Abstract
The increase in drug resistance and invasion caused by biofilm formation brings enormous challenges to the management of Candida infection. Aspirin's antibiofilm activity in vitro was discovered recently. The spectrophotometric method and the XTT {2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide} reduction assay used for data generation make it possible to evaluate fungal biofilm growth accurately. The combined use of the most commonly used methods, the fractional inhibitory concentration index (FICI) and a newly developed method, the ΔE model, which uses the concentration-effect relationship over the whole concentration range instead of using the MIC index alone, makes the interpretation of results more reliable. As an attractive tool for studying the pharmacodynamics of antimicrobial agents, time-kill curves can provide detailed information about antimicrobial efficacy as a function of both time and concentration. In the present study, in vitro interactions between aspirin (acetylsalicylic acid [ASA]) and amphotericin B (AMB) against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis were evaluated by the checkerboard microdilution method and the time-kill test. Synergistic and indifferent effects were found for the combination of ASA and AMB against planktonic cells, while strong synergy was found against biofilm cells analyzed by FICI. The ΔE model gave more consistent results with FICI. The positive interactions in concentration were also confirmed by the time-kill test. Moreover, this approach also revealed the pharmacodynamics changes of ASA and synergistic action on time. Our findings suggest a potential clinical use for combination therapy with ASA and AMB to augment activity against biofilm-associated infections.
INTRODUCTION
Infections caused by Candida species manifest in a number of diseases, including candidemia, vulvovaginal candidiasis, endocarditis, and peritonitis. Candida albicans and Candida parapsilosis are still two of the leading Candida species causing infections worldwide (45).
Microbial biofilm is a microbially derived sessile community characterized by cells that are irreversibly attached to a substratum or interface or to each other, are embedded in a matrix of extracellular polymeric substances, and exhibit an altered phenotype with respect to growth rate and gene transcription (12). Biofilms are ubiquitous in nature and are characterized by their recalcitrance toward antimicrobial treatment (42). They are notoriously resistant to antimicrobial agents of various types, including biocides, antibiotics, and antiseptics (17). Drug resistance has been demonstrated for Candida biofilms growing on surfaces such as cellulose, polystyrene, and denture acrylic, as well as polyvinyl chloride (3, 35). Therefore, there is a continuous need for the discovery of new antimicrobial agents that are effective against biofilms.
Among these efforts, one aspect is to explore “nonantibiotic drugs” for their antimicrobial and antibiofilm activities. The previous results show that aspirin, one of the oldest and most widely used anti-inflammatory drugs, dramatically decreases biofilm formation by C. albicans. Alem et al. found that aspirin was active against growing and fully mature (48-h) biofilms and that its effect was dose related (3).
Aspirin is known to have a variety of effects on microorganisms. In vitro studies on planktonic cultures demonstrated its antifungal (41), antibacterial (47), and antiviral (23) activities. These effects include changes in membrane potentials and production of virulence factors, reduction in extracellular polysaccharide, and prostaglandin production (3, 41), which may contribute to aspirin's antimicrobial activities against planktonic and biofilm cultures. Although some aspirin concentrations (50 to 200 μM) producing significant levels of antibiofilm activity in vitro fall within the range of those frequently achieved by therapeutic doses of aspirin in humans (48), significant effects of aspirin on growth and biofilm formation of Candida spp. were achieved only with suprapharmacological concentrations of the drug, which limits its clinical application (41).
Amphotericin B (AMB), a polyene macrolide agent used as the “the gold standard” antifungal drug since the 1960s, is a crucial agent in the management of serious systemic fungal infections. In spite of its proven track record, its well-known side effects and toxicity will sometimes require discontinuation of therapy despite a life-threatening systemic fungal infection. Conventional AMB, despite being a broad-spectrum fungicidal agent with little intrinsic or acquired resistance, is limited by its serious toxicities and lack of an oral formulation for systemic therapy. Although improvements in manufacturing over the last 40 years have enhanced tolerability, infusion-related reactions and renal dysfunction are still commonplace with the use of the deoxycholate solubilized formulation, which still imposes restrictions on its treatment in comparison to newer triazoles and echinocandins. What's more, the high prices of AMB's lipid complex limit its large-scale application (6, 21, 22).
Combination therapy is one approach that can be used to improve the efficacy of antimicrobial therapy for difficult-to-treat infections (1). Attempts have been made to cope with treatment failures either by combining different antifungals or by combining antifungals with nonantifungals (1, 26, 40). Aspirin, which possesses a weak and broad-spectrum antimicrobial activity toward some planktonic and biofilm cultures, may be useful in combined therapy with conventional antifungal agents to reduce their dose and improve their efficacy, while AMB, needs to be combined with agents that are cheaper, more effective, more tolerable, and less toxic, particularly less nephrotoxic than AMB deoxycholate. Considering these factors, the combination of aspirin and AMB is an excellent choice for clinical use.
In the present study, we investigated the MIC and combined effects of aspirin and AMB against planktonic cells and biofilm cells of C. albicans and C. parapsilosis by using the microdilution method and the checkerboard microdilution method, and we compared the differences in drug efficacy in different states. Furthermore, we obtained the detailed information about antimicrobial efficacy as a function of both time and concentration by using a time-kill test to assess the pharmacodynamics of each antimicrobial agent and their combined effects. There have been controversies over assessing the nature and intensity of drug interactions, and the observed in vitro interaction of two agents depends on different methodologies for data generation and analysis, resulting in variable and sometimes controversial conclusions (33). New methods and interpretation models such as the ΔE model were used in comparison to the fractional inhibitory concentration index (FICI). To improve the accuracy of results and reproducibility, we utilized the spectrophotometric method and the colorimetric method instead of the traditional methods of visual reading and colony counting.
MATERIALS AND METHODS
Candida strains.
Overnight cultures of the following microorganisms were used throughout the study: each one of the strains of C. albicans CCA10, C. albicans YEM30, and C. parapsilosis ATCC 22019. Long-term maintenance of the microbial strains was performed at −20°C using glycerol and short-term maintenance was on nutrient agar plates at 4°C. CCA10 was isolated from a patient with invasive candidiasis from QiLu hospital and was confirmed according to standard mycological methods by the Microbiological Research Laboratory, Center of Health Research and Epidemic Prevention, Shandong Province. C. albicans YEM30 was obtained from the Key Laboratory of Oral Biomedicine of Shandong Province. C. parapsilosis ATCC 22019 was obtained from the Key Laboratory for Experimental Teratology of Chinese Ministry of Education. All strains were maintained on slopes of Sabouraud dextrose agar (Difco) and subcultured monthly. Every 2 months, the cultures were replaced by new ones freshly grown from freeze-dried stocks.
Candida suspension.
Prior to each experiment, the yeast strain was cultured aerobically at 30°C for 18 h on YPD medium containing 1% (wt/vol) yeast extract, 1% (wt/vol) Bacto peptone, and 2% (wt/vol) glucose. The strains were cultured in the budding-yeast phase under these conditions. After 18 h of incubation, the cells, which were in the late exponential growth phase, were harvested, washed three times with phosphate-buffered saline (PBS; pH 7.2), and resuspended in YPD medium. The concentrations of the Candida suspensions were measured by blood cell plate counting.
Aspirin and AMB solutions.
A stock solution of AMB (NCPC, H13020284; 5 mg/ml) was freshly prepared in PBS that was sterilized by autoclaving. A stock solution of aspirin (Sigma; 900 mg/ml) was freshly prepared in dimethyl sulfoxide (DMSO, and then filtration was performed for sterilization. In experiments performed on planktonic cells and biofilm cells, aspirin was used at final concentrations ranging from 0.03125 to 16 mg/ml, and AMB was used at a final concentrations ranging from 0.015625 to 64 μg/ml.
MIC.
The MICs of aspirin and AMB were studied by the broth microdilution method in 96-well plates according to the CLSI standard M27-A2, using RPMI 1640 medium (buffered to pH 7.0 ± 0.1 with morpholinepropanesulfonic acid buffer) (30). For the MIC studies, stock solutions of aspirin at 160 mg ml−1 and AMB at 256 μg ml−1 were used. Each drug was first serially diluted 2-fold. A volume of 100 μl of either aspirin or AMB was added. Each suspension (5 × 106 CFU/ml) was diluted (1:50 dilution, followed by 1:20 dilution) in RPMI 1640 to obtain twice the final inoculum size of 2.5 × 103 CFU/ml, and then 100 μl of inoculum was added. The plates were incubated for 48 h at 37°C, and the MIC was expressed as the minimal concentration in the well showing 20% growth for planktonic cells (50% for biofilm cells) compared to positive control wells. Growth was detected as turbidity (492 nm) relative to an uninoculated well using a microtiter plate reader. Negative controls were performed with only RPMI 1640 in each well, and positive controls were performed with only microorganisms overnight culture in the wells. The positive control included DMSO in the same concentration (vol/vol) as that used in the experimental substances. Each MIC determination was performed in triplicate.
Measurement of antimicrobial combinations.
The in vitro interaction between aspirin and AMB was studied by a two-dimensional (8 by 12) checkerboard microdilution technique in sterile, 96-well flat-bottom microtitration plates as described below. Each isolate was tested three times on different days. The concentration of each antimicrobial agent in combination ranged from 1/32 times to 4 times the MIC. For the combination studies, each drug was first serially diluted 2-fold in the corresponding solvents according to the MIC in order to obtain four times the final concentration. A 50-μl aliquot of each drug concentration of AMB was added to columns 1 to10. Then, a 50-μl aliquot of each concentration of aspirin was added to rows A to G. In the wells of column 10, 50 μl of the medium containing aspirin solvent was added, and in the wells of row H, 50 μl of the medium containing AMB solvent was added. Thus, row H and column 11 contained only AMB and aspirin, respectively, and the wells at column 12 and the intersection of row H and column 11 (i.e., well H11) were the drug-free wells that served as the growth control. The final concentrations of aspirin and AMB were confirmed according to the MICs. Each well was inoculated with a final inoculum size of 2.5 × 103 CFU of the tested microorganisms ml−1. The setup was incubated at 37°C for 48 h. A microtiter plate reader was then used to detect growth as the turbidity (at 492 nm) relative to an uninoculated well.
Candida biofilm development.
Candida biofilm formation was performed as described by Da Silva et al. (10). Briefly, aliquots of 100 μl of standard cell suspensions of yeasts (107 cells/ml in YPD medium) were transferred into each well of 96-well microtiter plates, followed by incubation for 1.5 h (adhesion phase) at 37°C at 75 rpm in an orbital shaker. After the adhesion phase, the cell suspensions were gently aspirated, and each well was washed twice with PBS to remove any remaining planktonic. Adhered cells remained undisturbed during the whole process. In order to allow the growth of biofilm (biofilm phase), 200 μl of freshly prepared YPD medium was added to each well. The plates were incubated for 24 to 48 h at 37°C at 75 rpm in an orbital shaker. At 24 h of incubation, the medium was aspirated, and the biofilms were washed twice with PBS, followed by the addition of 200 μl of fresh medium.
Drug addition and biofilm susceptibility testing.
At 48 h of incubation (maturation of the biofilm), the medium was aspirated, and the biofilms were washed twice with PBS, followed by the addition of 100 μl of fresh medium. Then, each serially diluted drug was added. For the MIC studies, 100 μl of either aspirin or AMB was added. For the combination studies, checkerboard assay was used to evaluate the antimicrobial efficacy of aspirin and AMB (acetylsalicylic acid [ASA]-AMB) upon combination as described above. We chose the appropriate ranges of drug concentrations for the combination studies according to the biofilm MICs of the individual drugs, which were markedly different from those of planktonic cells. The setup was incubated at 37°C and 75 rpm in an orbital shaker for another 24 h. Then, the biofilms would be quantified using an 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay (Sigma-Aldrich). All assays were repeated three times on two separate occasions.
Oxidative activity assay.
Quantitation of Candida biofilms was performed as described previously (10, 20) by using the XTT assay reduction assay. XTT was reduced by mitochondrial dehydrogenase into a water-soluble formazan product that was measured spectrophotometrically. XTT was dissolved in PBS (without glucose) at a final concentration of 1 mg/ml. The solution was filter sterilized and stored frozen at −70°C until use. A menadione solution (0.4 mM; Sigma-Aldrich) was prepared immediately before each assay. Anhydrous D(+) glucose was dissolved in sterile PBS to obtain PBS with 200 mM glucose. For each assay, the XTT solution was thawed on ice and mixed with menadione solution at a volume ratio of 20:1. After two washes with 200 μl of PBS to remove nonadherent cells, 158 μl of PBS with 200 mM glucose, 40 μl of XTT, and 2 μl of menadione was transferred to each well of 96-well plates, and then the plates were covered with aluminum foil and incubated in dark at 37°C for 3 h. Thereafter, 100 μl of the solution was transferred to each well of new 96-well plates. The colorimetric changes were measured at 492 nm using a microtiter plate reader.
Time-kill curve.
To further investigate the effect of concentration and exposure time on the drug activities and to assess the pharmacodynamics of each antimicrobial agent and the combines agents, we performed a time-kill test in vitro to investigate the activity of serially diluted aspirin alone or combined with AMB against the biofilms of CCA10, YEM30, and ATCC 22019. This methodology was first used in planktonic cells by Ernst et al. (13). We utilized it to study biofilm cells with some modifications. Briefly, a C. albicans suspension in YPD medium (107 cells/ml) was prepared, and mature biofilms in 96-well microtiter plates were developed as described previously. At 48 h of incubation, the medium was aspirated, and biofilms were washed twice with PBS, followed by the addition of 100 μl of fresh medium. Aspirin was used at concentrations of 0.25, 0.5, 1, 2.0, and 4.0 mg/ml for CCA10 and ATCC 22019 and at concentrations of 0.5, 2.0, 4.0, and 8.0 mg/ml for YEM30. AMB was used at 8.0 μg/ml for these strains (concentrations of 16 and 32 μg/ml were also used for YEM30). Time-kill studies (for example, CCA10) were conducted in 12 treatment groups: (i) control (no drugs added), (ii) ASA 0.25 (i.e., aspirin [ASA] at 0.25 mg/ml), (iii) ASA 0.5, (iv) ASA 1, (v) ASA 2, (vi) ASA 4, (vii) AMB 8, (viii) AMB 8 + ASA 0.25, (ix) AMB 8 + ASA 0.5, (x) AMB 8 + ASA 1, (xi) AMB 8 + ASA 2, and (xii) AMB 8 + ASA 4. Microtiter plates covered with aluminum foil were placed on an orbital shaker (75 rpm) and incubated at 37°C during the experiment. At predetermined time points (3, 6, 9, 12, 18, 24, 36, and 48 h), the media of the corresponding wells were aspirated and washed twice with PBS. Then, 200-μl aliquots of XTT-menadione solution were added. After 3 h of incubation, 100 μl of the XTT solution was transferred to corresponding well of new 96-well plates. Thereafter, the colorimetric changes were measured at 492 nm by microtiter plate reader.
Drug interaction modeling.
In order to assess the nature of the in vitro interactions between aspirin and AMB against each Candida strain, the data obtained by the spectrophotometric method were analyzed using two different models, FICI and ΔE, that have been used to characterize antifungal drug interactions 24, 40, 44. Among different models and approaches, the assumption of no interaction has a central position, since synergy and antagonism are defined as departures from this. In Loewe additivity (LA)-based models, the concentrations of the drugs, alone or in combination, that produce the same effect are compared. In the Bliss independence (BI)-based models, the estimates of the combined effect based on the effect of the individual drugs are compared to those obtained by the experiment. FICI and ΔE are nonparametric models based on LA theory and BI theory, respectively.
LA-based models (FICI).
The nonparametric approach is based on the FICI model expressed as ΣFIC = FICA + FICB = CAcomb/MICAalone + CBcomb/MICBalone, where MICAalone and MICBalone are the MICs of the drugs A and B when acting alone and CAcomb and CBcomb are concentrations of the drugs A and B at the iso-effective combinations, respectively. Among all ΣFICs calculated for each data set, the FIC index was determined as the ΣFICmin (the lowest ΣFIC) when the ΣFICmax (the highest ΣFIC) was <4; otherwise, the FIC index was determined as the ΣFICmax. Two MIC endpoints were used for the evaluation of each data set. The MIC-1 (MIC80) and MIC-2 (MIC50) were defined as the lowest drug concentrations showing 80 and 50% growth compared to the growth inhibition control of planktonic cells and biofilm cells, respectively. Off-scale MICs were converted to the next-highest or next-lowest doubling concentration. Finally, the median and the range of FICIs of the replicates were determined for MIC-1 and MIC-2. According to the work of Odds (31), for the interpretation of a single FICI, a value of ≥4 is usually considered antagonism, and a value of ≤0.5 is synergy, in all other cases indifference is concluded. In the present study, we interpreted the results according to the definition of Odds. In addition, isobolograms were plotted in order to visualize the departure from additivity (31).
BI-based models (ΔE model).
The BI theory is described by the equation Ii = (IA + IB) − (IA × IB), where Ii is the predicted percentage of inhibition of the theoretical noninteractive combination of the drugs A and B and IA and IB are the experimental percentages of inhibition of each drug acting alone, respectively. Since I = 1 − E, where E is the percentage of growth, by substituting into the former equation, the following equation is derived: Ei = EA × EB, where Ei is the predicted percentage of growth of the theoretical noninteractive combination of the drugs A and B, respectively, and EA and EB are the experimental percentages of growth of each drug acting alone, respectively. Interaction is described by the difference (ΔE) between the predicted and measured percentages of growth with drugs at various concentrations (i.e., ΔE = Epredicted − Emeasured). Because of the nature of interaction testing using microtiter plates with 2-fold dilutions of either drug, this results in a ΔE value for each drug combination. A three-dimensional plot with the ΔE depicted on the z axis results in a surface plot.
For each combination of the two drugs in each of the three independent experiments, the observed percentage of growth obtained from the experimental data was subtracted from the predicted percentage, calculated as described above for each model. When the average difference was positive and its 95% confidence interval (CI) among the three replicates did not include 0, synergy was claimed; when the difference was negative and its 95% CI did not include 0, antagonism was claimed. In any other case, BI was concluded. The values thus obtained for each combination were used to construct a three-dimensional plot. Peaks above and below the 0 plane indicate synergistic and antagonistic combinations, respectively, while the 0 plane indicates the absence of SS interaction (see Fig. 2). The contour plots were also constructed in order to visualize the drug concentrations producing an interaction.
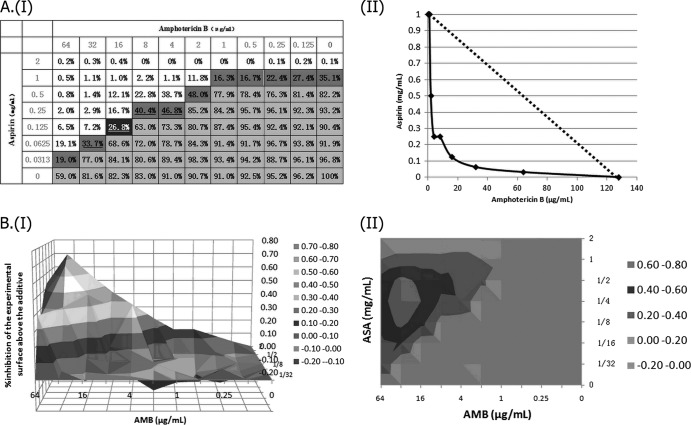
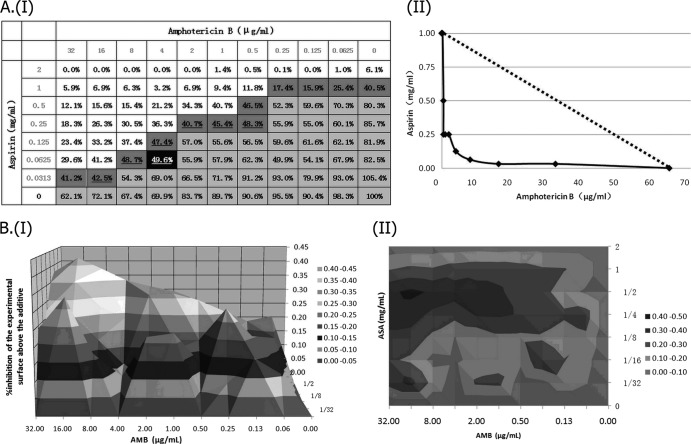
Fig 2.
Assessment of in vitro interaction between aspirin (ASA) and amphotericin B (AMB) against a standard C. albicans strain (YEM30) using LA-based models and BI-based models. (AI) Checkerboard showing the percentage of growth for each combination, combinations with >50% growth (light gray area), and the MIC-2 of aspirin (ASA) and amphotericin B (AMB) (percentages in boldface type), as well as the iso-effective combinations based on which the ΣFIC indices were calculated (dark gray area), combinations with ΣFIC indices lower than 0.5 (underlined percentages), and the combination with the lowest ΣFIC-2 index (0.25), corresponding to the FICI-2 (the percentage shown in the black cell). (AII) Corresponding isobologram with the additivity line (dashed line). (B) Three-dimensional (I) and contour (II) plots of the percent synergy calculated with the nonparametric approach, which resulted in 927% synergy. The difference between the predicted and measured percentages of fungal growth (ΔE = Epredicted − Emeasured) is shown on the z axis.
Since the plot only shows the interactions for each separate combination of the concentrations, a value is needed to summarize the interaction surface. This was achieved by calculating the sum percentage of all synergistic (ΣSyn) and antagonistic (ΣAnt) interactions. Interactions with <100% of interactions were considered weak, those with 100 to 200% of interactions were considered moderate, and those with 200% of interactions were considered strong. In addition, the numbers of synergistic and antagonistic combinations among all of the combinations tested were calculated for each strain.
RESULTS
Drug susceptibility alone and in combination.
CCA10, YEM30, and ATCC 22019 were selected in the present study. The MICs of two control strains, ATCC 22019 and ATCC 6528, were within the normal range. The results from the testing drug alone and in combination against the three tested microbial strains are summarized in Table 1. Testing the drug alone, in planktonic cells, showed that aspirin has weak effect on the tested strains and AMB has a strong fungicidal effect, whereas in biofilm cells, the highest level of resistance to AMB is observed, with the MIC-2 (50% inhibitory concentration [IC50]) to the corresponding strain increased up to 64- and 128-fold after biofilm formation, respectively, based on MICs determined by XTT assay. However, aspirin's fungistatic activity in biofilm cells seems to change little in comparison to planktonic cells, which is consistent with the previous report(s) and indicates dramatic antibiofilm activity. When it was combined with AMB a potent fungistatic effect was revealed, especially in biofilm cells. In terms of planktonic cells, the MICs of either individual agent were reduced by one to two dilutions against the tested strains, whereas remarked reductions were observed for AMB against biofilm cells when combined with aspirin. The geometric means of the MIC-2 to AMB and aspirin decreased up to 32- and 16-fold, respectively, based on the FICI values. The MIC-2 of AMB and aspirin to tested stains then shifted down to ca. 1 to 2 μg/ml and ca. 0.031 to 0.063 mg/ml, respectively. However, the MICs of AMB against biofilm cells were still a little higher than those against planktonic cells. The percentages of fungal growth for each combination were calculated by comparing the optical density (OD) of the drug-containing well to that of the drug-free well after subtracting the background OD obtained from the microorganism-free well. The interactions between AMB and aspirin against biofilm cells are depicted in Fig. 1, 2, and 3.
Table 1.
Susceptibilities of Candida strains against ASA alone and in combination with AMB as determined by the spectrophotometric methoda
| Drug combination (MIC) and strain | Median MIC (range) of drug |
|||
|---|---|---|---|---|
| Alone |
In combination |
|||
| ASA (mg/ml) | AMB (μg/ml) | ASA (mg/ml) | AMB (μg/ml) | |
| F/MIC-1 (MIC80) | ||||
| CCA10 | 2 (1-2) | 0.5 (0.25-0.5) | 1 (0.5-1) | 0.25 (0.125-0.25) |
| YEM30 | 4 (2-4) | 1 (0.5-1) | 2 (0.5-2) | 0.25 (0.125-0.25) |
| ATCC 22019 | 4 (2-4) | 2 (1-2) | 1 (1-2) | 0.5 (0.25-0.5) |
| BF/MIC-2 (MIC50) | ||||
| CCA10 | 1 (1-2) | >64 (>64) | 0.063 (0.031-0.5) | 4 (1-8) |
| YEM30 | 1 (1-2) | >128 (>128) | 0.125 (0.031-0.5) | 8 (2-32) |
| ATCC 22019 | 2 (1-2) | >64 (>64) | 0.063 (0.063-0.5) | 8 (1-16) |
The MICs of biofilm cells showed little change in comparison to planktonic cells for aspirin alone, whereas the MICs for the corresponding strain increased dramatically for AMB alone. When drugs were used in combination, for planktonic cells of the tested strains, the MICs of the cells showed no apparent changes based on the MIC-1, whereas for biofilm cells of the tested strains, the aspirin and AMB MICs decreased markedly based on the MIC-2. Abbreviations: ASA, aspirin; AMB, amphotericin B; F, planktonic cells; BF, biofilm cells.
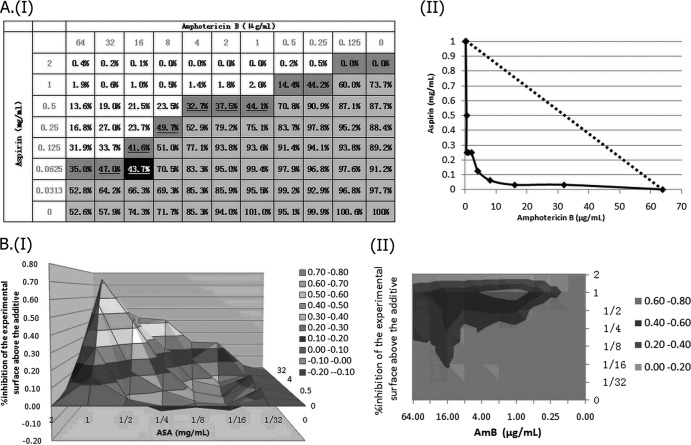
Fig 1.
Assessment of in vitro interaction between aspirin (ASA) and amphotericin B (AMB) against a standard C. parapsilosis strain (ATCC 22019) using LA-based models and BI-based models. (AI) Checkerboard showing the percentage of growth for each combination, combinations with >50% growth (light gray area), and the MIC-2 of aspirin (ASA) and amphotericin B (AMB) (percentages in boldface type), as well as the iso-effective combinations based on which the ΣFIC indices were calculated (dark gray area), combinations with ΣFIC indices lower than 0.5 (underlined percentages), and the combination with the lowest ΣFIC index (0.156), corresponding to the FICI (the percentage shown in the black cell). (AII) Corresponding isobologram with the additivity line (dashed line). (B) Three-dimensional (I) and contour (II) plots of the percent synergy calculated with the nonparametric approach, which resulted in 918% synergy. The difference between the predicted and measured percentages of fungal growth (ΔE = Epredicted − Emeasured) is shown on the z axis.
Fig 3.
Assessment of in vitro interaction between aspirin (ASA) and amphotericin B (AMB) against a clinical C. albicans strain (CCA10) using the LA-based models and BI-based models. (AI) Checkerboard showing the percentage of growth for each combination, combinations with >50% growth (light gray area), and the MIC-2 of aspirin (ASA) and amphotericin B (AMB) (the percentages in boldface type), as well as the iso-effective combinations based on which the ΣFIC indices were calculated (dark gray area), combinations with ΣFIC indices lower than 0.5 (underlined percentages) and the combination with the lowest ΣFIC-2 index (0.25), corresponding to the FICI-2 (the percentage shown in the black cell). (AII) Corresponding isobologram with the additivity line (dashed line). (B) Three-dimensional (I) and contour (II) plots of the percent synergy calculated with the nonparametric approach, which resulted in 1,225% synergy. The difference between the predicted and measured percentages of fungal growth (ΔE = Epredicted − Emeasured) is shown on the z axis.
Interpretation of drug interactions.
The results of the checkerboard analysis interpreted by the nonparametric methods based on both the LA and BI theories are summarized in Table 2. In the checkerboard microtiter plate format, strong synergism was observed in biofilm cells of all three strains analyzed by FICI and ΔE. The two models correlated very well. The FICI values were far less than 0.5, whereas all of the ΣSyn values of the three tested strains were beyond 900%, which indicates a super strong synergistic action between aspirin and AMB. As for the planktonic cells, synergisms were observed in ATCC 22019, the FICI is 0.5, the average ΔE was positive, and its 95% CI among three replicates did not include 0 and ΣSyn > 200%, and both of them showed a strong synergistic action. Indifference was observed in CCA10, the FICI index is 1.0, the 95% CI of the average ΔE included 0, and both of them revealed indifferent action. However, the interpretations for ATCC 22019 by two models seemed to be a little different. According to the definition of Odds, indifference was observed by FICI, whereas in the ΔE model the average ΔE was positive and its 95% CI among the three replicates did not include 0 and ΣSyn > 100%, which revealed a moderate synergistic action (Table 2). However, according to another standard, the FICIs of YEM30 in all three replicates were <1, and synergy can also be claimed. From that point, we also obtained consistent results by the two models. In addition, the values obtained for each combination based on BI theory were used to construct a three-dimensional plot. Thus, a surface plot was obtained by using a three-dimensional plot, with ΔE depicted on the z axis. The contour plot was also constructed in order to visualize the drug concentrations that produce an interaction (Fig. 1, 2, and 3).
Table 2.
In vitro interaction between ASA and AMB as determined by the nonparametric methoda
| Drug combination (MIC) and strain | FICI |
ΔE model (%) |
||||
|---|---|---|---|---|---|---|
| Mean (range) | Interpretation | Mean (range) | ΣSyn (n) | ΣAnt (n) | Interpretation | |
| F/MIC-1 (MIC80) | ||||||
| CCA10 | 1.000 (0.750-1.000) | Ind | 0.9 (−0.4-2.6) | 119 (11) | –74 (9) | Ind |
| YEM30 | 0.625 (0.563-0.750) | Ind | 2.3 (1.2-3.4) | 162 (15) | –16 (3) | Syn (M) |
| ATCC 22019 | 0.500 (0.375-0.500) | Syn | 4.5 (2.8-6.3) | 276 (15) | –8 (1) | Syn (S) |
| BF/MIC-2 (MIC50) | ||||||
| CCA10 | 0.125 (0.125-0.188) | Syn | 15.3 (12.8-17.8) | 1,225 (51) | 0 (0) | Syn (SS) |
| YEM30 | 0.250 (0.188-0.313) | Syn | 10.8 (6.5-15.1) | 927 (21) | 0 (0) | Syn (SS) |
| ATCC 22019 | 0.156 (0.156-0.1888) | Syn | 10.8 (6.6-14.9) | 918 (22) | 0 (0) | Syn (SS) |
F, planktonic cells; BF, biofilm cells; Syn, synergism; Ant, antagonism; Ind, indifference; (M), moderate synergism; (S), strong synergism; (SS), super strong synergism. For the FICI model, synergy was defined as a FICI of <0.5, antagonism was defined as a FICI of >4.0, and indifference was defined as a FICI of >0.5 to 4 (i.e., no interaction). For the ΔE model, ΣSyn and ΣAnt were the sums of the percentages of all statistically significant synergistic and antagonistic interactions. Interactions with 100% statistically significant interactions were considered weak synergism, those with 100 to 200% were considered moderate, and those with >200% were considered strong.
Time-kill curves.
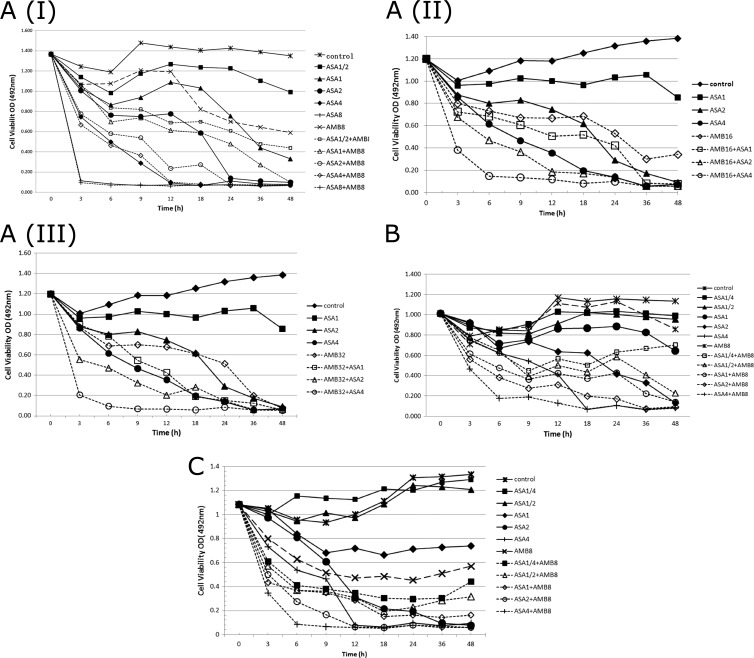
The concentration-dependent synergistic action of aspirin and AMB against biofilm cells that proved by checkerboard microdilution assay was confirmed by time-kill curves. Furthermore, the curves also revealed the time-dependent synergistic action between them. The time-kill curves, cell viability (i.e., the OD) versus time, were plotted and used for visual comparisons of the rate and extent of antifungal activity for aspirin and combination. The intensity and nature of the interactions between aspirin and AMB against YEM30, ATCC 22019, and CCA10 are shown in Fig. 4. With aspirin alone, although the rate of killing tested strains was gradual, it was heterogeneously changing versus time. Moreover, discernible improvement in the extent of fungistatic activity was noted as the amount of drug in solution was increased. Similarly, the rate of activity for each strain improved, and there was a trend toward a shorter time to the fungistatic endpoint as the concentration of aspirin in solution increased. Together, the curves reveal that AMB itself can hardly affect biofilm growth at 8 μg/ml over time, but the antibiofilm activity of aspirin against biofilm cells was dramatically enhanced by its addition. The combination of AMB and aspirin also resulted in a faster rate of killing, measured by the time necessary to achieve a fungicidal endpoint and the slope function of the curves in Fig. 4AI, B, and C. The slope of the curve for aspirin (4 mg/ml) against YEM30 was not influenced after the addition of AMB (8 μg/ml); however, the addition of AMB (16 or 32 μg/ml) to aspirin (4 mg/ml) enhanced its activity strikingly, which indicated that the intensity of interactions varies according to the drug dose. Moreover, when AMB was used alone, increasing the concentration of AMB in a small range displayed indifference in antifungal activity, whereas dramatic enhancement was found in combination with aspirin Fig. 4AI, II, and III. In summary, a strong concentration- and time-dependent synergistic action of ASA and AMB against Candida biofilms was found, and the most suitable therapeutic concentration of ASA and AMB for clinical use needs further optimization.
Fig 4.
(A) Representative time-kill curves of aspirin (ASA; 2-fold serially diluted) alone and in combination with amphotericin B (AMB) at 8 μg/ml (AI), 16 μg/ml (AII), or 32 μg/ml (AIII) against biofilm cells of a standard C. albicans strain (YEM30) versus time. (B) Representative time-kill curves of aspirin (ASA; 2-fold serially diluted) alone and in combination with amphotericin B (AMB; 8 μg/ml) against biofilm cells of a clinical strain of C. albicans (CCA10) versus time. (C) Representative time-kill curves of aspirin (ASA; 2-fold serially diluted) alone and in combination with amphotericin B (AMB; 8 μg/ml) against biofilm cells of a standard strain of C. parapsilosis (ATCC 22019) versus time.
DISCUSSION
C. albicans biofilm is a heterogeneous community of yeast, pseudohyphal, and hyphal cells embedded in an extracellular polymeric matrix, noticeably including soluble β-glucans (28). The formation of biofilms is intimately associated with C. albicans pathogenesis (34). C. albicans biofilms formed under clinical conditions display intrinsic resistance to antifungals. Consistent with these observations, a number of studies show that C. albicans biofilms obtained in vitro display decreased sensitivity to almost all available antifungals: amphotericin B (AMB), flucytosine, terbinafine, nystatin and, most notably, azoles (18). The 50% inhibitory concentrations (IC50s) are increased as much as 10-fold, while MICs are increased 30- to 20,000-fold; the highest level of resistance observed is that to azoles (7, 9).
The formation of biofilms as a source for antimicrobial treatment failure requires the discovery and development of new antimicrobial therapeutic agents. However, the efforts of identification and exploitation of novel targets and development of the related inhibitors have proved to be frustrating and not financially rewarding for the pharmaceutical industry, so the discovery of new medicinal value of the old classic drug is highly significant (43).
Recently, however, it has been reported that aspirin dramatically decreases biofilm formation by C. albicans in vitro (3, 48). Acetylsalicylic acid (ASA; aspirin) is a well-known nonsteroidal anti-inflammatory drug with analgesic, antipyretic, and anti-inflammatory properties. ASA significantly decreases C. albicans biofilm formation and reduces the viability of biofilm cells at concentrations that could be achieved in humans with therapeutic doses (3). Additional studies found that ASA also suppresses the biofilm formation of C. guilliermondii, C. kefyr, C. glabrata, and C. parapsilosis (41). Thus, it has been suggested to use ASA in combination with conventional antifungal agents in the management of biofilm-associated Candida infections (3).
In mammalian systems, arachidonic acid, formed by cleavage of phospholipids, is converted to prostaglandin H2 (PGH2) by the cyclooxygenase (COX) isoenzymes, COX-1 and COX-2. As a nonselective COX inhibitor, ASA inhibits COX-1 by hindering active-site accessibility by acetylating the serine-530 residue. Various COX inhibitors decreased biofilm formation by C. albicans, with aspirin producing the greatest effects (3). Biofilms of C. albicans produced much more prostaglandin than those of planktonic cells. In vivo, prostaglandin could significantly enhance fungal colonization and pathogenesis. The strong correlation between decreased prostaglandin level and decreased biofilm formation following exposure to COX inhibitors supports the notion that COX-dependent synthesis of prostaglandin may play a role in regulating biofilm development. However, the exact role of COX inhibitors and prostaglandin in fungal morphogenesis and biofilm development is obviously complex and remains unclear (4). Aspirin has also been verified to suppress the morphogenesis of C. albicans hyphae and filamentous structures by inhibiting the formation of 3(R)-hydroxyoxylipins, oxygenated fatty acid metabolites derived from arachidonic acid (11). Moreover, ASA may acetylate the active serine residue of the catalytic triad of the extracellular secreted lipase active site that prevents the nucleophilic residue from properly interacting with substrate, thus reducing damage to reconstituted human tissues infected with Candida species (46).
Clearly, it would be of interest to investigate combinations of antifungal agents and aspirin in Candida biofilm assays, with a view to their possible use in combined therapy for the management of some biofilm-associated infections. However, the growth of certain bacteria in the presence of salicylate can induce multiple resistances to antibiotics. Paradoxically, it can also reduce resistance to some antibiotics. The activities of antifungal agents can also be affected by salicylate. A combination of fluconazole with either sodium salicylate or ibuprofen results in synergistic activity against C. albicans planktonic cells (32, 39). Aspirin-EDTA combination has also shown synergistic activity against C. albicans biofilms and the exposure to the aspirin-EDTA combination (4 h) resulted in complete bacterial biofilm eradication (2). However, no results demonstrate that aspirin could be useful in combined therapy with conventional antifungal agents (such as amphotericin B) in the management of some biofilm-associated Candida infections in detail and whether the combination activity is synergistic or antagonistic. It is still unclear how aspirin can be used in related clinical application.
In the present study we applied checkerboard and time-kill curve experiments to Candida study through XTT reduction assay. We evaluated comprehensive interactions between aspirin and amphotericin B against planktonic cells and biofilm cells of ATCC 22019, YEM30, and newly clinically isolated C. albicans strains CCA10 by different methods and models. The XTT reduction assay is capable of detecting differences in the rate and the extent of antifungal activity and has been widely used in recent years (8, 14). XTT is a new yellow tetrazolium salt that can be converted to a colored formazan in the presence of metabolic activity. Since the formazan product is water soluble, it is easily measured in cellular supernatants, which is important in biofilm research because it allows the study of intact biofilms, as well as examination of biofilm drug susceptibility without disruption of biofilm structure (8). So, as an indicator substance, XTT makes it accessible to evaluate fungal biofilm growth. The interactions were evaluated with the checkerboard microdilution assay based on antifungal growth, the MIC50 was used as an endpoint for biofilm cells, while the MIC20 was used for planktonic cells. The results were interpreted by two nonparametric models, FICI, based on LA, and ΔE, based on BI no-interaction theories.
As we know, different results may be obtained by different methods. In recent years, considerable progress has been made in the methods of evaluating drug interactions. Spectrophotometry has been developed in recent years. These methods can quantify the fungal growth more precisely and are able to detect small changes in metabolic activity of fungi (25). Based on these methods, the effects of antifungal drugs either on the fungal biomass or on the metabolic status of fungi can be measured. FICI is the most frequently used model to interpret the interaction between antifungal drugs. Ease of use, simplicity, and feasibility of performance make FICI the method of choice for analyses of drug-drug interactions. However, assessing the nature of drug interactions using the FICI model presents several other problems besides the choice of the FIC indices and the MIC endpoints, such as the lack of a good summary and statistical interpretation of the results and the imprecise approximation of the real FICI when off-scale MICs are present. So, the ΔE model, as a nonparametric method based on BI theory, has also been developed as a useful approach in analyzing the nature of interactions between different drugs based on a checkerboard method. The model does not require the choice of the FICIs and MIC endpoints to obtain statistical measures and is less sensitive to intraexperimental errors than the FICI model. When they were used in assessing the interaction between aspirin and AMB in our study, the percentages of fungal growth were derived from the experimental data directly, and good agreement was found with the FICI model, particularly against biofilm cells. Therefore, it was sufficient to indicate the strong synergistic action between aspirin and AMB against biofilms of C. albicans and C. parapsilosis.
Data collected from time-kill studies have provided critical information regarding the rate and extent of bactericidal activity, pharmacodynamic characteristics (i.e., the relationship between concentration and effect and the postantibiotic effect), and potential antagonism or synergy between antibacterial agents administered concomitantly. These data that provide growth kinetic information over time and give a more detailed picture of the effect of drug combinations on cell viability have significantly enhanced our understanding of the dynamic relationships that exist between antimicrobial agents and their effects on bacteria (26). Our data from time-kill curves based on metabolism-inhibitory effects confirmed the positive interactions observed in the checkerboard test. The potency of all of the tested aspirin can be enhanced by combining them with AMB against C. albicans and C. parapsilosis. In aspirin alone, discernible improvement in the extent of fungistatic activity and the slope function of the time-kill curve to each strain was noted as the amount of drug in solution increased. Marked concentration-dependent fungistatic activity was also observed; moreover, the rate and extent of fungistatic activity varied over time and the time to achieve a fungistatic endpoint was shortened as the dose increased. In combination, the addition of AMB in low doses to various concentrations of aspirin resulted in striking improvement in the extent of activity and a trend toward a shorter time to the fungistatic endpoint versus the use of single agents. Thus, the combination therapy of AMB and aspirin can enhance their rate and extent of fungistatic activity and achieve maximal fungistatic activity in less time, whereas both drugs alone display weak fungistatic activity; this finding greatly enriched the meaning of combination therapy and produced significant therapeutic effects at a low concentrations (combination on concentration, reduced side effects, and improved efficacy) and in a short time (combination on time, reducing the time to fungistatic endpoint). Compared to animal models of infection, in vitro models offer significant advantages in cost, convenience, and time, as well as permitting direct investigation on the antimicrobial interaction in a controlled and reproducible manner. Time-kill testing can be an indispensable tool in the in vivo or in vitro study optimization of aspirin and AMB dosing regimens based on the antifungal pharmacodynamic properties described for these agents (38).
Extensive research has focused on the mechanisms of drug resistance in C. albicans biofilms. It is apparent that cells in a fungal biofilm represent an epigenetic modification of the cellular state compared to their planktonic counterparts, with changes in cellular morphology, cell-to-cell communication, and gene expression, as well as with the production of an extracellular matrix (15, 36). Multiple factors contribute to the elevated drug resistance of C. albicans biofilms. These factors include increased cell density, increased expression of drug efflux pumps, decreased ergosterol content, and elevated β-1,3 glucan levels in the cell wall and biofilm matrix, as well as signaling mediated by protein kinase C and the protein phosphatase calcineurin (36).
The pharmacological effects of aspirin on a mature biofilm remain unclear. Previous studies have shown that sodium salicylate inhibits biofilm formation and bacterial adhesion in a dose-dependent manner. It can also inhibit the production of some components of the extracellular polymeric matrix (ECM)—such as polysaccharides, proteins, and teichoic acid—by as much as 95% (3). C. albicans biofilms produce abundant ECM during biofilm growth under flow conditions which, in turn, increased resistance to antifungals (3, 5). It has been shown that, facilitated by the amphiphilic nature, AMB binds to β-1,3-glucans, a structural component of the fungal cell wall, as well as the biofilm ECM, and alters the β-1,3-glucans and increases the sensitivity of biofilms to AMB (3, 19). Although high concentrations of AMB could be reached within the biofilm, entrapment of AMB in the ECM might prevent them from reaching biofilm cells. Thus, despite accumulating in the biofilm at concentrations above the MIC, AMB is unable to kill biofilm cells completely (19). One explanation for the synergistic activity is likely that aspirin inhibits the production of ECM, especially β-1,3-glucans, and prevents them from binding with AMB, so high concentrations of AMB reach biofilm cells and it exert its antifungal effect. Decreased levels of ergosterol, whose biosynthesis is inhibited by azoles and which is bound by AMB, and diminished expression of ergosterol biosynthetic genes have also been reported in mature C. albicans biofilms (16, 19). It will be interesting to determine whether aspirin can disrupt the formation of mature biofilm and increase the content of ergosterol, which may increase the targets of AMB and result in synergistic activity. Exposure of C. albicans planktonic cells to azole antifungals is associated with the upregulation of genes that encode components of the ergosterol biosynthetic pathway and drug efflux pumps, thus providing a transient resistance mechanism (37). Genes encoding drug efflux pumps, namely, MDR1, CDR1, and CDR2, are upregulated upon attachment of C. albicans cells to a surface, which accounts for the resistance of early-stage biofilms to azole drugs. The upregulation in the caspofungin-exposed biofilms of several genes coding for cell wall proteins, in particular ALS3 and HWP1, plays an important role in biofilm formation (19, 27, 29, 49). Thus, the investigation of aspirin to biofilm resistance-related genes also helps explain the occurrence of synergistic activity. In addition, the increased activity shown by ASA-AMB combinations may also be explained by AMB, which can create a transmembrane channel and may therefore allow solutes such as aspirin to gain easier access and so demonstrate higher antimicrobial activity. Moreover, AMB's strong fungicidal effect can be very beneficial in counteracting the weak fungistatic activity of aspirin.
In summary, our work documented that the activity of AMB can be enhanced by aspirin, a weak and broad-spectrum antimicrobial agent, with surprisingly potent antibiofilm activity in vitro. The results were confirmed by the normal MIC test with different interpretation models and a time-kill study to demonstrate the combined effects against Candida, and the results also suggested different combination effects of the two drugs on planktonic cells and biofilm cells. Although a few assumptions have been offered here, further study is needed to determine the underlying mechanism of the synergistic action. However, this work leaves the door open for the clinical use of aspirin in combination with traditional antifungal drug (AMB) to treat infections based on biofilms in vivo, in vitro, and in situ, especially for superficial infections, where candidal biofilms colonizes several tissues, such as skin, vaginal, and oral epithelia, or inert surfaces, such as dental prostheses. However, the possibility of applying this combination therapy in the management of systemic fungal infections warrants further investigation.
ACKNOWLEDGMENTS
This study was supported by grants from the Natural Science Foundation of Shandong Province, China (ZR2009CM073, ZR2009CZ001, and ZR2009CM002), from the National Natural Science Foundation of China (81171536, 30972775, 30971151, and 81170514), and from the National Basic Research Program of China (973 Program 2012CB911202).
The analysis of drug interaction modeling was kindly guided by Qiongjie Guo (Department of Pharmacy, First Hospital of Qinhuangdao, Qinhuangdao, China). The standard strain of C. albicans (YEM30) was kindly provided by Qingguo Qi (Department of Oral Medicine, the Key Laboratory of Oral Biomedicine of Shandong Province, China).
Footnotes
Published ahead of print 5 March 2012
REFERENCES
- 1. Afeltra J, Vitale RG, Mouton JW, Verweij PE. 2004. Potent synergistic in vitro interaction between nonantimicrobial membrane-active compounds and itraconazole against clinical isolates of Aspergillus fumigatus resistant to itraconazole. Antimicrob. Agents Chemother. 48:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Bakri AG, Othman G, Bustanji Y. 2009. the assessment of the antibacterial and antifungal activities of aspirin, EDTA, and an aspirin-EDTA combination and their effectiveness as antibiofilm agents. J. Appl. Microbiol. 107:280–286 [DOI] [PubMed] [Google Scholar]
- 3. Alem MA, Douglas LJ. 2004. Effects of aspirin and other nonsteroidal anti-inflammatory drugs on biofilms and planktonic cells of Candida albicans. Antimicrob. Agents Chemother. 48:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alem MA, Douglas LJ. 2005. Prostaglandin production during growth of Candida albicans biofilms. J. Med. Microbiol. 54:1001–1005 [DOI] [PubMed] [Google Scholar]
- 5. Al-Fattani MA, Douglas LJ. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999–1008 [DOI] [PubMed] [Google Scholar]
- 6. Chapman SW, Sullivan DC, Cleary JD. 2008. In search of the holy grail of antifungal therapy. Trans. Am. Clin. Climatol. Assoc. 119:197–216 [PMC free article] [PubMed] [Google Scholar]
- 7. Cowen LE, Steinbach WJ. 2008. Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot. Cell 7:747–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA. 2003. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 41:506–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. d'Enfert C. 2006. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr. Drug Targets 7:465–470 [DOI] [PubMed] [Google Scholar]
- 10. Da Silva WJ, et al. 2008. Improvement of XTT assay performance for studies involving Candida albicans biofilms. Braz. Dent. J. 19:364–369 [DOI] [PubMed] [Google Scholar]
- 11. Deva R, Ciccoli R, Kock L, Nigam S. 2001. Involvement of aspirin-sensitive oxylipins in vulvovaginal candidiasis. FEMS Microbiol. Lett. 198:37–43 [DOI] [PubMed] [Google Scholar]
- 12. Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ernst EJ, Klepser ME, Pfaller MA. 1998. In vitro interaction of fluconazole and amphotericin B administered sequentially against Candida albicans: effect of concentration and exposure time. Diagn. Microbiol. Infect. Dis. 32:205–210 [DOI] [PubMed] [Google Scholar]
- 14. Ernst EJ, Roling EE, Petzold CR, Keele DJ, Klepser ME. 2002. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 46:3846–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Finkel JS, Mitchell AP. 2011. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 9:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Sańchez S, et al. 2004. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot. Cell 3:536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilbert P, Maira-Litran T, McBain AJ, Rickard AH, Whyte FW. 2002. The physiology and collective recalcitrance of microbial biofilm communities. Adv. Microb. Physiol. 46:203–256 [PubMed] [Google Scholar]
- 18. Govindsamy V, Rossignol T, d'Enfert C. 2010. Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to β-glucans. Antimicrob. Agents Chemother. 54:2096–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Granger BL, Flenniken ML, Davis DA, Mitchell AP, Cutler JE. 2005. Yeast wall protein 1 of Candida albicans. Microbiology 151:1631–1644 [DOI] [PubMed] [Google Scholar]
- 20. Jin Y, Samaranayake LP, Samaranayake Y, Yip HK. 2004. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch. Oral Biol. 49:789–798 [DOI] [PubMed] [Google Scholar]
- 21. Johnson RH, Einstein HE. 2007. Amphotericin B and coccidioidomicosis. Ann. N. Y. Acad. Sci. 1111:434–441 [DOI] [PubMed] [Google Scholar]
- 22. Laniado-Laborín R, Cabrales-Vargas MN. 2009. Amphotericin B: side effects and toxicity. Rev. Iberoam. Micol. 26:223–227 [DOI] [PubMed] [Google Scholar]
- 23. Mazur I, et al. 2007. Acetylsalicylic acid (ASA) blocks influenza virus propagation via its NF-κB-inhibiting activity. Cell Microbiol. 9:1683–1694 [DOI] [PubMed] [Google Scholar]
- 24. Meletiadis J, Mouton JW, Meis JFGM, Verweij PE. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meletiadis J, et al. 2001. Comparison of spectrophotometric and visual reading of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium hydroxide], for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. 2005. Combination treatment of invasive fungal infections. Clin. Microbiol. Rev. 18:163–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. 2003. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 71:4333–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nett J, et al. 2007. Putative role of β-1,3 glucans in Candida albicans biofilm resistance. Antimicrob. Agents Chemother. 51:510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nobile CJ, et al. 2006. Critical role of Bcr1-dependent adhesins in Candida albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Committee for Clinical Laboratory Standards 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. Document M27-A2 NCCLS, Wayne, PA [Google Scholar]
- 31. Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 32. Pina-Vaz C, et al. 2000. Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J. Med. Microbiol. 49:831–840 [DOI] [PubMed] [Google Scholar]
- 33. Polak A. 1999. The past, present and future of antimycotic combination therapy. Mycoses 42:355–370 [DOI] [PubMed] [Google Scholar]
- 34. Ramage G, Martinez JP, Lopez-Ribot JL. 2006. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 6:979–986 [DOI] [PubMed] [Google Scholar]
- 35. Ramage G, VandeWalle K, Wickes BL, Lopez-Ribot JL. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robbins N, et al. 2011. Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS Pathog. 7:e1002257 doi:10.1371/journal.ppat.1002257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanglard D, Odds FC. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73–85 [DOI] [PubMed] [Google Scholar]
- 38. Schuck EL, Derendorf H. 2005. Pharmacokinetic/pharmacodynamic evaluation of anti-infective agents. Expert Rev. Anti-Infect. Ther. 3:361–373 [DOI] [PubMed] [Google Scholar]
- 39. Scott EM, Tariq VN, McCrory RM. 1995. Demonstration of synergy with fluconazole and either ibuprofen, sodium salicylate, or propylparaben against Candida albicans in vitro. Antimicrob. Agents Chemother. 39:2610–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun, et al. 2008. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob. Agents Chemother. 52:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stepanovic S, Vukovic D, Jesic M, Ranin L. 2004. Influence of acetylsalicylic acid (aspirin) on biofilm production by Candida species. J. Chemother. 16:134–138 [DOI] [PubMed] [Google Scholar]
- 42. Stewart P, Costerton J. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138 [DOI] [PubMed] [Google Scholar]
- 43. Taha MO, Al-Bakri AG, Zalloum WA. 2006. Discovery of potent inhibitors of pseudomonal quorum sensing via pharmacophore modeling and in silico screening. Bioorg. Med. Chem. Lett. 16:5902–5906 [DOI] [PubMed] [Google Scholar]
- 44. Te Dorsthorst DT, et al. 2002. In vitro interaction of flucytosine combined with amphotericin B or fluconazole against thirty-five yeast isolates determined by both the fractional inhibitory concentration index and the response surface approach. Antimicrob. Agents Chemother. 46:2982–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trofa D, Gacser A, Nosanchuk JD. 2008. Candida parapsilosis, an emerging fungal pathogen. Clin. Microbiol. Rev. 21:606–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trofa D, et al. 2009. Acetylsalicylic acid (aspirin) reduces damage to reconstituted human tissues infected with Candida species by inhibiting extracellular fungal lipases. Microbes Infect. 11:1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang W, et al. 2003. Aspirin inhibits the growth of Helicobacter pylori and enhances its susceptibility to antimicrobial agents. Gut 52:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu KK. 2000. Aspirin and salicylate: an old remedy with a new twist. Circulation 102:2022–2023 [DOI] [PubMed] [Google Scholar]
- 49. Zhao X, et al. 2006. Candida albicans Als3p is required for wild-type biofilm formation on silicone elastomer surfaces. Microbiology 152:2287–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]