Abstract
Neonatal onset multisystem inflammatory disease (NOMID) is a rare autoinflammatory disorder, which manifests early in infancy. We describe a case of a 10-year-old boy who has been unwell since infancy. He presented with urticarial rash, intermittent fever and hepatosplenomegaly followed by progressive arthropathy. His joint symptoms started at two years of age, which progressively involved multiple joints, resulting in bone and joint deformities. A series of joint radiographs demonstrated bizarre enlarging physeal mass with heterogenous calcification. Magnetic resonance imaging (MRI) of the involved right ankle and knee showed characteristic thickened and calcified physeal lesions, which enhanced post-gadolinium. This debilitating disease is also known to involve the central nervous system and eyes. This case report aims to highlight the conventional radiographic and magnetic resonance imaging (MRI) findings of this physeal abnormality in NOMID syndrome.
Keywords: NOMID, CINCA, Neonate, radiograph, MRI
CASE REPORT
A 10-year-old boy was born of a non-consanguineous marriage by normal delivery. He had been chronically ill since infancy. He had multiple hospital admissions for recurrent fever, urticaria and poor weight gain. His developmental milestones were otherwise normal.
He started to have right ankle pain at the age of 2 years. His ankle radiograph showed cupping and flaring of the distal tibial metaphyses. The talus was normal. At four years old, he complained of right knee pain and swelling. Radiograph of the right knee at that time showed similar changes at the distal femoral metaphyses with an associated enlarged uncalcified physis. The patella is enlarged with an irregular outline. A repeat right ankle radiograph showed that the distal tibial physis had also enlarged with an associated heterogenously ossified physeal mass. Subsequent radiographs of the right ankle and right knee demonstrated progressive bulbous enlargement of the right distal tibial and distal femoral physes. The enlarged physis caused cupping of the metaphysis and compression of the epiphysis (Figures 1 & 2).
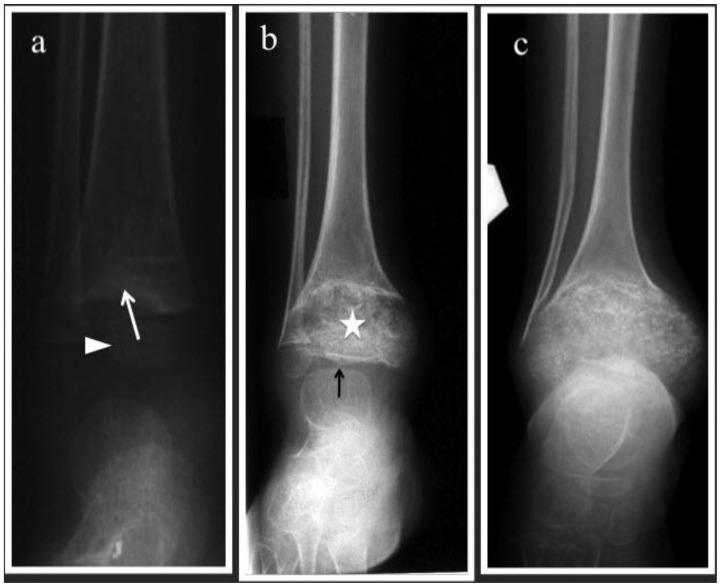
Figure 1.
10-year-old boy with Neonatal Onset Multisystem Inflammatory Disease (NOMID). A series of antero-posterior radiographs of the right ankle which were done at 2, 6 and 9 years of age (a-c) respectively.
(a) Eccentrically located uncalcified distal tibial physeal mass indenting the metaphysis. The metaphyseal margin appears sclerotic (white arrow). Epiphysis is normal and labeled with white arrowhead.
(b) Mass-like physis with heterogenous calcification (white star). The metaphysis shows cupping and flaring. The residual epiphysis is flattened and compressed (white arrow)
(c) Progressive enlargement of the calcified physis. The epiphysis is no longer seen and there is progressive cupping of the metaphysis to accommodate the enlarged physis.
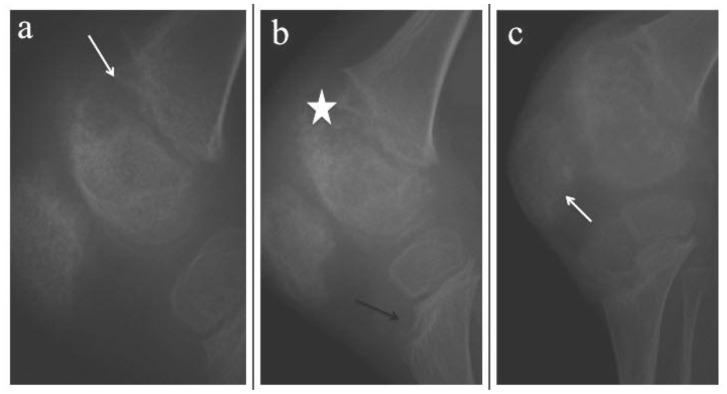
Figure 2.
10-year-old boy with Neonatal Onset Multisystem Inflammatory Disease (NOMID). Series of lateral right knee radiographs done at 5, 9 and 10 years of age respectively (a-c)
(a) At 5 years old, there is cupping of the metaphysis (white arrow) by uncalcified physeal mass with associated cupping of the distal epiphysis.
(b-c) By the age of 9 – 10 years old, the enlarged physis has calcified causing mass-like heterogenous ossification (white star), which is indistinct from the adjacent epiphysis. The proximal tibial metaphysis begins to show eccentric anterior cupping caused by uncalcified physeal mass (black arrow). Patella is enlarged with irregular outline (white arrow)
His condition worsened over the next few years. Despite a battery of blood and radiological investigations, the diagnosis was still evasive. He even had a percutaneous ankle biopsy at 8 years of age, which showed chronic inflammatory changes. He was treated empirically with antibiotics and even antituberculous therapy without any improvement in his symptoms. MRI of his right ankle was done at the age of 8 years of age showed an enlarged physis of distal tibia with presence of calcification within. There is associated cupping of the metaphysis and flattening of the epiphysis. He was finally referred to our medical center at the age of 10 years old for an infected right ankle biopsy wound and expert medical opinion. The infection resolved after a course of intravenous antibiotics. Physical examination revealed a thin boy with urticarial rash, hepatosplenomegaly and swelling of the right knee (Figure 3). MRI of right knee was performed to evaluate progression of disease, which showed an eccentrically enlarged physis of the distal femur, and proximal tibia, which were predominantly hypointense due to presence of calcification. An enlarged popliteal lymph node was also noted (Figure 4). Due to the uncertainty in diagnosis, an arthrotomy and debridement of the right knee was done in June 2008, which showed articular cartilage destruction resulting in bony ankylosis of the patellofemoral joint (Figure 5). HPE of the biopsied physeal tissue revealed mature disorganized chondrocytes with the intervening stroma infiltrated by lymphoplasmacytic infiltrate. PCR DNA for tuberculosis of the biopsied tissue was negative.
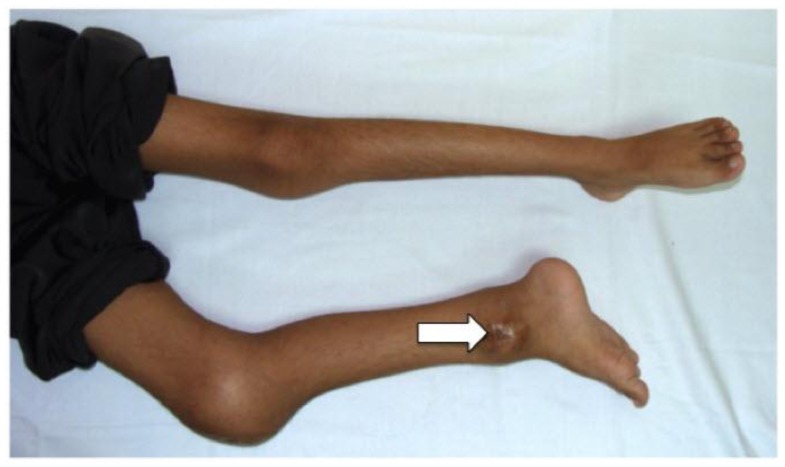
Figure 3.
10-year-old boy with Neonatal Onset Multisystem Inflammatory Disease (NOMID). Picture of the child’s lower limbs that shows swelling of the right knee and ankle joints. There is also flexion deformity of the right knee joint. An ulcer is seen at the enlarged right ankle due to a previous biopsy (white arrow).
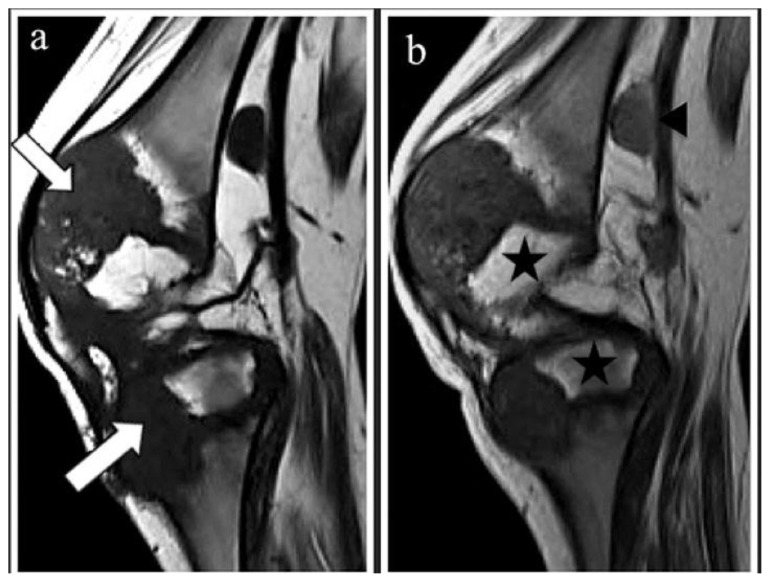
Figure 4.
10-year-old boy with Neonatal Onset Multisystem Inflammatory Disease (NOMID). Sagittal MRI of the right knee done at the age of 10 years old: (a) T1WI-pre gadolinium (1.5 Tesla, TR/TE=400msec/8msec, Slice Thickness=3mm); and (b) post gadolinium (1.5 Tesla, TR/TE=400msec/8msec, Slice Thickness=3mm; Omniscan®, 0.2mls/kg).
(a) The eccentrically enlarged physis of the distal femur and proximal tibia are predominantly hypointense due to the presence of calcification (white arrow)
(b) There is minimal enhancement post contrast. Note the compressed epiphysis of the distal femur and proximal tibia (black stars). An enlarged popliteal node is also noted, measuring 0.8cmx1.0cmx1.5cm (anteroposterior × width × craniocaudal)(black arrowhead). The patellofemoral joint space is obliterated.
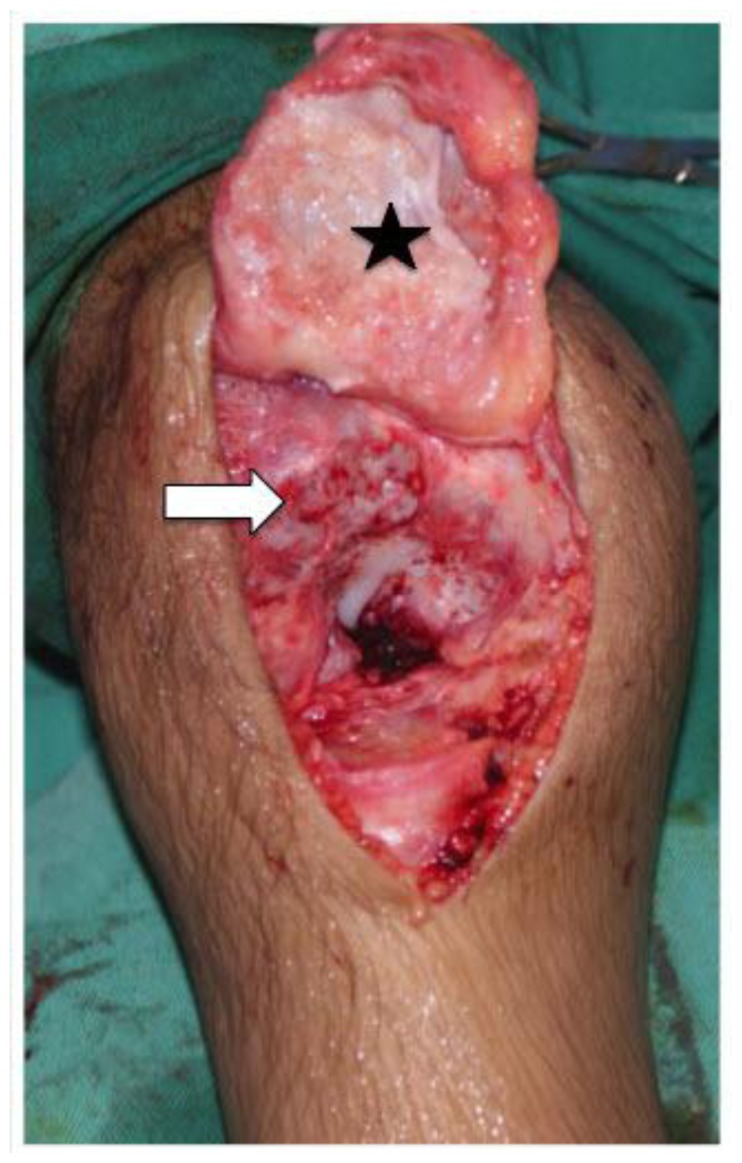
Figure 5.
10-year-old boy with Neonatal Onset Multisystem Inflammatory Disease (NOMID). Intraoperative photograph of the right knee shows the denuded cartilage of the patella & distal femur. The patellofemoral joint was ankylosed. The patella ligament was released (black star) & the ankylosed joint osteotomized to separate the patella from the femur (white arrow).
He also complained of intermittent headaches during his admission in our center. Ophthalmology review revealed blurring of the optic disc margins bilaterally and he was diagnosed as having pseudopapilloedema due to optic nerve Drusen. His MRI brain was normal.
Biochemical investigations revealed that he had hypochromic microcytic anemia with raised ESR (103mm/hr; normal 1–15mm/hr) and C-reactive protein (CRP) (17.33mg/dl; normal 0–0.5mg/dl). His white cell count was elevated at 18,900 g/dL, with a predominance of neutrophils (77%). His immunoglobulin studies showed high IgA and IgG (1070mg/dL and 3500mg/dL respectively; normal 70–473mg/dL and 931–1916mg/dL respectively). His complement (C3) level was slightly high (231mg/dL; normal 86–184mg/dL).
The diagnosis of Neonatal onset multisystem inflammatory disease (NOMID) was made based on the radiographic and MRI findings, supported by the clinical, histopathological and biochemical evidence. Currently, he has fixed flexion deformity of both right ankle and right knee and has started to complain of left knee pain. He has been started on oral Prednisolone and responded remarkably with resolution of his fever and both joint and bone pains. His inflammatory markers (ESR and CRP) also improved. He was also subsequently started on Methotrexate as he remained steroid dependant and anti-Interleukin-1(IL-1) therapy is not available in this country. He is currently showing improvement with occasional urticaric rash and is afebrile. He is able to ambulate independently but with a limping gait due to the fixed flexion deformity of his right knee.
DISCUSSION
Neonatal onset multisystem inflammatory disease (NOMID) was first described by Prier and Griscelli in 1987 (1, 2). The articular findings are the most striking and characteristic features of NOMID syndrome (3, 4). Central nervous system symptoms and ocular manifestation are other clinical findings in patients with NOMID (1–4).
The actual incidence and prevalence of the disease is difficult to determine. Hill et al described 20 patients with the disease, published in the year 2007 with equal gender predilection (4). The phenotypically milder forms of familial autoinflammatory syndromes, familial cold autoinflammatory syndrome (FCAS) and Muckle-Wells syndrome (MWS) have clinical similarities with NOMID. However, the rash in FCAS is cold induced but the rash in MWS and NOMID is constantly present. Nevertheless, neither FCAS nor MWS is associated with bony overgrowth or mental retardation (4). The discovery of mutations in CIAS1 gene in NOMID, FCAS and MWS has led to the idea that these diseases are part of a disease spectrum (4). These diseases are classified as cryopyrin-associated periodic syndromes (CAPS) as the mutation of the CIAS1 gene codes cryopyrin, a pyrin-like protein (5, 6). CAPS include these three allelic variants, ranging in order of increasing severity from FCAS, through MWS to NOMID/CINCA (5). However, NOMID/CINCA may be heterogenous and only 60% of affected individuals have NLRP3 (CIAS1) mutations (5).
The disease begins early in infancy with a generalized, evanescent macular or maculopapular rash which may have urticarial features (1–3). Studies have shown that skin rash appears in 100% of NOMID cases (1). Over months, failure to thrive, generalized lymphadenopathy, hepatosplenomegaly, intermittent fever, anemia, leukocytosis and painful arthropathy would develop (1–3). All of these findings were present in this patient except for generalized lymphadenopathy.
Arthropathy in NOMID is caused by abnormal endochondral bone formation and bony overgrowth (4). It is uncertain whether the epicenter of the lesion is in the physis or epiphysis as these sites were used interchangeably in previous reports. The latest report published by Hill et al in 2007 stressed that the abnormal endochondral bone formation would lead to bony overgrowth of the physis. The initial finding is visible enlargement of the non-ossified portion of the physis (4). This is followed by splaying and cupping of the metaphyses which is irregular in outline, simulating rickets. The hallmark of this disease is a bizarre enlargement of the ossified portions of the physis, presenting as a heterogenous calcified mass. Other radiographic manifestations include osteoporosis, delayed bone age, genu varus/valgus, limb length discrepancy and less commonly, symmetrical involvement of the large joints (4).
Further imaging by magnetic resonance imaging (MRI) supports that the abnormal bone mass originating in the physis, which deforms the adjacent epiphysis and metaphysis. It manifests as an abnormal area of ossification presenting as thickened physis that is hypointense on T1-weighted images and isointense on T2-weighted images, and invariably enhances post gadolinium. In our case, the thickened enlarged physis enhanced minimally post-gadolinium. Hill et al described that popliteal lymphadenopathy was present in 6 out of 18 cases they reviewed (4). That is to say, popliteal lymphadenopathy can be seen in about 1/3 of the cases, which was also present in this patient.
Ocular manifestations and central nervous system symptoms are also present in patients with NOMID. Ocular manifestations include papillitis, anterior and posterior uveitis, papilledema, pseudopapilledema and optic atrophy (1, 2). Tobiak et al summarize clinical findings in 32 NOMID cases and found that the incidence of sensorineural hearing loss, seizures, intellectual impairment, hydrocephalus/atrophy and chronic meningitis ranges from 22% to 93% of cases in ascending frequency (7).
Reported laboratory abnormalities include persistent hypochromic microcytic anaemia, leukocytosis (granulocytic) and elevated sedimentation rate (1, 7); all of which were noted in our patient. Other positive laboratory findings were increased complement and serum immunoglobulin levels, which were seen in 60% and 75% of cases respectively (7). Hill at al described two tissue biopsy results from his two NOMID patients, which revealed disorganized chondrocytes columns on hematoxylin and eosin staining that supported the idea that the pathogenesis of NOMID is related to derangement of the normal progression of endochondral ossification (4).
The differential diagnoses of this disease include rickets, chronic recurrent multiple osteomyelitis (CRMO), systemic Juvenile Idiopathic Arthritis (JIA) and tuberculosis. Early changes of NOMID are characterized by enlarged non-calcified physes that may simulate rickets. In rickets, the pathologic changes result from failure of enchondral ossification and disruption of the physeal arrangement which are more or less similar to NOMID (8). However, later in the disease progression, NOMID is rather different from rickets as the physeal mass becomes calcified giving rise to a bizarre heterogenous mass. In CRMO, the epicenter of the lesion is in the metaphysis rather than the epiphysis or physis (9). The primary clinical problem is differentiation from systemic-onset juvenile idiopathic arthritis (JIA) (10). NOMID bears some clinical similarities to JIA whereby both exhibit spiking fever, hepatosplenomegaly and prominent arthropathy. In fact, the major pathogenesis of these two diseases is different. JIA primarily affects the synovium resulting in synovial proliferation. On the other hand, NOMID showed abnormal bone growth affecting the physis causing the deforming arthropathy. There is no articular surface erosion in NOMID as seen in JIA (3,5). This would explain why only the distal tibia was affected and not the talus in our patient. Patients with NOMID demonstrate heterogenous calcification of the physis, which is discernible by virtue of its contrast with the normal trabecular bone in the epiphysis. This indicates distortion of the physeal/epiphyseal interface by the mass rather than invasion (4).
Treatment of NOMID is challenging for both orthopedic surgeons and rheumatologists, as there is no bone-specific medical treatment for NOMID (4). It has been well documented that the IL-1 receptor antagonist anakinra (Kineret®) helps mitigate systemic inflammation in NOMID (4, 10). Recently, researchers from Japan have stressed on the importance of early treatment with anakinra for better outcome of arthropathy in NOMID. They also concluded that once bone deformities develop, they are difficult to reverse even with anakinra (5). Meanwhile, surgical treatment such as osteotomy may be necessary in order to correct varus/valgus deformity resulting from NOMID (4).
TEACHING POINT
Children with rashes and arthropathy should be screened for any neurological symptoms to exclude NOMID. The progressively enlarging and heterogeneously calcified physeal mass, which can be diagnosed radiologically, is one of the radiological features of NOMID.
Table 1.
Summary table of Neonatal onset multisystem inflammatory disease (NOMID)
| ETIOLOGY | NOMID is an autoinflammatory disorder characterized by the triad of neonatal onset of cutaneous symptoms, arthropathy and central nervous system symptoms. |
| INCIDENCE | Since NOMID is a newly discovered condition and is a very rare condition, the actual incidence and prevalence of the disease is difficult to determine. |
| GENDER RATIO | Males and females are affected equally |
| AGE PREDILECTION | Most children are affected before age of 10 years old |
| RISK FACTORS | It is discovered to be due to alterations in a gene identified as the Cold-induced Auto-inflammatory Syndrome 1 (CIAS1) gene (Autosomal Dominant). |
| TREATMENT | The treatment is supportive, or aimed solely at controlling symptoms and maximizing function. Some reports showed that early treatment with anakinra related with better outcome of arthropathy. |
| PROGNOSIS | The prognosis for patients with NOMID is not good and debilitating especially due to the arthropathy. |
| FINDINGS ON IMAGING | Bulky mass in the physis with heterogenous calcification within. |
Table 2.
Differential table of Neonatal onset multisystem inflammatory disease (NOMID)
| Radiograph | MRI | |
|---|---|---|
| NOMID/CINCA | Epicenter of the lesion is in the physis. Early radiograph will show enlargement of the unossified physis, giving rise to mass-like lesion. Later, it will show stippled ossification/calcification causing deformed adjacent epiphysis. It can involve multiple joints simultaneously or at different times. | The enlarged physis will appear heterogenous with hypointense calcifications on T1 & T2 WI. Post-gadolinium images show heterogenous enhancement at the physis. Popliteal lymph node is another recognized feature. No bony erosion seen. |
| Juvenile Rheumatoid Arthritis (JRA) | It involves the articular surface of the joints. Initially, it will cause soft tissue swelling and enlarged epiphysis due to hyperaemia. As the disease progresses, the dominant features would be joint space narrowing and bony erosion. | Joint effusion, cartilage thinning and loss. Later, there will be joint erosion and evidence of secondary osteoarthritis as well as complications of avascular necrosis. |
| Chronic Recurrent Multifocal Osteomyelitis (CRMO) | Multiple metaphyseal lesions, which are normally symmetrical. The appearance of CRMO lesions can range between purely osteolytic, osteolytic with a sclerotic rim, mixed lytic and sclerotic, and purely sclerotic. | In the early phases, the disease will show marrow edema, which appears hypointense on T1-weighted images and hyperintense on T2-weighted images with synovial thickening, joint effusion, or destruction of joint cartilage and subchondral bone. |
| Rickets | There is widening, cupping and fraying of the metaphysis with irregular and widened epiphyseal plate. It is also associated with periosteal reaction. | MRI shows high signal intensity of unossified cartilage in the physis of the primary and secondary ossification centers. These can be easily differentiated from the lower signal intensity of epiphyseal cartilage on T2WI. However, there is no mass lesion or ossification in the physis. |
ACKNOWLEDGEMENTS
The authors would like to thank Encik Mohamad Norman B. Mohd Nordin for helping us with the excellent graphic work.
ABBREVIATIONS
- CINCA
Chronic infantile neurological, cutaneous and articular syndrome
- CRMO
chronic recurrent multiple osteomyelitis
- DNA
Deoxyribonucleic acid
- ESR
Erythrocyte sedimentation rate
- HPE
Histopathological examination
- JIA/JRA
Juvenile idiopathic/rheumatoid arthritis
- MR
Magnetic resonance imaging
- NOMID
Neonatal-onset multisystem inflammatory disease
- PCR
Polymerase chain reaction
REFERENCES
- 1.Prieur AM, Griscelli C, Lampert F, Trunkenbrodt H, Guggenheim MA, Lovell DJ, et al. A Chronic, Infantile, Neurological, Cutaneous and Articular (CINCA) Syndrome. A specific entity analysed in 30 patients. Scand J Rheumatology. 1987;(Suppl 66):57–68. doi: 10.3109/03009748709102523. [DOI] [PubMed] [Google Scholar]
- 2.Prieur AM, Griscelli C. Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. The Journal of Pediatrics. 1981;99(1):79–83. doi: 10.1016/s0022-3476(81)80961-4. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman RA, Lovell DJ. Infantile-onset Multisystem Inflammatory Disease: Radiologic findings. Radiology. 1986;160:741–746. doi: 10.1148/radiology.160.3.3737913. [DOI] [PubMed] [Google Scholar]
- 4.Hill SC, Namde M, Dwyer A, Poznanski A, Canna S, Goldbach-Mansky R. Arthropathy of neonatal onset multisystem inflammatory disease (NOMID/CINCA) Pediatric Radiology. 2007;37:145–152. doi: 10.1007/s00247-006-0358-0. [DOI] [PubMed] [Google Scholar]
- 5.Miyamae T, Inaba Y, Nishimura G, Kikuchi M, Kishi T, Hara R, et al. Effect of anakinra on arthropathy in CINCA/NOMID syndrome. Pediatric Rheumatology. 2001;8:9. doi: 10.1186/1546-0096-8-9. Available from http://www.ped-rheum.com/content/8/1/9.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieur AM. CINCA syndrome. Orphanet Encyclopedia. Oct, 2003. Available from http://www.orpha.net/data/patho/GB/uk-cinca.pdf.
- 7.Torbiak RP, Dent PB, Cockshott WP. NOMID - a neonatal syndrome of multisystem inflammation. Skeletal Radiology. 1989;18:359–364. doi: 10.1007/BF00361425. [DOI] [PubMed] [Google Scholar]
- 8.Ecklund K, Doria AS, Jaramillo D. Rickets on MR images. Pediatr Radiol. 1999;29:673–675. doi: 10.1007/s002470050673. [DOI] [PubMed] [Google Scholar]
- 9.Khanna G, Sato TSP, Ferguson P. Imaging of Chronic Recurrent Multifocal Osteomyelitis. Radiographics. 2009;29:1159–1177. doi: 10.1148/rg.294085244. [DOI] [PubMed] [Google Scholar]
- 10.Kilcline C, Shinkai K, Bree A, Modica R, Scheven EV, Frieden IJ. Neonatal-Onset Multisystem Inflammatory Disorder: The Emerging Role of Pyrin Genes in Autoinflammatory Diseases. Arch Dermatol. 2005;141:248–253. doi: 10.1001/archderm.141.2.248. [DOI] [PubMed] [Google Scholar]