, 14]. MRI also typically yields nonspecific results, although it can be very useful to characterize the lesion and exclude other disorders. Lesions of RDD enhance with gadolinium-based contrast agents, showing moderate to slightly increased signal density on T1-weighted imaging but present with variable signal intensity on T2-weighted imaging [11]. A review of central nervous system RDD lesions noted most were visible as isointense relative to gray matter on diffusion-weighted and apparent diffusion coefficient imaging [12]. While these findings again are not unique to RDD, they can be utilized to assist in excluding other disorders [11, 12, 14]. In this reported case, it was determined that new neurologic symptoms were not due to intracranial involvement or progression of RDD. Systemically, RDD lesions are isointense to skeletal muscle on T1-weighted images but comparatively hyperintense with on T2 imaging [4]. Even though there are no distinguishing features in RDD adenopathy by ultrasound, CT or MRI, RDD lesions are hypermetabolic, thus are predicatively quite positive on positron emission tomography [11]. This increased glucose utilization in RDD allows PET-CT to not only localize tissue involvement not demonstrated on CT alone, but to monitor efficacy of treatment and disease progress [15, 16]. This is a valuable tool as comparisons of patients with confirmed RDD in one anatomical location invariably had involvement at another site [12], although FDG uptake might be site-variable[4]. In general, earlier detection of subtle site involvement may help determine what if any intervention in indicated, based on the organs involved.
Treatment of RDD may be indicated if there is morbidity due to the specific organ system involved, during disruptive exacerbations or if the case is rapidly progressing. Scenarios such as these are associated with many more complications, including death and occur most frequently in cases presenting with major or primary extranodal involvement [3]. As the causative factor is unknown, and the disease is too rare for randomized case studies of any significant size, treatment efficacy is difficult to determine. Although no definitive link has been established, both EBV and HHV-6 have been postulated as causative [3, 9] with RDD basically a maladaptive reaction to the viral infection. As such it is not surprising that treatment with antiviral drugs has been reported with dramatic success [17], but with only a sample size of one, and with no follow up trials or studies found. Treatment with various immunosuppressant medications have been used; from high dose steroids to an assortment of chemotherapeutic drugs with overall minimal success at best [3]. Surgical resection of lesions has been far superior to chemotherapy, and in many cases complete resection is curative [3, 13]. In situations where invasive lesions cannot be surgically treated, radiotherapy has been used with some success [3]. Invasion of the myocardium presents with a dilemma of being both poorly resectable and difficult to treat by radiation as well [4, 18]. Invasive cardiac presentation of the disease is very rare with exceptionally few reported cases and a poor prognosis [18, 19, 20]. Difficult to resect disease in extra nodal sites such as intracranial, laryngeal and other locations have shown good response to radiotherapy [21] even if the sample size is small. Although the rarity of the disease limits sample size and inhibits generalizations about efficacy to be drawn, analysis suggests that surgical treatment is generally superior to pharmacological management [1, 3, 13]. Pediatric populations are more likely to have invasive disease [19], as well as a progressive course. As many of the chemotherapy drugs are themselves carcinogens, course monitoring and surgical control of lesions would seem to be the most prudent course.
TEACHING POINT
Rosai-Dorfman disease is a rare entity and even fewer cases with cardiac involvement being reported. Imaging is important in the diagnosis and management of Rosai-Dorfman disease despite generally non-specific findings. Plain film, CT, and MRI imaging are extremely useful in detecting and characterizing masses, but are not specific for Rosai-Dorfman disease with a wide differential diagnoses including lymphoma, inflammatory and infectious processes. The radiologist should be aware of this rare entity and include it in the differential diagnosis.
Table 1.
Summary table of Rosai-Dorfman Disease (RDD)
| Etiology | The etiology of RDD is poorly understood at best. There is a suggested link with HHV-6 and EBV but no concrete evidence. Cells with histiocytic linage migrate to lymph nodes and proliferate in a seemingly unregulated manner. This is a distinct entity from Langerhans Cell Histiocytosis with a better prognosis but poorer response to treatment. Emperipolesis is a key feature of RDD, but its function and regulation is not currently understood. |
| Incidence and prevalence | Specific incidence and prevalence information is not known for this very rare disease with only about 1000 reported cases, however the broader class of histiocytosis type diseases has an incidence of about 3 per million. The disease seems to be less prevalent in persons of Asian descent. Although there is conflicting evidence, RDD may also be more common in those with African descent than Caucasians. |
| Gender Predilection | There is a strong male gender predilection of approximately 4:1. |
| Age Predilection | RDD spans all age groups but is most common in the second and third decades of life with a mean age of 20 years at diagnosis. |
| Risk Factors | There are no clearly defined risk factors for RDD. However extranodal and aggressive disease are more common with a younger presentation. |
| Treatment | Surgical treatment is the only method with clear efficacy although some success with radiation therapy has been noted and antiviral therapy in early disease holds promise. Chemotherapy is ineffective. |
| Prognosis | RDD generally has a slow course and most cases spontaneously resolve. Extranodal cases are much more aggressive. |
| Imagining findings | Nonspecific imaging on CT, MRI and US, similar to inflammatory fibrosing lesions. PET with FDG is strongly positive. |
Table 2.
Differential table of Rosai-Dorfman Disease (RDD)
| Differential Dx | US | CT | MRI | PET |
|---|---|---|---|---|
| RDD | Nonspecific. Lymph nodes enlarged and hypoechoic. Echogenic hilum preserved | Poorly defined masses on noncontrast CT, Contrast enhancing, heterogenous enhancement, nodal enlargement. | Contrast enhancing, intensity varies with lesion location. Variable signal in T1 and T2. | Markedly elevated metabolism of FDG |
| Gaucher’s Disease | Both hyper- and hypoechoic in splenic and hepatic tissues with occasionally mixed lesions. Hyperechoic basal ganglia in older patients. | Nonspecific and wide variations, including ground glass opacities, reticular nodular and interstitial lung changes. | Low T1 signal of marrow spaces, especially in pediatric patients. Generally nonspecific. | Decreased F- DOPA in patients with IPD like symptoms. Otherwise not significant. |
| Langerhans Cell Histiocytosis (LCH) | Nonspecific. Lymph nodes enlarged, may have abnormal shape, and are hypoechoic, with occasional loss of echogenic hilum. | Poorly defined masses, heterogeneously contrast enhancing, often with nodal enlargement. Lytic bone lesions common. | Contrast enhancing. Intensity varies with lesion location. Variable signal in T1 and T2. | Elevated FDG metabolism. |
| Metastatic carcinoma | Generally nonspecific. Lymph nodes enlarged often with abnormal shape, hypoechoic. Thyroid papillary carcinoma is unique in being hyperechoic. | Varies with carcinoma type, usually contrast enhancing, may be homogenous or heterogeneously enhancing. | Usually hypointense T1 and hyperintense T2 but may vary with carcinoma type (melanoma is classically hyperintense on T1). Contrast enhancing. CNS lesions usually show ring enhancement. | Extremely elevated FDG uptake in most carcinomas. May have positive Tc-99m colloid uptake. |
| Inflammatory pseudo tumor | Nonspecific and variable, often with mixed echogenicity within lesions. | Nonspecific and variable, usually with heterogeneous enhancement and attenuation. | Usually intermediate T1 signal and high intensity on T2 weighted MRI, but may vary with lesion site. Contrast enhancing. | Positive Tc-99m colloid SPECT and Ga-67 scintigraphy. |
| Lymphoma | Non specific. Lymph nodes enlarged often with abnormal shape, hypoechoic, frequently with loss of echogenic hilum. | Homogeneously contrast enhancing. Non specific lymph node enlargement. Lytic bone lesions rare. | Homogeneously enhancing with contrast. Homogenous hypointense mass (T2) with surrounding vasogenic edema (CNS) without contrast. | Markedly elevated metabolism of FDG. |
ABBREVIATIONS
- ACS
Acute Chest Syndrome
- CD1a
Cluster of Differentiation 1a
- CD3
Cluster of Differentiation 3
- CT
Computerized tomography
- EBV
Epstein - Barr virus
- F-18 FDG
Fludeoxyglucose F 18
- HHV-6
Human Herpes Virus 6
- IgG4
Immunoglobulin G
- mCi
millicuries
- MRI
magnetic resonance Imaging
- PET
Positron Emission Tomography
- RDD
Rosai-Dorfman Disease
- SUV
Standard Uptake Value
- US
Ultrasound
REFERENCES
- 1.McClain K, Natkunam Y, Swerdlow S. Atypical Cellular Disorders Hematology the American Society of Hematology Education Program Book. 2004:283–96. doi: 10.1182/asheducation-2004.1.283. [DOI] [PubMed] [Google Scholar]
- 2.Rosia J, Dorfman RF. Sinus Histiocytosis with Massive Lymphadenophathy. A newly recognized benign clinicopathological entity. Archives of Pathology. 1969;87(1):63–70. [PubMed] [Google Scholar]
- 3.Pulsoni A, et al. Treatment of Sinus Histiocytosis with Massive Lymphadenopathy (Rosai-Dorfman Disease): Report of a case and Literature Review. American Journal of Hematology. 2002;69:67–71. doi: 10.1002/ajh.10008. [DOI] [PubMed] [Google Scholar]
- 4.La Barge D, III, Salzman K, Harnsberger H, et al. Sinus Histiocytosis with Massive Lymphadenopathy (Rosai-Dorfman Disease): Imaging Manifestations in the Head and Neck. American Journal of Roentgenology. 2008;191:W299–306. doi: 10.2214/AJR.08.1114. [DOI] [PubMed] [Google Scholar]
- 5.Molina-Garrido MJ. Guillén-Ponce C, Extranodal Rosai-Dorfman Disease with Cutaneous and Periodontal Involvement: A Rare Presentation. Case Reports Oncology. 2011;4:96–100. doi: 10.1159/000324760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stebbing C, van der Walt J, Ramadan G, Inusa B. Rosai-Dorfman Disease: A previously unreported association with Sickle Cell Disease. BMC Clinical Pathology. 2007;7:3. doi: 10.1186/1472-6890-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halushka M, et al. Extranodal Rosai-Dorfman Disease Involving the Heart: report of two cases. Cardiovascular Pathology. 2010;19:380–384. doi: 10.1016/j.carpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Huang Q, Chang K, Weiss L. Extranodal Rosi-Dorfman Disease Involving the Bone Marrow. American Journal of Surgical Pathology. 2006;30(9):1189–1192. doi: 10.1097/01.pas.0000209846.52046.62. [DOI] [PubMed] [Google Scholar]
- 9.Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Seminars in Diagnostic Pathology. 1990 Feb;7(1):19–73. [PubMed] [Google Scholar]
- 10.Xia P, et al. Emperipolesis, Entosis and Beyond: Dance with Fate. Cell Research. 2008;18:705–707. doi: 10.1038/cr.2008.64. [DOI] [PubMed] [Google Scholar]
- 11.Sodhi KS, Suri S, Nijhawan R, et al. Rosai-Dorfman Disease: Unusual Cause of Diffuse and Massive Retroperitoneal Lymphadenopathy. The British Journal of Radiology. 2005;78:845–847. doi: 10.1259/bjr/23127241. [DOI] [PubMed] [Google Scholar]
- 12.Schellingerhout D, et al. Rosai-Dorfman Disease in Neuroradiology: Image Findings in a Series of 10 patients. American Journal of Roentgenology. 2011;196:W187–193. doi: 10.2214/AJR.10.4778. [DOI] [PubMed] [Google Scholar]
- 13.Zivin S, Atieh M, Mosier M, et al. Rosai-Dorfman Disease (Sinus Histiocytosis with Massive Lymphadenopathy) of the Pancreas: Second Case Report. Journal of Gastrointestinal Surgery. 2009;13:806–809. doi: 10.1007/s11605-008-0752-z. [DOI] [PubMed] [Google Scholar]
- 14.Udono H, Fukuyama K, Okamoto H, et al. Rosai-Dorfman Disease Presenting Multiple Intracranial Lesions With Unique Findings on Magnetic Resonance Imaging. Journal of Neurosurgery. 1991;91:335–339. doi: 10.3171/jns.1999.91.2.0335. [DOI] [PubMed] [Google Scholar]
- 15.Haung J, et al. FDG/PET-CT Findings in Purely Cutaneous Rosai-Dorfman Disease. Clinical Nuclear Medicine. 2011;36:e13–e15. doi: 10.1097/RLU.0b013e31820aa36d. [DOI] [PubMed] [Google Scholar]
- 16.Menzel C, Hamscho N, Dobert N, et al. PET imaging of Rosai-Dorfman Disease: correlation with histopathology and ex-vivo beta-imaging. Archives of Dermatology Research. 2003;295:280–283. doi: 10.1007/s00403-003-0431-6. [DOI] [PubMed] [Google Scholar]
- 17.Baildam EM, D’Souza S, Stevens R, et al. Sinus Histiocytosis with Massive Lymphadenopathy (Rosai- Dorfman Disease): Response to Acyclovir. Journal of the Royal Society of Medicine. 1992;85:179–180. [PMC free article] [PubMed] [Google Scholar]
- 18.Maleszewski J, Hristov A, Halushka M, et al. Extranodal Rosai-Dorfman disease involving the heart: report of two cases. Journal of Cardiovascular Pathology. 2010;19:380–384. doi: 10.1016/j.carpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Richter J, et al. Extranodal Rosai-Dorfman Disease Presenting as a Cardiac Mass in an Adult: Report of a Unique Case and Lack of Relationship to IgG4-related Sclerosing Lesions. Hum Pathol. 2010 Feb;41(2):297–301. doi: 10.1016/j.humpath.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Ajise OE, Stahl-Herz J, Goozner B, Cassai N, McRae G, Wieczorek R. Extranodal Rosai-Dorfman Disease Arising in the Right Atrium: A Case Report With Literature Review. Int J Surg Pathol. 2011 Jun 1; doi: 10.1177/1066896911409577. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Toguri D, Louie AV, Rizkalla K, et al. Radiotherapy for Steroid Resistant Laryngeal Rosai-Dorfman Disease. Current Oncology. 2011;18(3):e158–e162. doi: 10.3747/co.v18i3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]