Abstract
The pharmacokinetics and safety of ceftolozane, a novel cephalosporin, and tazobactam, a β-lactamase inhibitor, alone and in combination as a 2:1 ratio in single doses of up to 2,000 and 1,000 mg of ceftolozane and tazobactam, respectively, and multiple doses of up to 3,000 and 1,500 mg of ceftolozane and tazobactam, respectively, per day were evaluated in healthy adult subjects. In part 1, groups of six subjects each received single ascending doses of ceftolozane, tazobactam, and ceftolozane-tazobactam in a within-cohort crossover design. In part 2, groups of 5 or 10 subjects each received multiple doses of ceftolozane, tazobactam, or ceftolozane-tazobactam for 10 days. After a single dose of ceftolozane alone, the ranges of mean values for half-life (2.48 to 2.64 h), the total clearance (4.35 to 6.01 liters/h), and the volume of distribution at steady state (11.0 to 14.1 liters) were consistent across dose levels and similar to those observed when ceftolozane was coadministered with tazobactam. Mean values after multiple doses for ceftolozane alone and ceftolozane-tazobactam were similar to those seen following a single dose. The pharmacokinetics of the dosing regimens evaluated were dose proportional and linear. Ceftolozane-tazobactam was well tolerated and systemic adverse events were uncommon. Mild infusion-related adverse events were the most commonly observed following multiple-dose administration. Adverse events were not dose related, and no dose-limiting toxicity was identified.
INTRODUCTION
The emergence of drug resistance in common pathogens has become a major medical issue; increased resistance in Gram-negative pathogens, such as Pseudomonas aeruginosa and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, is especially concerning due to the increased morbidity and mortality associated with such infections (5). However, the number of antimicrobial products currently being developed to address these unmet medical needs appears to be limited (1).
Ceftolozane-tazobactam, formerly referred to as CXA-201, is a novel oxyimino-aminothiazolyl cephalosporin (ceftolozane) and β-lactamase inhibitor (tazobactam) combination being developed for the treatment of serious Gram-negative infections (6, 7, 8). The addition of tazobactam extends its spectrum of activity to cover ESBL-producing organisms. The pharmacokinetics (PK) and safety of multiple doses of ceftolozane alone has already been established (3) but needs to be investigated for the coadministration of ceftolozane and tazobactam. In the present study, the PK and safety of single and multiple doses of ceftolozane and tazobactam alone and in combination at a 2:1 ratio were investigated in healthy adult subjects.
MATERIALS AND METHODS
Study population and study design.
Healthy male and female subjects, 18 to 65 years of age, were enrolled in a single-center, prospective, randomized, double blind study of single ascending doses (part 1) and multiple ascending doses (part 2) of intravenous (i.v.) ceftolozane, tazobactam, and ceftolozane-tazobactam. In part 1, three cohorts of six subjects each received single ascending doses of ceftolozane, tazobactam, and ceftolozane-tazobactam as a 1-h infusion, with a minimum of 2 days separating each subsequent treatment (days 1, 4, and 7) in a within-cohort crossover design. Cohorts 1, 2, and 3 received 500, 1,000, and 2,000 mg of ceftolozane, respectively, 250, 500, and 1,000 mg of tazobactam, respectively, and 500/250, 1,000/500, and 2,000/1,000 mg of ceftolozane-tazobactam, respectively. In part 2, two cohorts of 20 subjects each, in a within-cohort parallel design, received multiple doses of study drug for 10 days; on days 1 and 10, each cohort of subjects received only a single dose of study medication. In cohort 4, five subjects received 1,000 mg of ceftolozane every 8 h (q8h), five subjects received 500 mg of tazobactam q8h, and 10 subjects received 1,000/500 mg of ceftolozane-tazobactam q8h. In cohort 5, five subjects received 1,500 mg of ceftolozane every 12 h (q12h), five subjects received 750 mg of tazobactam q12h, and 10 subjects received 1,500/750 mg of ceftolozane-tazobactam q12h.
Safety monitoring.
During the course of the study, all subjects were monitored for the occurrence of adverse events that included physical examinations, vital sign assessments, and laboratory assessments, including hematology, serum chemistry, coagulation, and urinalysis tests. In subjects receiving multiple doses of ceftolozane-tazobactam, direct Coombs' testing was conducted at screening, on day 11, and at follow-up.
Pharmacokinetic evaluations.
PK analyses were performed for ceftolozane, tazobactam, and metabolite M1 to assess the PK of each component alone and to determine what effect coadministration of ceftolozane-tazobactam had on each component's PK parameters.
In part 1, plasma samples were collected on days 1, 4, and 7 after single doses of ceftolozane, tazobactam, and ceftolozane-tazobactam, respectively. On each day, samples were collected predose and at 30, 60, 65, 75, and 90 min and 2, 3, 4, 6, 8, 10, 12, 16, and 24 h after the start of study drug infusion. On the same days, urine samples for PK analysis were collected over a 24-h period.
In part 2, plasma samples were collected on days 1 and 10. On each day, samples were collected predose and at 30, 60, 65, 75, and 90 min and 2, 3, 4, 6, 8, 10, 12, 16, and 24 h after the start of study drug infusion. Urine samples for PK analysis were collected on days 1 and 10 over a 24-h period.
A liquid chromatography-tandem mass spectrometry assay by Microconstants, Inc., analyzed ceftolozane, tazobactam, and its metabolite M1 (known to be pharmacologically and microbiologically inactive) using solid-phase extraction of plasma and urine, followed by separation with reversed-phase chromatography and detection by tandem mass spectrometry. All assays and PK samples were validated and tested according to U.S. Food and Drug Administration guidelines and Good Laboratory Practice procedures. The lower limits of quantification of ceftolozane, tazobactam, and metabolite M1 were 100 and 5,000 ng/ml, 100 and 10,000 ng/ml, and 50 and 5,000 ng/ml for plasma and urine, respectively. The assay accuracy and precision values for ceftolozane, tazobactam, and metabolite M1 were ±7.33% and 4.15 to 10.3%, ±5.00% and 3.11 to 4.27%, and ±7.00% and 7.47 to 8.5%, respectively. The PK parameters of ceftolozane, tazobactam, (including metabolite M1), and ceftolozane-tazobactam in plasma were calculated by noncompartmental methods using a validated version of WinNonlin Enterprise (v5.2). The PK parameters included Cmax (the maximum plasma concentration), tmax (the time of maximum plasma concentration), AUC0-∞ (the area under the plasma concentration-time curve from time zero to the time of last quantifiable concentration), t1/2 (the plasma half-life), CL (clearance), CLR (renal clearance), and Vss (the volume of distribution at steady state). In part 2, the accumulation index (AI) was also calculated as a ratio of AUC0-τ on day 10 to AUC0-τ on day 1. The AUC was determined using the linear trapezoidal method. Statistical analyses to assess a drug-drug interaction were performed using WinNonlin v.5.2 (Average Bioequivalence Module). Exclusion of subjects from the descriptive statistics was based on individual subject values that were greater than 3 standard deviations from the mean of the group without the outlier.
RESULTS
Demographics and disposition.
A total of 58 subjects received study drug. In part 2, one subject withdrew consent after study day 6; the subject's PK samples were obtained up to the time of withdrawal and were included in the PK analysis. The demographic characteristics of subjects included in the PK analyses were similar between the single and multiple ascending dose groups, with a larger percentage of males being enrolled in part 2. Demographic data are presented in Table 1.
Table 1.
Baseline subject demographics
| Parametera | Mean subject demographics (range)b |
|
|---|---|---|
| Part 1 (single i.v. doses [n = 18]) | Part 2 (multiple ascending i.v. doses [n = 40]) | |
| Age (yr) | 38.4 (25–59) | 34.2 (21–62) |
| Wt (kg) | 71.4 (59.6–95.9) | 81.3 (55.9–93.7) |
| BMI (kg/m2) | 26.1 (22.6–29.4) | 26.0 (19.5–29.6) |
| CLCR (ml/min) | 137 (101–181) | 134 (86–238) |
| % Male | 56 | 70 |
CLCR, creatinine clearance calculated by Cockcroft-Gault method; BMI, body mass index.
Except as noted in column 1 for the percent male subjects.
Safety.
No serious adverse events or deaths were reported in the study, and no subject prematurely discontinued study drug or withdrew from the study as a result of an adverse event.
In part 1, 11/18 (61%) subjects experienced a total of 16 adverse events. Of these, 15 (94%) adverse events were mild in severity, and 1 was moderate (generalized body aches). The most common adverse event in part 1 was constipation, reported in 6/18 (33%) subjects. Adverse events did not appear to be dose dependent. Six subjects in cohort 1 experienced 10 adverse events, three subjects in cohort 2 experienced four adverse events, and two subjects in cohort 3 experienced two adverse events.
In part 2, 29/40 (73%) subjects experienced 95 adverse events. Almost all adverse events were mild in severity (94/95 [99%]), with only one adverse event reported as moderate (menstrual cramps). Of the 95 adverse events reported in part 2, 48 (51%) were related to the study drug, with mild i.v. infusion-related events, including erythema and pruritus, being the most common (33/48 [69%]). As in part 1, adverse events did not appear to be dose dependent. Fifteen subjects in cohort 4 experienced 53 adverse events, and 14 subjects in cohort 5 experienced 42 adverse events. No dose-limiting toxicity was identified with the doses evaluated, and no abnormal laboratory result was judged to be an adverse event. Gastrointestinal disorders (nausea and vomiting), along with headache, were the only systemic adverse events reported in two subjects receiving ceftolozane-tazobactam in part 2 of the study. No direct Coombs' test was positive at baseline or at any subsequent testing.
Overall, the clinical laboratory results showed no correlation to study drug assignment, and no laboratory results were deemed to be an adverse event. Of all of the laboratory tests collected during the study, only three results were potentially clinically significant (using criteria specified a priori in the statistical analysis plan). These three results were low blood glucose concentrations reported by two subjects in part 2. Each episode was asymptomatic and was believed to most likely represent normal variation seen in healthy subjects at different stages of fasting.
Pharmacokinetic summary.
The PK parameters for ceftolozane when given alone and with tazobactam as single ascending and multiple ascending doses are given in Tables 2 and 3, respectively, and the PK parameters for tazobactam and metabolite M1 following multiple ascending doses are given in Table 4. The PK parameters for tazobactam and metabolite M1 following single ascending doses are not shown but were similar to those shown for multiple ascending doses.
Table 2.
Mean PK values for ceftolozane alone and in combination with tazobactam after a single dose (part 1)
| Parameter | Mean (% CV) PK value ata: |
|||||
|---|---|---|---|---|---|---|
| 500 mg (C) (n = 6) | 500/250 mg (C/T) (n = 6) | 1,000 mg (C) (n = 6) | 1,000/500 mg (C/T) (n = 6) | 2,000 mg (C) (n = 6) | 2,000/1,000 mg (C/T) (n = 6) | |
| Cmax (μg/ml) | 42.6 (13.5) | 40.2 (12.6) | 92.3 (12.9) | 90.2 (10.6) | 153 (10.8) | 140 (14.9) |
| tmax (h)b | 1.00 (1.00–1.09) | 1.00 (1.00–1.01) | 1.01 (1.00–1.08) | 1.05 (1.00–1.10) | 1.01 (1.00–1.09) | 1.01 (1.00–1.09) |
| AUC0-∞ (μg·h/ml) | 98.6 (16.3) | 97.3 (15.0) | 230 (5.8) | 209 (9.0) | 375 (16.4) | 353 (18.0) |
| t1/2 (h) | 2.48 (8.2) | 2.43 (18.9) | 2.64 (19.6) | 2.58 (18.6) | 2.62 (16.9) | 2.62 (18.2) |
| CL (liters/h) | 5.18 (15.2) | 5.23 (13.2) | 4.35 (6.0) | 4.82 (10.4) | 5.43 (13.7) | 5.81 (15.5) |
| CLR (liters/h) | 5.54 (14.1) | 5.44 (17.6) | 4.61 (5.5) | 5.10 (12.4) | 5.53 (17.3) | 5.93 (28.8) |
| Vss (liters) | 11.8 (13.2) | 11.7 (13.7) | 11.0 (18.9) | 11.8 (15.7) | 13.3 (14.9) | 14.0 (18.4) |
C, ceftolozane; C/T, ceftolozane-tazobactam. CV, coefficient of variation.
The median (range) is indicated.
Table 3.
Mean PK values for ceftolozane alone and in combination with tazobactam after single (day 1) and multiple doses (part 2)
| Parameter | Mean (% CV) PK value ata: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1,000 mg (C) (n = 5) |
1,000/500 mg (C/T, q8h) (n = 10) |
1,500 mg (C, q12h) (n = 5) |
1,500/750 mg (C/T, q12h) (n = 10) |
|||||
| Day 1 | Day 10 | Day 1b | Day 10 | Day 1 | Day 10 | Day 1 | Day 10 | |
| Cmax (μg/ml) | 68.8 (17.0) | 73.4 (15.2) | 69.1 (11.3) | 74.4 (13.6) | 110 (11.2) | 110 (13.1) | 122 (10.8) | 124 (11.1) |
| tmax (h)c | 1.03 (1.02–1.09) | 1.00 (1.00–1.04) | 1.02 (1.01–1.1) | 1.07 (1.0–1.1) | 1.01 (1.0–1.09) | 1.01 (1.0–1.03) | 1.01 (1.0–1.1) | 1.02 (1.0–1.11) |
| AUC0-last (μg·h/ml) | 168 (17.0) | 195 (15.2) | 172 (13.8) | 197 (16.6) | 259 (12.7) | 266 (20.3) | 308 (10.4) | 313 (9.6) |
| t1/2 (h) | 2.30 (17.1) | 2.73 (24.2) | 2.77 (30.0) | 3.12 (21.9) | 2.52 (9.4) | 2.48 (29.5) | 2.89 (13.3) | 3.18 (13.4) |
| CL (liters/h) | 6.01 (14.0) | 5.54 (13.3) | 5.86 (13.7) | 5.58 (12.6) | 5.85 (11.5) | 5.88 (17.4) | 4.90 (9.7) | 4.97 (10.6) |
| CLR (liters/h) | 6.42 (3.1)d | 5.28 (17.4)d | 5.58 (24.0)e | 6.80 (49.4) | 5.89 (16.8)f | 4.55 (35.8) | 4.80 (15.1) | 4.71 (11.8) |
| Vss (liters) | 14.1 (18.1) | 13.4 (18.1) | 14.6 (16.0) | 14.2 (16.6) | 12.9 (11.4) | 13.0 (9.3) | 12.0 (10.4) | 12.2 (11.1) |
| AI | NA | 1.15 (2.0) | NA | 1.14 (5.7) | NA | 1.02 (7.6) | NA | 1.02 (9.6) |
C, ceftolozane; C/T, ceftolozane-tazobactam. CV, coefficient of variation. NA, not applicable.
n = 9 (one subject was excluded due to outlying higher-than-expected plasma results for the administered dose).
The median (range) is indicated.
n = 4 (one subject was excluded from the calculation of descriptive statistics due to incomplete urine collection over 24 h).
n = 8 (one subject was excluded from the calculation of descriptive statistics due to outlying higher-than-expected urine results for the administered dose).
n = 4 (one subject was excluded from the calculation of descriptive statistics due to outlying lower-than-expected urine results for the administered dose).
Table 4.
Mean PK values for tazobactam and its major metabolite M1 alone and in combination with ceftolozane after single (day 1) and multiple doses (part 2)
| Specimen and parameter | Mean (% CV) PK value ata: |
|||||||
|---|---|---|---|---|---|---|---|---|
| 500 mg (T, q8h) (n = 5) |
1,000/500 mg (C/T, q8h) (n = 10) |
750 mg (T, q12h) |
1,500/750 mg (C/T, q12h) (n = 10) |
|||||
| Day 1 | Day 10 | Day 1b | Day 10 | Day 1 (n = 5) | Day 10 (n = 4) | Day 1 | Day 10 | |
| Tazobactam | ||||||||
| Cmax (μg/ml) | 17.8 (9.9) | 18.0 (8.7) | 18.4 (15.9) | 18.0 (8.0) | 29.9 (20.6) | 29.8 (15.4) | 30.2 (13.6) | 27.5 (15.2) |
| tmax (h)c | 1.01 (1.01–1.08) | 1.0 (1.0–1.03) | 1.02 (0.99–1.03) | 1.01 (1.0–1.1) | 1.0 (1.0–1.03) | 1.01 (1.0–1.01) | 1.01 (1.0–1.03) | 1.02 (1.0–1.04) |
| AUC0-last (μg·h/ml) | 24.1 (18.0) | 25.6 (12.1) | 24.4 (17.9) | 24.8 (15.5) | 38.8 (23.8) | 41.5 (17.3) | 40.1 (10.4) | 36.1 (13.3) |
| t1/2 (h) | 0.970 (35.9) | 1.10 (27.4) | 0.91 (26.2)e | 1.03 (18.6) | 0.98 (18.9) | 0.94 (18.2) | 0.992 (11.3) | 1.04 (19.0) |
| CL (liters/h) | 21.2 (20.6) | 19.7 (12.0) | 20.6 (17.8)e | 20.4 (13.6) | 20.0 (23.2) | 18.4 (17.2) | 18.9 (10.2) | 21.0 (12.6) |
| CLR (liters/h) | 14.9 (26.6) | 14.3 (13.8)d | 12.3 (23.5)f | 16.3 (12.2)g | 12.4 (23.6) | 12.6 (11.1) | 12.2 (21.6) | 15.0 (11.5) |
| Vss (liters) | 18.8 (17.3) | 18.8 (17.2) | 18.1 (12.6)e | 17.9 (9.7) | 18.0 (23.0) | 16.7 (17.3) | 16.6 (13.3) | 17.7 (11.6) |
| AI | NA | 1.08 (9.8) | NA | 0.93 (33.1) | NA | 1.03 (8.3) | NA | 0.91 (6.2) |
| M1 | ||||||||
| Cmax (μg/ml) | 0.78 (27.7) | 1.32 (21.1) | 0.70 (25.4) | 1.10 (17.0) | 1.36 (15.6) | 1.72 (10.5) | 1.45 (23.4) | 1.61 (22.7) |
| tmax (h)c | 4.03 (3.01–4.11) | 3.0 (2.0–4.01) | 4.03 (3.01–4.09) | 2.51 (1.25–4.0) | 4.02 (3.01–4.03) | 3.02 (3.0–4.0) | 3.0 (3.0–4.01) | 3.03 (3.0–4.0) |
| AUC0-last (μg·h/ml) | 5.88 (23.6) | 11.2 (30.3) | 5.64 (22.4) | 9.51 (20.6) | 10.1 (24.5) | 13.7 (19.2) | 11.6 (24.5) | 13.4 (26.7) |
| t1/2 (h) | 3.44 (14.0) | 3.61 (19.4) | 3.67 (37.2) | 4.50 (23.2) | 3.39 (20.5) | 4.45 (19.4) | 3.64 (12.1) | 4.09 (21.2) |
| CL/Fm (liters/h) | 68.9 (24.0) | 52.9 (26.2) | 69.5 (17.3) | 61.8 (18.7) | 61.3 (26.4) | 52.2 (17.5) | 54.8 (29.0) | 56.3 (31.8) |
| CLR (liters/h) | 9.31 (20.3) | 8.54 (21.6)h | 9.43 (24.1) | 8.96 (15.8)i | 6.80 (29.4) | 5.79 (22.6) | 7.70 (21.7) | 6.81 (23.7) |
| Vss/Fm (liters)j | 451 (27.1) | 331 (17.6) | 480 (34.3) | 419 (23.4) | 369 (12.1) | 323 (10.2) | 349 (25.2) | 344 (22.2) |
| AI | NA | 1.95 (13.6) | NA | 1.69 (13.6) | NA | 1.38 (8.3) | NA | 1.15 (7.1) |
T, tazobactam; C/T, ceftolozane-tazobactam. CV, coefficient of variation. NA, not applicable.
n = 9 (one subject was excluded due to outlying higher-than-expected plasma results for the administered dose).
The median (range) is indicated.
n = 3 (two subjects were excluded due to outlying higher-than-expected urine results for the administered dose).
n = 8 (one subject was excluded from the calculation of descriptive statistics, since the concentration-time profile did not exhibit a terminal log-linear phase and t1/2, CL, CLR, and Vss could not be calculated).
n = 6 (one subject was excluded due to outlying higher-than-expected plasma results for the administered dose, one subject was excluded due to incomplete urine collection over 24 h, and one subject was excluded due to outlying higher-than-expected urine results for the administered dose).
n = 8 (two subjects were excluded due to outlying higher-than-expected urine results for the administered dose).
n = 4 (one subject was excluded due to outlying higher-than-expected urine results for the administered dose).
n = 9 (one subject was excluded due to outlying higher-than-expected urine results for the administered dose).
Fmfraction metabolized.
Single ascending doses.
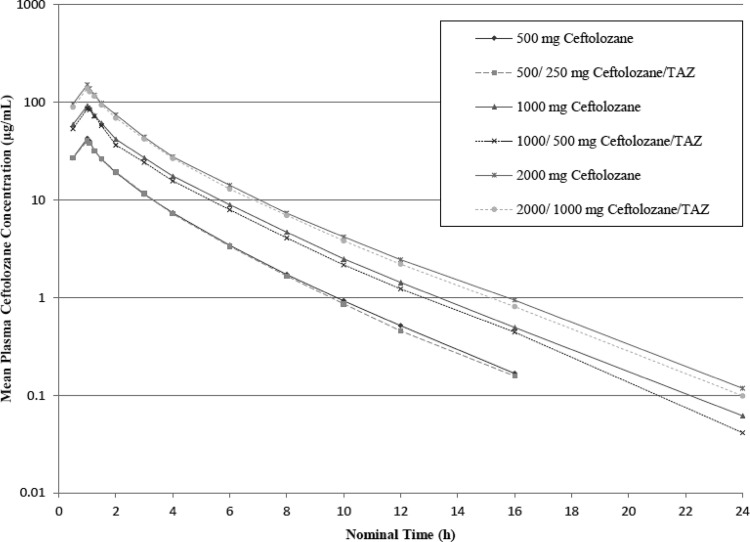
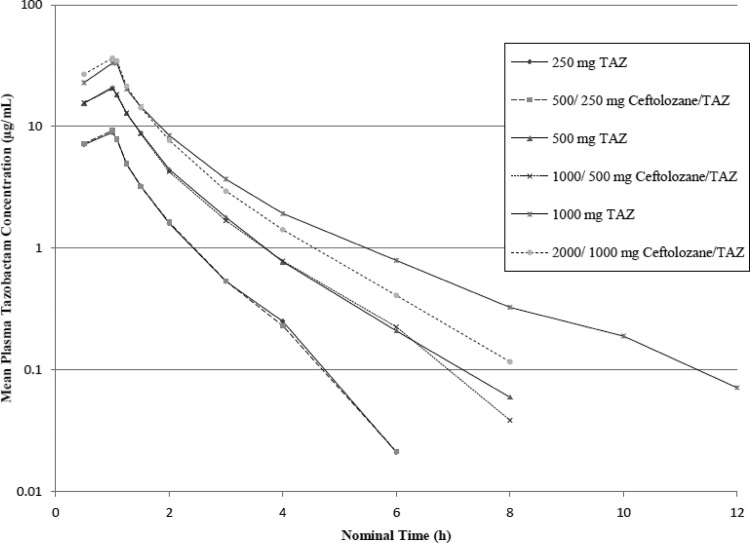
Ceftolozane demonstrated linear PK up to 2,000 mg as a single dose. After a single-dose administration of ceftolozane alone and in combination with tazobactam, the mean plasma elimination t1/2 of ceftolozane was 2.6 h across the doses studied; the half-life values for ceftolozane when given alone and when given with tazobactam were similar. In addition, the mean CL and Vss values determined for ceftolozane alone and in combination with tazobactam were 5.1 liters/h and 12.3 liters, respectively. The CL and Vss values for ceftolozane were comparable with or without the coadministration of tazobactam over the dose range of 500 to 2,000 mg. The mean CLR of ceftolozane across all doses alone or in combination with tazobactam was 5.4 liters/h and similar to the plasma clearance, suggesting that the systemic elimination of ceftolozane was primarily attributed to the kidney. After single-dose administration of ceftolozane alone, ca. 100% of the dose was recovered in the urine in the subsequent 24 h for doses between 500 and 2,000 mg; similar results were observed when ceftolozane was coadministered with tazobactam. These findings, as well as the similarity between the overall clearance and the CLR, indicate that clearance of ceftolozane occurs exclusively via renal elimination. Semilog concentration-time profiles of ceftolozane and tazobactam alone and when coadministered after single-dose administration are shown in Fig. 1 and 2, respectively.
Fig 1.
Mean concentration-time profiles of ceftolozane in plasma (semilog plot, part 1).
Fig 2.
Mean concentration-time profiles of tazobactam in plasma (semilog plot, part 1).
Multiple ascending doses.
After multiple-dose administration across all doses, the mean half-life of ceftolozane alone or in combination with tazobactam was 3.0 h (on study day 10). This value was similar for groups that received ceftolozane-tazobactam or ceftolozane alone and was also similar to the value on study day 1. The mean CL and Vss values of ceftolozane alone or in combination with tazobactam after multiple doses were 5.4 liters/h and 13.2 liters, respectively, across all doses. The clearance and Vss values for ceftolozane when coadministered with tazobactam were similar to the values observed after multiple doses of ceftolozane alone. The mean CLR of ceftolozane after 10 days of dosing was 5.5 liters/h across all doses and was similar to the overall mean plasma CL, suggesting that the systemic elimination of ceftolozane was primarily attributed to the kidney. As a result, the wide range of creatinine clearance (CLCR) values observed in part 2 of the study (86 to 238 ml/min) may have increased the intersubject variability of the PK parameters in this group of healthy volunteers. After multiple-dose administration of ceftolozane administered alone or coadministered with tazobactam, the mean AI across dose levels ranged from 1.02 to 1.15, indicating no relevant accumulation of ceftolozane in healthy subjects after repeated dosing.
Both ceftolozane and tazobactam (including metabolite M1) PK parameters were unaffected by coadministration. The mean half-life of tazobactam alone or in combination with ceftolozane after multiple doses was 1.0 h, and the CL and Vss were 20.2 liters/h and 17.8 liters, respectively, across all doses. Values were similar whether doses were administered alone or in combination with ceftolozane. Furthermore, it was noted that the urinary excretion of ceftolozane and tazobactam was unaffected by the coadministration of the two drugs. The M1 metabolite accumulated slightly with repeated dosing, as indicated by the range of AI values (1.15 to 1.95).
DISCUSSION
Doses of ceftolozane-tazobactam up to 3,000/1,500 mg per day administered for 10 days were generally safe and well tolerated. Systemic drug-related adverse events were infrequent and mild; most events were infusion related and were observed in all three treatment arms (ceftolozane, tazobactam, and ceftolozane-tazobactam). The fairly high incidence of adverse events in normal healthy subjects was not unexpected given the close monitoring of subjects that typically occurs in a phase 1 investigational unit. Furthermore, most infusion-related adverse events that were drug related occurred after having an i.v. line in place for more than 24 h. The nature and incidence of adverse events did not appear to be dose related, and no dose-limiting toxicities were identified for ceftolozane-tazobactam in single doses up to 2,000/1,000 mg and multiple doses up to 3,000/1,500 mg daily.
Ceftolozane was apparently eliminated exclusively in the urine, and its clearance was similar whether administered alone or in combination with tazobactam, suggesting tazobactam had no effect on the clearance of ceftolozane. Furthermore, other PK parameters, such as t1/2, CL, AUC, Vss, and Cmax, were not affected by the coadministration of ceftolozane-tazobactam. Some PK differences were observed between part 1 and day 1 of part 2. For the 1,000/500-mg dose of ceftolozane-tazobactam, the AUC0-∞ and Cmax were increased, while CL and Vss were decreased in part 1 of the study compared to day 1 of part 2. In part 2, mean CLCR for subjects who received 1,000 mg of ceftolozane on day 1, alone or in combination with tazobactam, were slightly more elevated than the mean CLCR observed in subjects dosed in part 1 of the study. The median (range) CLCR was 113.5 ml/min (101 to 162 ml/min). In part 2, the median CLCR was 142 ml/min (106 to 166 ml/min) for 1,000 mg of ceftolozane and 127 (105 to 196 ml/min) for 1,000/500 mg of ceftolozane-tazobactam. Since the plasma CL and CLR for ceftolozane increased with increasing CLCR, the slightly decreased exposure in part 2 was consistent with the increased CL in part 2. Thus, no dose adjustment need be considered when these two drugs are coadministered.
Tazobactam was excreted mostly renally, with clearance decreasing as renal function declined, but metabolism to metabolite M1 was also observed. The M1 metabolite accumulated to a minor degree with multiple doses; however, the accumulation was not affected by concurrent ceftolozane administration, and the M1 metabolite has not demonstrated any pharmacological or antibacterial activity (4). Ceftolozane had no impact on the PK of tazobactam.
The PK profile of ceftolozane coadministered with tazobactam was very similar to that of ceftolozane administered alone after single- and multiple-dose administration. In the dosing regimens evaluated, the PK profile was predictable and dose proportional. This finding is in contrast to tazobactam's PK profile when coadministered with piperacillin since the clearance of tazobactam is decreased and its AUC is increased (9). It is likely that the lack of interaction between ceftolozane and tazobactam is the result of ceftolozane being eliminated almost entirely via glomerular filtration, as evidenced by the similarity between its clearance and the glomerular filtration rate in normal healthy subjects. Thus, since ceftolozane does not appear to undergo active tubular secretion, it does not interfere with the known active renal tubular secretion of tazobactam.
The mean plasma t1/2 of ceftolozane was ∼2.7 h after single or multiple doses, with no meaningful accumulation of ceftolozane observed after multiple dosing. The PK/pharmacodynamic index that best correlates with the therapeutic efficacy of cephalosporins is the time above the MIC of the infecting pathogen (T > MIC), the longer half-life of ceftolozane compared to that of some other cephalosporins is potentially advantageous (2). The ceftolozane Vss (12.9 liters) was similar to that of the average human's extracellular volume, indicating that it concentrates well at extracellular sites of infection. These potential PK advantages, in addition to the reported in vitro activity of ceftolozane-tazobactam against Enterobacteriaceae, including those that produce ESBLs or overexpress AmpC, and against multidrug-resistant P. aeruginosa supports the use of ceftolozane-tazobactam as a treatment option for difficult-to-treat Gram-negative infections. The results from the present study support the further clinical development of ceftolozane-tazobactam.
ACKNOWLEDGMENTS
We thank MDS Pharma Services, Inc., for conducting the clinical portion of the study. We thank MicroConstants for the bioanalytical analyses and Pharsight for the pharmacokinetic analyses. We thank Axistat, Inc., for performing the data management and statistical analyses. We also thank Michael Bedenbaugh for his assistance in preparing the manuscript.
This study was supported by Cubist Pharmaceuticals, Lexington, MA.
B.M., E.H., D.B., and I.F. are employees of Cubist. M.T. is an employee of Pharsight and received consulting fees.
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Boucher HW, et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 2. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–12 [DOI] [PubMed] [Google Scholar]
- 3. Ge Y, Whitehouse MJ, Friedland I, Talbot GH. 2010. Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions. Antimicrob. Agents Chemother. 54:3427–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halstenson CE, et al. 1994. Pharmacokinetics of tazobactam M1 metabolite after administration of piperacillin-tazobactam in subjects with renal impairment. J. Clin. Pharmacol. 34:1208–1217 [DOI] [PubMed] [Google Scholar]
- 5. Maragakis LL, Perencevich EN, Cosgrove SE. 2008. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 6:751–763 [DOI] [PubMed] [Google Scholar]
- 6. Moya B, et al. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against β-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 54:1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sader HS, Rhomberg PR, Farrell DJ, Jones RN. 2011. Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes. Antimicrob. Agents Chemother. 55:2390–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Titelman E, Karlsson IM, Ge Y, Giske CG. 2011. In vitro activity of CXA-101 plus tazobactam (CXA-201) against CTX-M-14- and CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 70:137–141 [DOI] [PubMed] [Google Scholar]
- 9. Wise R, Logan M, Cooper M, Andrews JM. 1991. Pharmacokinetics and tissue penetration of tazobactam administered alone and with piperacillin. Antimicrob. Agents Chemother. 35:1081–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]