Abstract
Breast fibromatosis is a rare entity responsible for 0.2% of all solid breast tumors. It has been associated with scars, pregnancy, implants, and familial adenomatous polyposis. We present an interesting case of breast fibromatosis in a 29 year old woman which encroached upon her saline implant and subsequently filled its cavity once the implant was removed. The patient was put on tamoxifen therapy and at 14 month follow-up there was a significant decrease in the size of the mass. Dynamic MRI images are offered for review and current treatment options are discussed.
Keywords: Breast fibromatosis, desmoid, tamoxifen, Dynamic MRI
CASE REPORT
A 29 year old G2P1A1 female presented with history of bilateral retropectroral saline breast implants placed two years prior, who developed increasing right breast pain. The patient had a mammogram at an outside institution that demonstrated a slight focal bulge of the right breast implant (Fig. 1). The patient was then seen by a surgeon who diagnosed a capsular contraction of the right implant and two months prior to presentation at our institution underwent surgery for implant removal with replacement. The left breast implant was removed and replaced without complication. On the right side, the surgeon noted firmness and superior displacement of the breast which was consistent with periprosthetic capsular contracture. The implant was removed however immediately caudal to the implant was a grossly smooth, glistening mass which was biopsied. Pathology revealed fibromatosis and was negative for estrogen and progesterone receptors (Fig. 7). The patient was subsequently referred to our institution for evaluation and treatment.
Figure 1.
29 year old female with right breast retropectoral saline implant. Right breast MLO projection scanned analogue mammogram from outside institution demonstrates focal bulge in the contour of the implant denoted by white arrow.
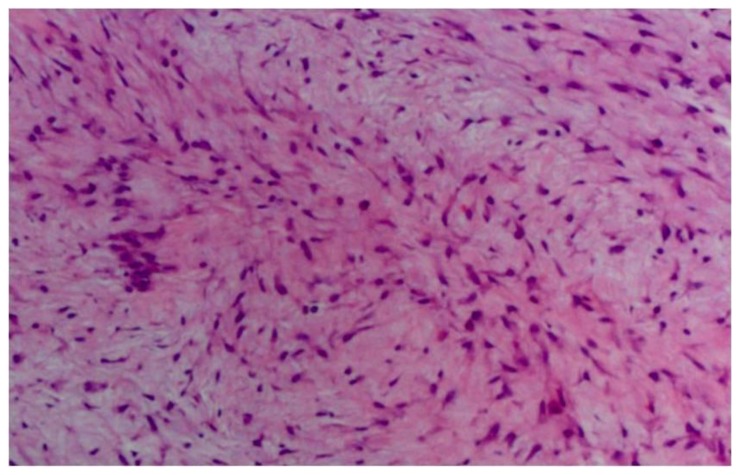
Figure 7.
29 year old female with right breast fibromatosis. Medium power view of a core biopsy sample demonstrating a spindle cell neoplasm (H&E stain, 10x).
On physical exam, her right breast demonstrated diffuse tenderness and a mass which was difficult to appreciate. There was no axillary lymphadenopathy and the skin appeared normal. In addition, despite the reported history of right breast implant explantation, the breasts appeared symmetrical in size and contour.
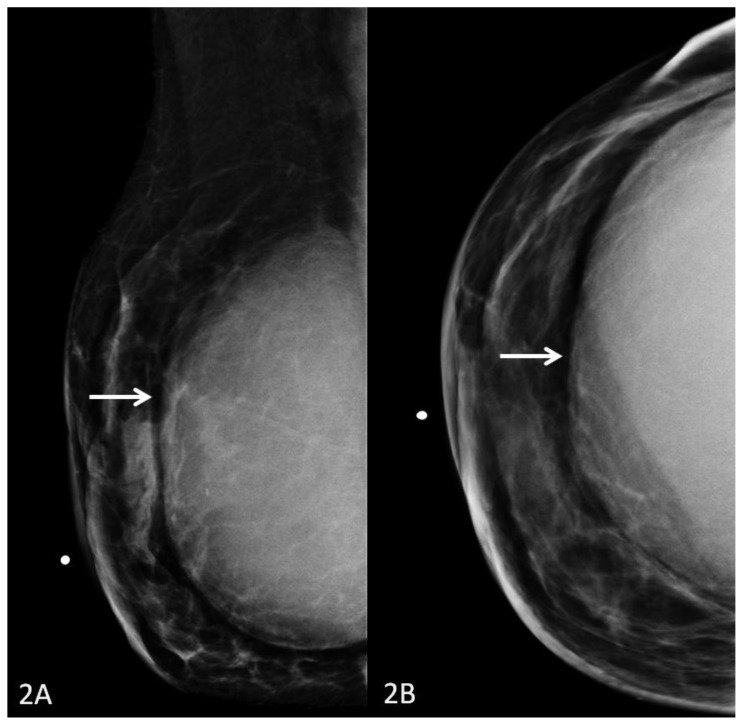
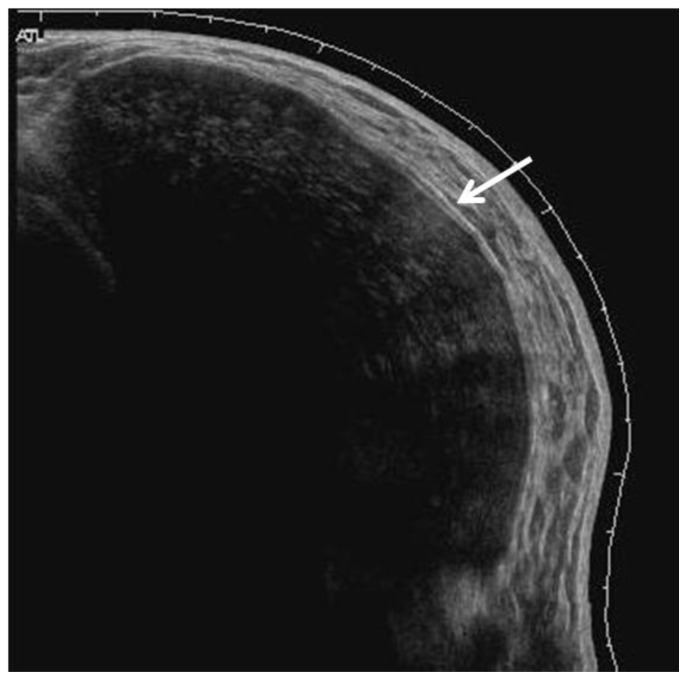
The patient had multiple imaging studies performed at our institution. Mammography demonstrated a partially visualized large soft tissue mass with anterior circumscribed margins, which was retropectoral in location and conformed to the contour of the prior implant cavity (Fig. 2a, Fig. 2b). Ultrasound demonstrated a heterogeneous but primarily hypoechoic, circumscribed solid mass retropectoral in location (Fig. 3).
Figure 2.
29 year old female with right breast fibromatosis. 2A: Right breast MLO projection digital mammogram (Hologic Selenia Digital Mammography Unit). 2B: Right breast CC projection digital mammogram. Images demonstrate a partially imaged large retropectoral soft tissue density mass in the right breast with well-circumscribed anterior margins measuring 11.8 × 11.7 × 5.8cm.
Figure 3.
29 year old female with right breast fibromatosis. Right breast panoramic view ultrasound demonstrates a hypoechoic solid mass with circumscribed anterior margins measuring 11.3 × 5.7 cm (GE Healthcare LOGIQ E9 Breast Ultrasound Unit utilizing a linear 7.5–15MHz probe).
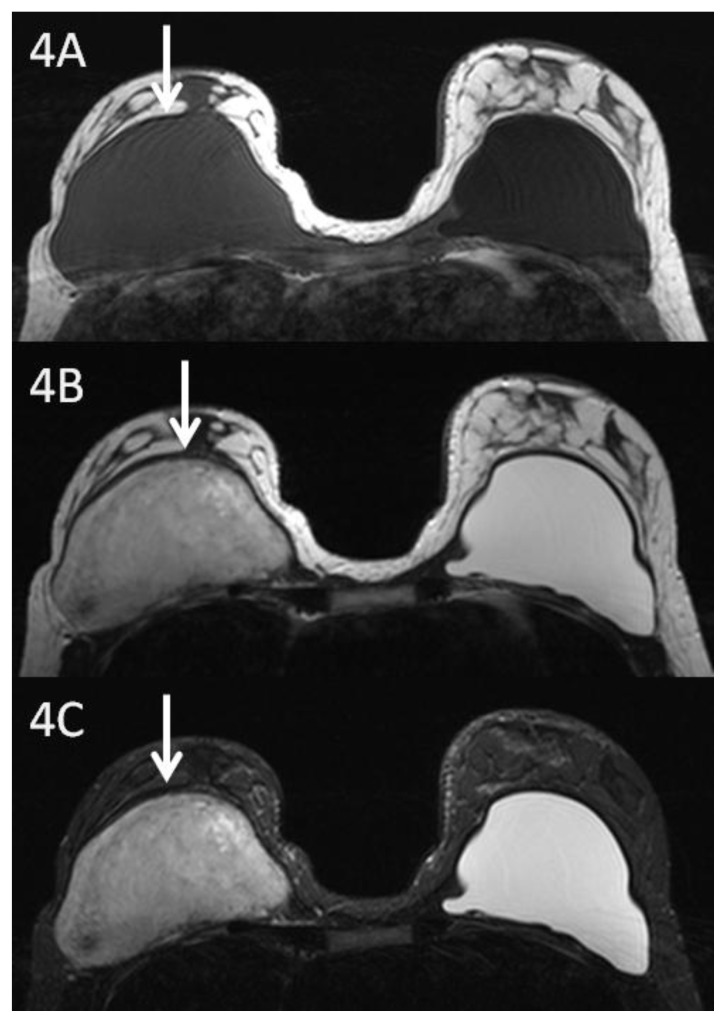
Magnetic resonance imaging demonstrated a retropectoral right breast mass measuring 10.5 × 6.2 × 10.5 cm. The mass was isointense to muscle on T1, heterogeneous but primarily hyperintense on T2, and heterogeneous but predominantly hyperintense on short tau inversion recovery (STIR) images (Fig. 4a, Fig. 4b, Fig. 4c). T1 fat suppressed post contrast images demonstrated a heterogeneously enhancing mass with involvement of the surrounding pectoralis major muscle and no clear separation from the chest wall (Fig. 5a, Fig. 5b). In addition, dynamic sequences obtained before and one minute, two, and six minutes after contrast administration demonstrated gradual progressive enhancement consistent with a type I kinetic curve (Fig. 6).
Figure 4.
29 year old female with right breast fibromatosis and left breast saline implant. 4A: Axial T1 weighted breast MR image (TR 565 TE 12; 1.5 Tesla Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 256×256) showing a right retropectoral breast mass isointense to muscle measuring 10.5 × 6.2 × 10.5 cm. Left breast retropectoral saline implant is hypointense relative to muscle. 4B: Axial T2 weighted MR image (TR 3570 TE 93) showing right breast retropectoral mass with heterogeneous signal intensity. 4C: Axial STIR MR image (TR 5470 TE 70) showing right breast mass with heterogeneous signal intensity.
Figure 5.
29 year old female with right breast fibromatosis before and after treatment with tamoxifen.
5A: Axial T1 weighted MR 6 minutes post contrast subtraction image before tamoxifen therapy (TR 6.44 TE 1.4; 1.5T Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 512×287; 15cc of Magnevist) shows heterogeneously enhancing right retropectoral breast mass measuring 10.5 × 6.2 × 10.5 cm. Left breast saline implant is seen.
5B: Sagittal T1 weighted fat suppressed post contrast image of the right breast before tamoxifen therapy(TR 11.9 TE 5.54; matrix 320×221).
5C: Axial T1 weighted MR 5 minutes post contrast subtraction image 14 months after tamoxifen therapy (TR 4.3 TE 1.51; 1.5T Siemens MAGNETOM Sonata MRI scanner using breast phased array coil; matrix 384×381; 15 cc of Prohance) shows heterogeneously enhancing right retropectoral breast mass that is significantly smaller in size measuring 1.8 × 4.9 × 5.9 cm. Left breast saline implant remains unchanged.
5D: Sagittal T1 weighted fat suppressed post contrast image of the right breast 14 months after tamoxifen therapy(TR 11.9 TE 5.4; matrix 320×221).
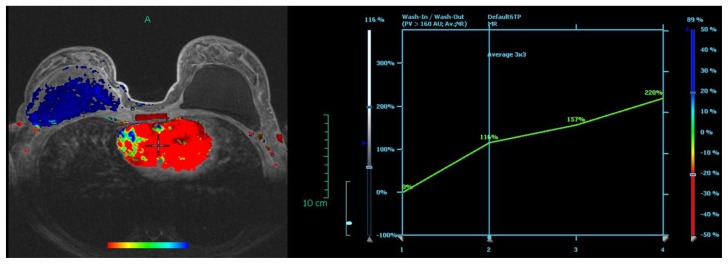
Figure 6.
29 year old female with right breast fibromatosis. Axial post contrast subtraction image with color mapping (1.5T Siemens MAGNETOM Sonata MRI scanner utilized with dedicated breast coil; evaluation of contrast uptake performed with DYNACAD). The retropectoral right breast mass is blue denoting progressive enhancement. The enhancement curve on the right is confirmatory.
Upon surgical evaluation, it was deemed that reconstruction would be complex due to the size of the mass; therefore, it was recommended that the patient undergo neoadjuvant therapy prior to surgical resection. She was given tamoxifen 10 mg twice daily in view of the fact that the fibromatosis may have been related to elevated estrogen levels during pregnancy. 14 month follow-up breast MRI demonstrated considerable decrease in the size of the mass which now measured 1.8 × 4.9 × 5.9 cm and the patient was continued on the tamoxifen regimen (Fig. 5c, Fig. 5d).
DISCUSSION
Fibromatosis or desmoid tumors are rare entities and are even more unusual when found in the breast as they are typically intraabdominal. Breast fibromatosis accounts for 0.2% of all breast tumors [1]. The age at diagnosis is variable; however, the mean age is 37 with the majority of patients ranging between ages 20–35 [2, 3]. Fibromatosis has a 2:1 predilection for women and has been associated with high estrogen states such as pregnancy. However, the evidence linking high estrogen levels with the development of desmoids tumors is mostly anecdotal [4]. In addition, although most desmoid tumors arise sporadically, there has been an association with familial adenomatosis polyposis as 10–20% of these patients will develop a desmoid. Last, 30% of patients have a prior history of surgical trauma at the site of the development of a desmoid tumor [5]. To date there have been a total of 12 documented cases of fibromatosis associated with breast implants with an average time to diagnosis of 2.5 years after augmentation [6].
The clinical and radiographic appearance of breast fibromatosis is suspicious as it usually presents as an enlarging palpable mass and may appear spiculated on mammography [1]. The main differential consideration of a spiculated mass on mammography is an invasive ductal carcinoma. Radial scar may have a similar appearance, but is classically described as an area of architectural distortion with central lucency.
The sonographic appearance of fibromatosis is also typically suspicious and usually warrants descriptors such as spiculated, microlobulated, and irregular. The main differential consideration is again invasive ductal carcinoma. Fat necrosis often mimics other entities and may be suggested in the setting of trauma or prior surgery. Calcifications and central area of hyperechogenicity representing fat is also suggestive of fat necrosis on ultrasound. In a patient with long standing type I diabetes, diabetic mastopathy may have a similar sonographic appearance. A phyllodes tumor should be considered in women with a large rapidly growing breast mass, but this type of mass will be circumscribed with smooth margins, and without calcifications. Spindle cell carcinoma and fibrosarcoma are also in the differential, but are indistinguishable from fibromatosis on imaging. The case presented here appears to have smooth margins anteriorly because it is encased anteriorly by the pectoralis major muscle.
MRI is useful for imaging the extent of tumor and for evaluating involvement of the chest wall. Accurately describing the presence or absence of chest wall involvement is critical as it guides surgical planning [1, 7]. MRI appearance of breast fibromatosis is hypo- to isointense to muscle on T1, heterogeneous with areas of high and low signal intensity on T2, and heterogeneously enhancing [1, 8, 9]. The areas of hypointensity on T2 weighted imaging correspond to fibrous tissue, while the areas of high signal correspond to myxoid tissue. On dynamic sequences, breast fibromatosis has been previously described with either type I, II, or III kinetics [8, 9]. Imaging characteristics on MR are also nonspecific and indistinguishable from invasive ductal or lobular carcinoma. A sarcomatous mass is also in the differential and cannot be readily distinguished from fibromatosis. Diabetic mastopathy is typically symmetric, but in cases of asymmetry, MR may show a heterogeneously enhancing mass with type I kinetics. Fat necrosis is distinguished by showing fat signal centrally within the lesion. Another differential consideration is pseudoangiomatous stromal hyperplasia (PASH) which is benign and seen most commonly in premenopausal women. On T2 weighted images it may contain areas of low and high signal and may demonstrate heterogeneous enhancement which is similar to what we see with fibromatosis. A major difference from the classic presentation of breast fibromatosis is that PASH is usually oval shaped and with smooth well defined borders.
At histopathology it is important to differentiate fibromatosis from other breast spindle cell tumors such as fibrosarcoma or malignant myoepithelioma as treatment varies depending on the pathology. The pathognomic appearance of fibromatosis has been described as bland-looking spindle cells forming interlacing bundles with positivity of immunohistochemical markers for vimentin and smooth muscle actin supporting the diagnosis [10].
The first line of treatment for breast fibromatosis is surgical excision with wide margins. Positive surgical margins increase the likelihood of recurrence, but in a select number of cases there is no recurrence despite incomplete excision and there have been reported cases of spontaneous regression [7]. Conversely, negative surgical margins decreases the likelihood of recurrence as evidenced in a series of 32 patients with breast fibromatosis in which there was a trend towards less recurrence in the setting of negative margins [11]. There is agreement of how to surgically manage fibromatosis involving the breast alone, however in cases where the chest wall is involved there remains controversy as to how surgically aggressive one should be in order to balance between adequate excision and patient morbidity [7]. There is no reliable way to accurately predict the chance of recurrence, however the local recurrence rate of breast fibromatosis has been reported to be 21–27% and usually within 3 years [12].
There is growing evidence supporting the use of radiation therapy with desmoid tumors. The data is mostly in regards to extramammary desmoids and has reported local control rates ranging from 73–94% [13, 14]. Control rates with radiation were better than surgery alone and trended towards being higher in the setting of negative surgical margins and for primary tumors rather than recurrent cases. There is controversy regarding the use of postoperative radiation treatment with some advocating the use of the post-operative radiation therapy regardless of whether there are negative or positive margins, while others say it should be reserved only for cases where there are positive surgical margins. Guidelines from the National Comprehensive Cancer Network recommend considering postoperative radiation therapy in “large” primary tumors. Radiation therapy should be considered an option in recurrent desmoids, but only if they are extra-abdominal in location [15].
Indications for systemic therapy in fibromatosis include unresectability, patients that cannot tolerate surgery, unacceptable morbidity which can be seen with chest wall involvement, or prevention of recurrence. The use of systemic therapy as a neoadjuvant measure in an attempt to shrink the size prior to excision is relatively new with little data available. Systemic therapy includes non-cytotoxic and cytotoxic approaches with the majority of data being derived from reports and small series evaluating response in extramammary fibromatosis. Clinical benefit is defined in most reports as stable disease, partial regression in tumor size, or complete regression of the tumor.
Non-cytotoxic approaches include the use of antiestrogen agents such as tamoxifen used alone or in combination with nonsteroidal anti-inflammatories (NSAIDS) such as sulindac. 15–20% of desmoid tumors objectively regress in response to tamoxifen with another 25–30% stabilizing in size [16]. The largest series investigating the clinical benefit of tamoxifen by Brooks et al. included 20 patients with desmoid tumors and demonstrated clinical benefit in 65% of patients which ranged from stabilization of disease to complete resolution [17]. The exact mechanism of hormonal agents is unknown and may be related to estrogen-receptor modulation or deprivation of a growth signal or cytokine in the Wnt/beta-catenin tumor suppressor pathway [12]. Most cases of breast fibromatosis lack estrogen or progesterone receptor positivity and thus any benefit would likely be through the Wnt/beta-catenin tumor suppressor pathway [12, 16]. In addition, combination of NSAIDs with antihormonal therapy tends to show a higher response rate than monotherapy [18]. Imatinib is another form of noncytotoxic systemic therapy for which there is growing data supporting its use in patients with desmoid tumors. It appears useful in stabilization of disease with a 1 and 2 year progression-free rate of 66 and 55 % respectively, while objective partial or complete regression is much lower and ranges from 6–9% [19, 20].
Response to systemic noncytotoxic therapy may take 6–8 months and in some cases the tumor may initially increase in size before shrinking or stabilizing [18]. Therefore, a small objective increase in size of a breast fibromatosis tumor should not be considered a treatment failure in the early treatment cycles.
Cytotoxic chemotherapy is typically a last resort in cases in which there is an urgency to elicit a response or if noncytotoxic systemic therapy options have been exhausted. Doxorubicin-based combinations are most often used with response rates being highly variable and ranging from 40–100% [18]. Anthracycline monotherapy has been studied in intraabdominal desmoids with response rates of up 75% in some series [21]. In one series of 30 patients by Skapek et al., methotrexate plus a vinca alkaloid demonstrated clinical benefit in 70% of patients with a median follow-up of 62 months [22]. The patients with clinical benefit did not progress while they were on the chemotherapy or after the last treatment cycle [22]. Doxorubicin-based combinations generally have greater likelihood of response, yet patient toxicity is the limiting factor.
In conclusion, breast fibromatosis is a rare breast tumor that clinically and radiographically mimics breast carcinoma. Biopsy is required for accurate diagnosis and breast MRI is warranted to examine extent of disease, guide treatment, and evaluate response to therapy. First choice of treatment is surgical resection, however there are several other options including radiation therapy, noncytotoxic systemic therapy, and cytotoxic systemic therapy, in patients who are not surgical candidates. The data surrounding nonsurgical options is primarily with regards to intraabdominal desmoids, but it is reasonable to apply them to mammary desmoid tumors since pathologically they are identical. The choice of nonsurgical treatment should be tailored to the specific clinical scenario keeping in mind age, desire to bear children, toxicity from the medication, whether it is a primary or recurrent desmoid, and how aggressive the tumor is and whether invasion of adjacent vital structures is imminent. Recurrence of disease cannot be accurately predicted by surgical margins or pathologic features. The use of neoadjuvant radiation therapy or systemic therapy has limited experience.
TEACHING POINT
Breast fibromatosis is a rare solid breast tumor that radiographically mimics breast carcinoma on all imaging modalities and warrants biopsy. First choice of treatment is surgical resection; however, patients who are not surgical candidates may respond to one of several medical therapy options, including tamoxifen.
Table 1.
Summary table for breast fibromatosis
| Etiology: | Spindle cell proliferation often associated with post-surgical trauma |
| Incidence: | 0.2% of breast neoplasms |
| Age predilection: | Mean age 37, range 13–80 years |
| Risk factors: | Trauma, post-surgical trauma, exogenous hormone treatments, association with familial adenomatous polyposis |
| Treatment: | Wide local excision is standard Radiation therapy in selected cases Hormonal therapy in selected cases Chemotherapy in selected cases |
| Prognosis: | No metastatic potential Local recurrence rate 21–27% usually within 3 years Response to medical treatment variable Spontaneous regression rare |
Table 2.
Differential table for breast fibromatosis
| Modality | Breast fibromatosis | Invasive Ductal Carcinoma | Fat Necrosis | Diabetic mastopathy |
|---|---|---|---|---|
| Mammography | Irregular dense mass with spiculated margins, typically close to or arising from the pectoralis major muscle | Irregular spiculated mass | Lucent center, peripheral rim of calcification, may have spiculated or circumscribed margins | Symmetric areas of homogeneous dense parenchyma |
| Ultrasound | Hypoechoic, irregular shape, spiculated margins; rarely oval and circumscribed; hypervascular | Irregular hypoechoic shadowing mass; rarely circumscribed or rounded; hypervascular | Evolves with time; acute phase: echogenic structure, subacute phase: ill-defined complex cyst, late phase: calcified echogenic walls with posterior acoustic shadowing; no hypervascularity | Hypoechoic region or mass with indistinct margins; no hypervascularity |
| MRI T1 | Isointense to muscle | Hypointense if visible | Hyperintense central fat | Iso/hypointense |
| MRI T2 | Heterogeneous signal intensity, hypointense fibroid tissue and hyperintense myxoid component | Hypointense mass | Low signal centrally with fat suppression, peripheral high signal edema | Iso/hypointense |
| Dynamic contrast enhanced MRI | Heterogeneous enhancement with variable enhancement curves | ~90% rapidly enhance with rapid washout | Thin rim of peripheral enhancement up to 18 months post trauma/surgery | Focal progressive heterogeneous enhancement |
ABBREVIATIONS
- MRI
Magnetic resonance imaging
- NSAID
Nonsteroidal anti-inflammatory drug
- STIR
Short tau inversion recovery
REFERENCES
- 1.Glazebrook KN, Reynolds CA. Mammary fibromatosis. AJR Am J Roentgenol. 2009 Sep;193(3):856–60. doi: 10.2214/AJR.08.1892. [DOI] [PubMed] [Google Scholar]
- 2.Goel NB, et al. Fibrous lesions of the breast: imaging-pathologic correlation. Radiographics. 2005 Nov-Dec;25(6):1547–59. doi: 10.1148/rg.256045183. [DOI] [PubMed] [Google Scholar]
- 3.Aaron AD, et al. Chest wall fibromatosis associated with silicone breast implants. Surg Oncol. 1996 Apr;5(2):93–9. doi: 10.1016/s0960-7404(96)80006-5. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JJ, et al. The enigma of desmoid tumors. Ann Surg. 1999 Jun;229(6):866–72. doi: 10.1097/00000658-199906000-00014. discussion 872–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlemmer M. Desmoid tumors and deep fibromatoses. Hematol Oncol Clin North Am. 2005 Jun;19(3):565–71. vii–viii. doi: 10.1016/j.hoc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Henderson PW, Singh SP, Spector JA. Chest wall spindle cell fibromatosis after breast augmentation. Plast Reconstr Surg. 2010 Aug;126(2):94e–5e. doi: 10.1097/PRS.0b013e3181de23ce. [DOI] [PubMed] [Google Scholar]
- 7.Povoski SP, Jimenez RE. Fibromatosis (desmoid tumor) of the breast mimicking a case of ipsilateral metachronous breast cancer. World J Surg Oncol. 2006 Aug 22;4:57. doi: 10.1186/1477-7819-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesurolle B, et al. Dynamic breast MRI in recurrent fibromatosis. AJR Am J Roentgenol. 2005 Feb;184(2):696–7. doi: 10.2214/ajr.184.2.01840696. author reply 697. [DOI] [PubMed] [Google Scholar]
- 9.Nakazono T, et al. Dynamic MRI of fibromatosis of the breast. AJR Am J Roentgenol. 2003 Dec;181(6):1718–9. doi: 10.2214/ajr.181.6.1811718. [DOI] [PubMed] [Google Scholar]
- 10.Abd el-All HS. Breast spindle cell tumours: about eight cases. Diagn Pathol. 2006 Jul 22;1:13. doi: 10.1186/1746-1596-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuman HB, et al. Desmoid tumors (fibromatoses) of the breast: a 25-year experience. Ann Surg Oncol. 2008 Jan;15(1):274–80. doi: 10.1245/s10434-007-9580-8. Epub 2007 Sep 26. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz GS, et al. Fibromatosis of the breast: case report and current concepts in the management of an uncommon lesion. Breast J. 2006 Jan-Feb;12(1):66–71. doi: 10.1111/j.1075-122X.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 13.Micke O, Seegenschmeidt MH. Radiation therapy for aggressive fibromatosis (desmoids tumors): results of a national Patterns of Care Study. Int J Radiation Oncology Biol Phys. 2005;61:882. doi: 10.1016/j.ijrobp.2004.07.705. [DOI] [PubMed] [Google Scholar]
- 14.Nuyttens JJ, et al. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoids tumors: A comparative review of 22 articles. Cancer. 2000;88:1517. [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network guidelines. available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
- 16.Patel SR, Benjamin RS. Desmoid tumors respond to chemotherapy: defying the dogman in oncology. J Clin Oncol. 2006 Jan 1;24(1):102–5. doi: 10.1200/JCO.2005.03.6566. [DOI] [PubMed] [Google Scholar]
- 17.Brooks MD, Ebbs SR, Colletta AA, Baum M. Desmoid tumours treated with triphenylethylenes. Eur J Cancer. 1992;28A(6–7):1014–8. doi: 10.1016/0959-8049(92)90445-8. [DOI] [PubMed] [Google Scholar]
- 18.Ravi V. Desmoid tumors: epidemiology, risk factors, molecular pathogenesis, clinical presentation, diagnosis, and local therapy. In: Maki R, Berman RS, editors. UpToDate. UpToDate; Waltham MA: 2011. [Google Scholar]
- 19.Penel N, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol. 2010 Jul 9; doi: 10.1093/annonc/mdq341. [DOI] [PubMed] [Google Scholar]
- 20.Chugh R, et al. Efficacy of imatinib in aggressive fibromatosis: results of a phase II multicenter Sarcoma Alliance for Research through Collaboration trial. Clin Cancer Res. 2010 Oct 1;16(19):4884–91. doi: 10.1158/1078-0432.CCR-10-1177. Epub 2010 Aug 19. [DOI] [PubMed] [Google Scholar]
- 21.Bertagnolli MM, et al. Multimodality treatment of mesenteric desmoid tumours. Eur J Cancer. 2008 Nov;44(16):2404–10. doi: 10.1016/j.ejca.2008.06.038. Epub 2008 Aug 14. [DOI] [PubMed] [Google Scholar]
- 22.Skapek S, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol. 2007 Feb 10;25(5):501–6. doi: 10.1200/JCO.2006.08.2966. [DOI] [PubMed] [Google Scholar]