Abstract
Cerebrospinal fluid (CSF) shunts used to treat hydrocephalus have an overall infection rate of about 10% of operations. The commonest causative bacteria are Staphylococcus epidermidis, followed by Staphylococcus aureus and enterococci. Major difficulties are encountered with nonsurgical treatment due to biofilm development in the shunt tubing and inability to achieve sufficiently high CSF drug levels by intravenous administration. Recently, three cases of S. epidermidis CSF shunt infection have been treated by intravenous linezolid without surgical shunt removal, and we therefore investigated vancomycin and linezolid against biofilms of these bacteria in vitro. A continuous-perfusion model of shunt catheter biofilms was used to establish mature (1-week) biofilms of Staphylococcus aureus, Staphylococcus epidermidis (both methicillin resistant [MRSA and MRSE]), Enterococcus faecalis, and Enterococcus faecium. They were then “treated” with either vancomycin or linezolid in concentrations achievable in CSF for 14 days. The biofilms were then monitored for 1 week for eradication and for regrowth. Enterococcal biofilms were not eradicated by either vancomycin or linezolid. Staphylococcal biofilms were eradicated by both antibiotics after 2 days and did not regrow. No resistance was seen. Linezolid at concentrations achievable by intravenous or oral administration was able to eradicate biofilms of both S. epidermidis (MRSE) and S. aureus (MRSA). Neither vancomycin at concentrations achievable by intrathecal administration nor linezolid was able to eradicate enterococcal biofilms. It is hoped that these in vitro results will stimulate further clinical trials with linezolid, avoiding surgical shunt removal.
INTRODUCTION
Since their introduction in the 1950s, hydrocephalus shunts have been subject to infection, which has seriously compromised their benefit. Despite general and specific improvements in infection control, approximately 10% of shunt procedures result in infection (11). This rate is higher when infants under 6 months of age are shunted (22). Most shunt infections are caused by staphylococci, with the Staphylococcus epidermidis group predominating, followed by Staphylococcus aureus. Less commonly, Enterococcus spp. are involved (7, 17). Historically, staphylococcal shunt infections were treated with long courses of intravenous antistaphylococcal antibiotics, such as methicillin (8), with no success. Methicillin was undetectable in the cerebrospinal fluid (CSF), but even intraventricular administration was unsuccessful, and the shunts had to be removed to effect a cure. Little has changed since in therapy of shunt infections, with attempts to treat them without shunt removal showing poor results (1, 18). There are two main reasons for this. First, most shunt infections are associated with a low-grade inflammatory response in the ventricular system, and most antibiotics when administered intravenously do not penetrate the blood-CSF barrier sufficiently to provide therapeutic CSF concentrations. Second, the seat of a shunt infection is inside the shunt tubing itself. The causative bacteria attach to the shunt surface and develop a biofilm, a situation that became apparent only in 1972 (4) but which has been confirmed since (12, 16). Biofilms are complex communities of bacteria that have adopted a dormant mode in order to survive. As almost all antibiotics act on synthetic systems of actively multiplying bacteria, these biofilm bacteria become insusceptible to therapy (13). There are therefore two reasons for treatment failure in shunt infections. Current recommendations are to remove or externalize the shunt and to give intravenous antibiotics until the CSF is clear of infection before considering shunt replacement. The guidelines of the British Society for Antimicrobial Chemotherapy (BSAC) (27) suggest using intraventricular vancomycin in order to ensure adequate CSF concentrations, and for staphylococcal infections, this usually allows reshunting where necessary within about 10 to 14 days, but regimens vary considerably, and long hospital stays with relapse are not infrequent (20).
Recently, intravenous linezolid has shown value in treating posttraumatic meningitis due to multiresistant S. epidermidis, where over 90% serum concentrations of the drug were detected in the CSF despite minimal ventricular inflammatory response (21, 29). There are few reports in the literature of the use of linezolid in shunt infections (9, 10, 14, 15, 17, 26, 28, 29), and in only three cases (9, 28) was the infection treated without shunt removal. In these three cases (two due to methicillin-resistant S. epidermidis [MRSE] and one to methicillin-resistant S. aureus [MRSA]), cure was achieved.
This led us to investigate the ability of therapeutically achievable CSF linezolid concentrations to eradicate biofilms of staphylococci and enterococci using an established in vitro model of shunt colonization (3). (G. Ullas submitted part of this work in fulfillment of the requirements for the degree of Bachelor of Medical Science from the University of Nottingham School of Medicine and Health Sciences, Nottingham, United Kingdom.)
MATERIALS AND METHODS
Test bacteria.
Strains of MRSA and MRSE and of Enterococcus faecium and Enterococcus faecalis, both susceptible to vancomycin, were used. The MRSA, MRSE, and E. faecium strains were from shunt infections; the E. faecalis strain was from a urinary-catheter-related infection, as a shunt isolate was not available. All test strains were shown by microtiter plate assay to be biofilm producers. The test strains were characterized and antimicrobial susceptibilities were determined by standard methods, including determination of MICs by Etest (AB Biodisk, Solna, Sweden).
In vitro challenge model.
The in vitro challenge model was a modification of an established apparatus that we have used previously to study biofilm growth in shunt catheters and its prevention (3). The modifications were to hardware and pumps and did not change the fundamental design. Briefly, 35-cm lengths of medical-grade silicone tubing of the type used in hydrocephalus shunt catheters were sterilized by autoclaving and aseptically introduced into controlled-environment tubes to maintain hydration and a temperature of 37°C. Each was aseptically connected distally to an outlet tube draining to waste and proximally to an inlet tube. This was then connected via a peristaltic pump to a reservoir containing tryptone soy broth (TSB) (Oxoid, Basingstoke, United Kingdom). The test catheters were perfused with TSB at a rate of 20 ml/hour, approximating the CSF production rate. Control catheters were set up identically except for the antibiotics.
Bacterial challenge.
Cultures of the test bacteria were prepared in TSB by incubation on an orbital shaker at 200 rpm for 5 h. The resulting suspension was then diluted to give 108 CFU/ml, and 1 ml of this was aseptically injected into the relevant test catheter. The catheter was clamped for 1 h to allow bacterial attachment, and perfusion was then restarted.
Monitoring of biofilm development and treatment effects.
Samples were taken daily to ensure establishment of biofilms, and quantitative cultures were carried out. On day 7, the antibiotics were introduced to the reservoir (representing the ventricle), and perfusion was continued for 14 days, followed by a 7-day antibiotic-free perfusion. Each day, a 1-ml sample was taken from the distal end of each test catheter after aseptic disconnection of the outlet tube, and after vortex mixing, 200 μl was spread over a blood agar plate and incubated for 48 h at 37°C for colony counting. Where samples were visibly cloudy, appropriate dilutions were made before plating. When there was no decrease in counts; where there were persistent positive cultures over the treatment period, indicating failure; or when regrowth occurred, the identities and MICs of the isolates were checked.
Antibiotic dosing.
Concentrations of each antibiotic in the reservoir (“ventricle”) were calculated to reflect those achieved after intravenous administration (linezolid) or intraventricular administration (vancomycin). The concentrations were as follows: vancomycin, 50 mg/liter (2), and linezolid, 5 mg/liter (5). Vancomycin hydrochloride was purchased from Wockhardt Ltd., Wrexham, United Kingdom, and linezolid from Pharmacia Ltd., Sandwich, United Kingdom.
All colonized catheters were treated with linezolid or vancomycin. All antibiotic treatment was continued for 2 weeks, after which perfusion was switched to plain TSB for the detection of regrowth.
Assay of antibiotic activity.
Samples from each ventricle were taken at day 7, day 8, and day 21 to determine whether the drugs were still fully active. Standards for each drug were made, and the samples were tested by the agar dilution bioassay technique using a laboratory strain of S. epidermidis.
RESULTS
The MICs of the antibiotics for the test bacteria are shown in Table 1. All were susceptible to linezolid and vancomycin.
Table 1.
MICs of linezolid and vancomycin for test bacteria
| Bacteriuma | MIC (mg/liter) |
|
|---|---|---|
| Linezolid | Vancomycin | |
| MRSA | 1.0 | 2.0 |
| MRSE | 0.5 | 2.0 |
| E. faecalis | 1.0 | 4.0 |
| E. faecium | 1.0 | 4.0 |
E. faecalis and E. faecium were vancomycin susceptible.
The results of treatment with each drug regimen are shown in Fig. 1 to 4. In the case of E. faecium, vancomycin appeared to eradicate colonization, but regrowth occurred. Though bacterial counts were considerably reduced, linezolid did not eradicate E. faecium, and regrowth occurred. Neither linezolid nor vancomycin eradicated E. faecalis. Both linezolid and vancomycin eradicated S. epidermidis within 3 days, and no regrowth occurred. In the case of MRSA, both drug treatments eradicated the biofilms in 2 days, and no regrowth occurred. No resistance was seen to either of the antibiotics.
Fig 1.
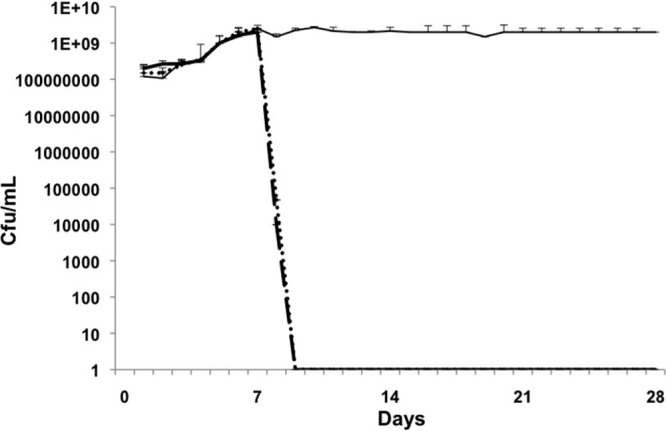
MRSA. Days 1 to 7, biofilm growth and maturation; days 8 to 21, treatment; days 22 to 28, regrowth/relapse phase. Control biofilm, solid line; linezolid, dotted line; vancomycin, dashed line. Linezolid and vancomycin eradicated colonization on day 9 (day 2 of treatment), and there was no regrowth. The error bars indicate standard deviations.
Fig 4.
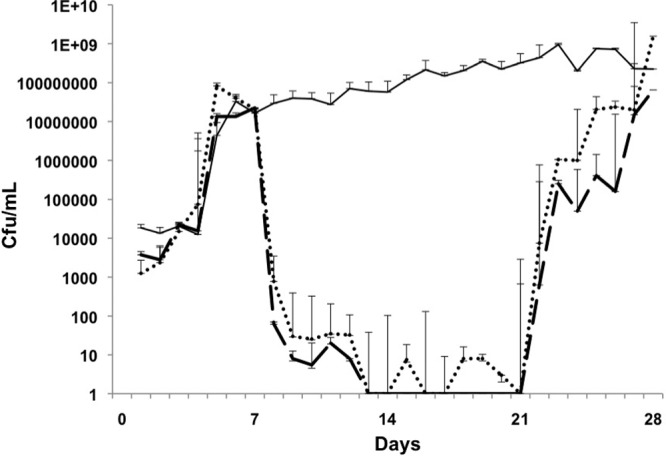
E. faecium. Days 1 to 7, biofilm growth and maturation; days 8 to 21, treatment; days 22 to 28, regrowth/relapse phase. Control biofilm, solid line; linezolid, dotted line; vancomycin, dashed line. Linezolid and vancomycin reduced colonization between days 8 and 21, but this was not eradication, and growth increased again during the relapse phase. The error bars indicate standard deviations.
Fig 2.
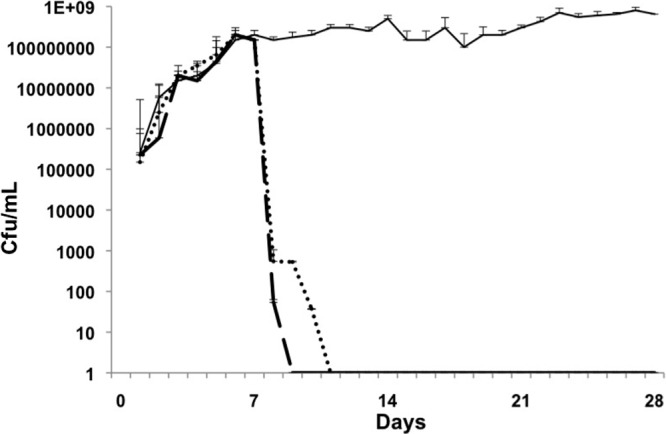
Methicillin-resistant S. epidermidis. Days 1 to 7, biofilm growth and maturation; days 8 to 21, treatment; days 22 to 28, regrowth/relapse phase. Control biofilm, solid line; linezolid, dotted line; vancomycin, dashed line. Linezolid and vancomycin eradicated colonization on day 9 (day 2 of treatment), and there was no regrowth. The error bars indicate standard deviations.
Fig 3.
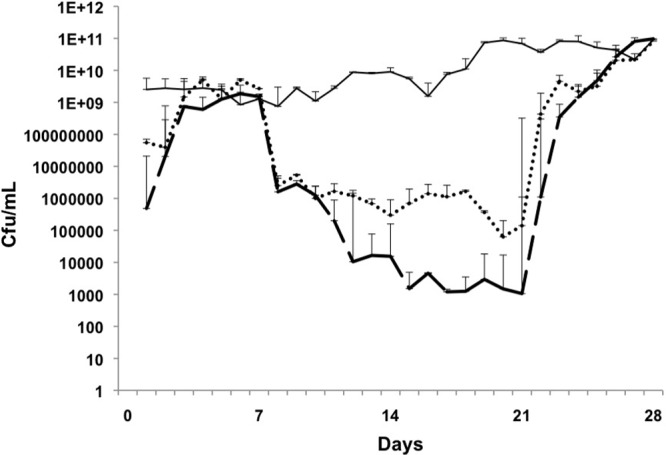
E. faecalis. Days 1 to 7, biofilm growth and maturation; days 8 to 21, treatment; days 22 to 28, regrowth/relapse phase. Control biofilm, solid line; linezolid, dotted line; vancomycin, dashed line. Linezolid and vancomycin reduced colonization between days 8 and 21, but this was not eradication, and growth increased again during the relapse phase. The error bars indicate standard deviations.
Bioassay of vancomycin and linezolid concentrations before, during, and at the end of the perfusion period showed no reduction in antimicrobial activity (data not shown).
DISCUSSION
Current treatment of shunt infection depends on shunt removal and intravenous antibiotics, often extending to several weeks. It can be complicated by secondary infections during external ventricular drainage to control intracranial pressure and by failure to eradicate the intracranial infection before reshunting. The reinfection rate has been reported to be 15% (25), and each reinfection requires another round of shunt removal, intravenous antibiotics, and reshunting. In some cases, several attempts are necessary in order to establish a functioning, uninfected shunt, and in such cases, there is evidence of poorer outcome. Intravenous administration of most of the common antimicrobials does not reliably lead to therapeutic antibiotic levels in the CSF (1, 19, 23, 24). Intraventricular antibiotic administration involves a risk of introduction of infection, and administration of some antibiotics by this route is not possible due to neurotoxicity. In almost all studies, even when intraventricular administration has been used, shunt removal has still been necessary. This is because the seat of the infection is bacterial colonization of the shunt tubing with biofilm formation. To our knowledge, only one study has reported the consistently successful use of a nonsurgical approach based on intravenous and intraventricular antibiotics (6). The treatment failed in only 2 of 30 patients with coagulase-negative staphylococcus (CoNS) shunt infections, though the regimen was not useful for S. aureus infections. For enterococcal infections, intraventricular gentamicin was added to the vancomycin. In each case, a contralateral reservoir was inserted for ventricular access. We could find no other reports of the successful use of this regimen in other hands. Obviously, if a regimen were available that eradicated shunt colonization without shunt removal, and preferably without intraventricular administration of antibiotics, it would be a significant therapeutic advance. Linezolid achieves therapeutic CSF concentrations when administered intravenously (21, 29). For eradication of the biofilms in the shunt, concentrations in excess of the MIC are required, and these have been shown to be achievable (17).
The BSAC guidelines (27) recommend the use of intraventricular vancomycin, and this is acknowledged to be more effective than intravenous administration (1, 2, 24). The results of this in vitro investigation show that both vancomycin and linezolid, at concentrations found in CSF and under flow conditions, are active against biofilms of S. epidermidis and methicillin-resistant S. aureus, but not against those of E. faecalis or E. faecium. While both vancomycin and linezolid were able to reduce the enterococcal biofilms, only linezolid reduced the effluent counts to zero, and in all cases, even with linezolid, they regrew during the relapse phase. It is possible that longer treatment with linezolid would have led to their eradication. The staphylococcal biofilms were apparently eradicated and did not regrow during the relapse phase. The effluent counts have been shown to closely reflect the biofilm density in the catheters (3), but where they fall to zero, a period of antibiotic-free perfusion is necessary to exclude their persistence and subsequent regrowth. In clinical terms, this would, of course, lead to treatment failure with relapse after cessation of treatment.
The results suggest that both linezolid and vancomycin might be effective against staphylococcal shunt infections without shunt removal. The advantage of linezolid over vancomycin is that therapeutic CSF levels can be achieved by intravenous administration, while vancomycin must be administered intraventricularly to obtain the CSF levels used here, and it may be necessary to insert a contralateral reservoir for this purpose to ensure success (6). The possibility has also been raised of reverting to oral linezolid therapy, perhaps even as an outpatient, subject to the patient's general condition. Further clinical trials of the use of linezolid for nonsurgical treatment of staphylococcal shunt infections should be stimulated by these results. However, it should be recognized that the data were generated using an in vitro system, and while they support previous clinical findings, they should be interpreted with caution.
ACKNOWLEDGMENTS
We are grateful to the University of Nottingham for financial support for G.U. This work was supported by the University of Nottingham.
We have no conflict of interest to declare.
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Arnell K, Enblad P, Wester T, Sjölin J. 2007. Treatment of cerebrospinal fluid shunt infections in children using systemic and intraventricular antibiotic therapy in combination with externalization of the ventricular catheter: efficacy in 34 consecutively treated infections. J. Neurosurg. 107:213–219 [DOI] [PubMed] [Google Scholar]
- 2. Bafeltowska JJ, Buszman E, Mandat KM, Hawranek JK. 2004. Therapeutic vancomycin monitoring in children with hydrocephalus during treatment of shunt infections. Surg. Neurol. 62:142–150 [DOI] [PubMed] [Google Scholar]
- 3. Bayston R, Lambert E. 1997. Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. J. Neurosurg. 87:247–251 [DOI] [PubMed] [Google Scholar]
- 4. Bayston R, Penny SR. 1972. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev. Med. Child Neurol. Suppl. 27:25–28 [DOI] [PubMed] [Google Scholar]
- 5. Beer R, et al. 2007. Pharmacokinetics of intravenous linezolid in cerebrospinal fluid and plasma in neurointensive care patients with staphylococcal ventriculitis associated with external ventricular drains. Antimicrob. Agents Chemother. 51:379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown EM, Edwards RJ, Pople IK. 2006. Conservative management of patients with cerebrospinal fluid shunt infections. Neurosurgery 58:657–665 [DOI] [PubMed] [Google Scholar]
- 7. Bruinsma N, et al. 2000. Subcutaneous ventricular catheter reservoir and ventriculoperitoneal drain-related infections in preterm infants and young children. Clin. Microbiol. Infect. 6:202–206 [DOI] [PubMed] [Google Scholar]
- 8. Callaghan RP, Cohen SJ, Stewart GT. 1961. Septicaemia due to colonization of Spitz-Holter valves by staphylococci. Br. Med. J. 5229:860–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castro P, et al. 2005. Linezolid treatment of ventriculoperitoneal shunt infection without implant removal. Eur. J. Clin. Microbiol. Infect. Dis. 24:603–606 [DOI] [PubMed] [Google Scholar]
- 10. Cook AM, Ramsey CN, Martin CA, Pittman T. 2005. Linezolid for the treatment of a heteroresistant Staphylococcus aureus shunt infection. Pediatr. Neurosurg. 41:102–104 [DOI] [PubMed] [Google Scholar]
- 11. Duhaime A-C. 2006. Evaluation and management of shunt infections in children with hydrocephalus. Clin. Pediatr. 45:705–713 [DOI] [PubMed] [Google Scholar]
- 12. Fux CA, et al. 2006. Biofilm-related infections of cerebrospinal fluid shunts. Clin. Microbiol. Infect. 12:331–337 [DOI] [PubMed] [Google Scholar]
- 13. Gilbert P, Maira-Litran T, McBain AJ, Rickard AH, Whyte FW. 2002. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol. 46:202–256 [PubMed] [Google Scholar]
- 14. Gill CJ, Murphy MA, Hamer DH. 2002. Treatment of Staphylococcus epidermidis ventriculo-peritoneal shunt infection with linezolid. J. Infect. 45:129–132 [DOI] [PubMed] [Google Scholar]
- 15. Graham PL, Ampofo K, Saiman L. 2002. Linezolid treatment of vancomycin-resistant Enterococcus faecium ventriculitis. Pediatr. Infect. Dis. J. 21:798–800 [DOI] [PubMed] [Google Scholar]
- 16. Guevara JA, Zuccaro G, Trevisan A, Denoya CD. 1987. Bacterial adhesion to cerebrospinal fluid shunts. J. Neurosurg. 67:438–445 [DOI] [PubMed] [Google Scholar]
- 17. Hachem R, Afif C, Gokaslan Z, Raad IR. 2001. Successful treatment of vancomycin-resistant Enterococcus meningitis with linezolid. Eur. J. Clin. Microbiol. Infect. Dis. 20:432–434 [DOI] [PubMed] [Google Scholar]
- 18. James HE, Bradley JS. 2008. Management of complicated shunt infections: a clinical report. J. Neurosurg. Pediatr. 1:223–228 [DOI] [PubMed] [Google Scholar]
- 19. Jorgenson L, et al. 2007. Vancomycin disposition and penetration into ventricular fluid of the central nervous system following intravenous therapy in patients with cerebrospinal devices. Pediatr. Neurosurg. 43:449–455 [DOI] [PubMed] [Google Scholar]
- 20. Kestle JRW, et al. 2006. Management of shunt infections: a multicenter pilot study. J. Neurosurg. Pediatr. 105:177–181 [DOI] [PubMed] [Google Scholar]
- 21. Krueger WA, et al. 2004. Treatment of meningitis due to methicillin-resistant Staphylococcus epidermidis with linezolid. J. Clin. Microbiol. 42:929–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kulkarni AV, Drake JM, Lamberti-Pasculli M. 2001. Cerebrospinal fluid shunt infection: a prospective study of risk factors. J. Neurosurg. 94:195–201 [DOI] [PubMed] [Google Scholar]
- 23. Nau R, Sörgel F, Eiffert H. 2010. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin. Microbiol. Rev. 23:858–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pfausler B, et al. 2003. Treatment of staphylococcal ventriculitis associated with external cerebrospinal fluid drains: a prospective randomized trial of intravenous compared with intraventricular vancomycin therapy. J. Neurosurg. 98:1040–1044 [DOI] [PubMed] [Google Scholar]
- 25. Simon TD, Hall M, Dean JM, Kestle JR, Riva-Cambrin J. 2010. Reinfection following initial cerebrospinal fluid shunt infection. J. Neurosurg. Pediatr. 6:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viale PL, et al. 2002. Linezolid for the treatment of central nervous system infections in neurosurgical patients. Scand. J. Infect. Dis. 34:456–459 [DOI] [PubMed] [Google Scholar]
- 27. Working Party on the Use of Antibiotics in Neurosurgery of the British Society for Antimicrobial Chemotherapy 1995. Treatment of infection associated with shunting for hydrocephalus. Br. J. Hosp. Med. 53:368–373 [PubMed] [Google Scholar]
- 28. Yilmaz A, et al. 2010. Linezolid treatment of shunt-related cerebrospinal fluid infections in children. J. Neurosurg. Pediatr. 5:443–448 [DOI] [PubMed] [Google Scholar]
- 29. Zeana C, Kubin CJ, Della-Latta P, Hammer SM. 2001. Vancomycin-resistant Enterococcus faecium meningitis successfully managed with linezolid: case report and review of the literature. Clin. Infect. Dis. 33:477–482 [DOI] [PubMed] [Google Scholar]


