Abstract
Coadministration of moxifloxacin and rifampin was evaluated in a murine model of Mycobacterium tuberculosis pulmonary infection to determine whether the finding of antagonism documented in a hollow-fiber infection model could be recapitulated in vivo. Colony counts were followed in a no-treatment control group, groups administered moxifloxacin or rifampin monotherapy, and a group administered a combination of the two agents. Following 18 days of once-daily oral administration to mice infected with M. tuberculosis, there was a reduction in the plasma exposure to rifampin that decreased further when rifampin was coadministered with moxifloxacin. Pharmacodynamic analysis demonstrated a mild antagonistic interaction between moxifloxacin and rifampin with respect to cell kill in the mouse model for tuberculosis (TB). No emergence of resistance was noted over 28 days of therapy, even with monotherapy. This was true even though one of the agents in the combination (moxifloxacin) induces error-prone replication. The previously noted antagonism with respect to cell kill shown in the hollow-fiber infection model was recapitulated in the murine TB lung model, although to a lesser extent.
INTRODUCTION
Shortening the duration of chemotherapy for Mycobacterium tuberculosis is a critical issue, as a long duration makes therapy difficult and reduces patient compliance. Recently, we studied the combination of rifampin and moxifloxacin in a hollow-fiber infection model in log-phase growth as well as with respect to the Non-Replicating Persistence (NRP) phenotype (2). We demonstrated that the combination of moxifloxacin plus rifampin suppressed emergence of resistance quite efficiently. We also demonstrated that the combination was mildly but statistically significantly antagonistic with regard to cell kill. While the hollow-fiber system (HFS) is a valuable evaluation tool, making it possible to quickly elucidate the pharmacodynamics of antibacterial agents alone and in combination, it completely lacks an immune system, as well as the barriers associated with the lesions that drugs need to penetrate to access the bacteria in the disease state. Consequently, we extended those observations to a standard Mycobacterium tuberculosis murine challenge model that we have previously employed (9, 11). We evaluated moxifloxacin and rifampin alone and in combination for a 28-day treatment duration and developed information about cell kill rates and the emergence of resistance.
MATERIALS AND METHODS
Much of the methodology employed here was published by us previously (9, 11).
Reagents.
Moxifloxacin was purchased from Sai Quest Laboratories, Hyderabad, India. Moxifloxacin stock solutions were made in distilled water (0.02 N NaOH in water). Hydroxypropylmethylcellulose (HPMC) (lot no. 1240274) was purchased from Fluka Biochemika. EDTA (lot no. 5-4514) was purchased from Hi-Media Laboratories, Mumbai, India. Acetonitrile (high-performance-liquid-chromatography [HPLC] grade) was obtained from Spectrochem Pvt. Ltd., Mumbai, India.
Microbial cultures and cell lines.
The challenge strain, M. tuberculosis H37Rv (ATCC 27294), was prepared for animal infection studies as described previously (9). The inoculum used for all of the experiments was derived from a single seed lot maintained at −70°C. This was made from infected mouse lungs, after which a single round of broth amplification was performed. M. tuberculosis H37Rv (ATCC 27294), a strain sensitive to all standard antituberculosis (anti-TB) agents, was grown in roller bottles in Middlebrook 7H9 broth supplemented with 0.2% glycerol, 0.25% Tween 80 (Sigma, St. Louis, MO), and 10% albumin dextrose catalase (Difco Laboratories) at 37°C for 7 to 10 days. Cells were centrifuged, washed in 7H9 broth, and then resuspended in fresh 7H9 broth. Aliquots (0.5 ml each) were dispensed, and the seed lot suspensions were stored at −70°C.
Animals.
The Institutional Animal Ethics Committee, which is registered with the government of India (registration no. CPCSEA 99/5), approved all animal experimental protocols and usage. Six- to 8-week-old BALB/c mice purchased from RCC Ltd., Hyderabad, India, were randomly assigned at five per cage, with the restriction that the weights of all cage members were within 1 to 2 g. The mice were allowed 2 weeks of acclimatization before intake into experiments. Feed and water were given ad libitum.
Pharmacokinetics of moxifloxacin in infected mice.
The pharmacokinetics (PK) of moxifloxacin and rifampin was analyzed in infected mice on day 18 after initiation of treatment in all the three dose groups, with rifampin administered alone at 10 mg/kg, moxifloxacin at 400 mg/kg, and the two drugs in combination at those doses. The drugs were administered orally as suspensions in 0.5% hydroxypropylmethylcellulose (HPMC). The dose of rifampin was given 2 h before the dose of moxifloxacin when the two were given in combination, since Weiner et al. have shown that these drugs interact in humans (12). Blood samples were collected by saphenous venipuncture at 0, 5, 15, 30, 60, 120, 180, 240, 480, and 576 min after dosing, and plasma was harvested as described previously (9, 11). Three animals were used per time point. Drug concentrations in plasma were determined by LC-mass spectroscopy (LC-MS). Pharmacokinetic analyses of the plasma concentration-time relationships were performed using WinNonLin software (Pharsight; version 5.2.1). A noncompartmental library model (model 200) was used to calculate the area under the concentration-time curve from time zero to infinity (AUC0-∞). The fAUC0-∞ values were obtained by multiplying the total AUC0-∞ values by the fraction unbound with respect to moxifloxacin (0.5; see reference 11) or rifampin (0.15; see reference 9). Free-drug AUC/MIC ratios were calculated by dividing the fAUC0-∞ by the MIC value for each drug (0.5 mg/liter for moxifloxacin and 0.1 mg/liter for rifampin [6, 9]).
Aerosol infection experiment in mice.
Mice were exposed to a Mycobacterium tuberculosis challenge via the inhalation route, as described previously (9), in an aerosol infection chamber designed and constructed in the Mechanical Engineering Shop, University of Wisconsin—Madison. Treatment was initiated 28 days postinfection, when the bacterial burden was approximately 107 CFU/lung homogenate. There were 5 animals per group per time point, and treatment was administered orally 7 days per week for a period of 28 days. Groups were sacrificed on a weekly basis from day 0 through day 56. Doses administered were moxifloxacin monotherapy at 400 mg/kg and rifampin monotherapy at 10 mg/kg. These doses were also employed for the combination regimen. There was a matching no-treatment control group for each time point in the study.
Lungs were dissected free and homogenized in gel saline, the homogenized mixture was appropriately diluted, and 100 μl of the lung homogenate was plated on Middlebrook 7H11 agar plates. Samples were also plated on antibiotic-containing agar plates (rifampin at 4× MIC [0.4 mg/liter] and moxifloxacin at 4× MIC [2.0 mg/liter]). Colonies were counted at 28 days after plating for total counts and at 35 days for antibiotic-containing plates.
Determination of theoretical additive effect.
The null reference model employed was the Loewe additivity model (5). Because there is a pharmacokinetic interaction when moxifloxacin and rifampin are administered together, we multiplied the observed theoretical additive-effect time line value by the ratio of AUC/MIC at day 18 of the combination pharmacokinetics regimen for both moxifloxacin and rifampin. This is a very conservative evaluation and provides a high degree of certitude that a finding of antagonism is warranted (since the moxifloxacin exposure on day 18 in combination was marginally higher, we did not use this correction for that agent). The significance of the pharmacodynamic interaction was demonstrated by generating the lower 95% confidence boundary around the point estimate of the colony counts for the drugs in combination. When this boundary did not overlap the theoretical additive-effect time line, the interaction was determined to be statistically significant and antagonistic interaction was declared.
RESULTS
Oral exposure to rifampin and moxifloxacin.
Following once-daily dosing of rifampin, the AUC decreased from 110 mg · h/liter on day 1 to 31.6 mg · h/liter on day 18 for the infected mice (Table 1). When the drug was coadministered with moxifloxacin, oral exposure to rifampin was reduced further to 19.5 mg · h/liter. On the other hand, the moxifloxacin AUC did not change significantly after multiple-day dosing or in combination with rifampin. The free-drug oral exposures to these drugs are also reported in Table 1, following correction for the free fraction in plasma.
Table 1.
Pharmacokinetic/pharmacodynamic values for moxifloxacin and rifampin alone and in combination
| Treatment | MIC (mg/liter) | Fraction unbound | AUC0–24 (mg · h/liter) | SE (AUC) | fAUC/MIC (h) |
|---|---|---|---|---|---|
| Moxifloxacin alone (400 mg/kg) | 0.5 | 0.5 | 56 | 10.0 | 56 |
| Moxifloxacin alone (400 mg/kg) after 18 doses | 0.5 | 0.5 | 50 | 3.3 | 50 |
| Moxifloxacin (400 mg/kg) plus rifampin (10 mg/kg) after 18 doses | 0.5 | 0.5 | 59 | 5.4 | 59 |
| Rifampin alone (10 mg/kg) after 1 dose | 0.10 | 0.15 | 110.0 | 16.0 | 165 |
| Rifampin alone (10 mg/kg) after 18 dosesa | 0.10 | 0.15 | 31.6 | 5.6 | 47 |
| Rifampin (10 mg/kg) plus moxifloxacin (400 mg/kg) after 18 dosesb | 0.10 | 0.15 | 19.5 | 3.2 | 29 |
The rifampin AUC after multiple doses was 3- to 4-fold lower than that seen after a single dose (P < 0.01; Dunnett's multiple-comparison test).
The rifampin AUC after multiple doses was reduced further in the presence of moxifloxacin (P < 0.01; Dunnett's multiple-comparison test).
Pharmacodynamics of moxifloxacin and rifampin alone and in combination.
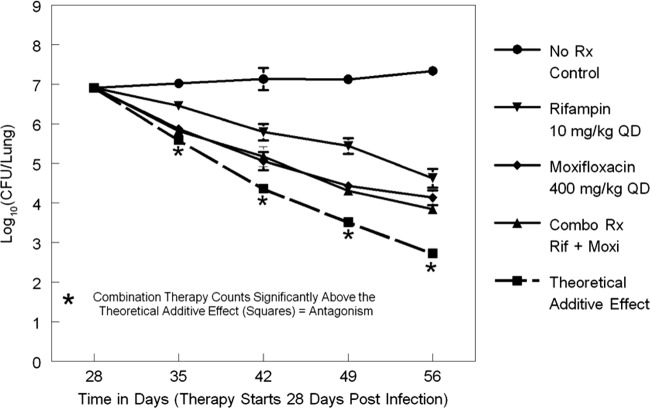
The lung colony counts of Mycobacterium tuberculosis for all treatment regimens as determined at the start of therapy (day 28) and weekly through day 56 are displayed in Fig. 1. The combination therapy effect on the lung colony counts closely followed that of moxifloxacin alone. The lower 95% confidence boundary did not overlap the theoretical additive interaction time line at days 35, 42, 49, and 56. This indicated that the rate of cell kill in the case of moxifloxacin-rifampin combination therapy is slowed over time and that the interaction is antagonistic.
Fig 1.
Colony counts for moxifloxacin and rifampin alone and in combination (Combo Rx Rif + Moxi) relative to a no-treatment control (No Rx Control) and also relative to a theoretical additive-effect time line. QD, once daily.
Lack of emergence of resistance to either moxifloxacin or rifampin.
None of the drug-containing plates had any countable M. tuberculosis colonies for moxifloxacin alone or for rifampin alone or for the two drugs in combination.
DISCUSSION
We had previously shown antagonism with respect to cell kill for the combination of moxifloxacin plus rifampin in a hollow-fiber bioreactor system (2). Since our hollow-fiber system lacks an immune response component, it was important to evaluate this combination in a standard murine model of Mycobacterium tuberculosis infection.
Combination therapy is central to achieving the goal of markedly shortening the current standard of a 6-month duration of therapy to 2 to 8 weeks. Optimal pharmacodynamically based drug-drug interaction is key to deriving a combination that not only achieves rapid cell kill leading to sterilization but also suppresses resistance. Moxifloxacin plus rifampin is one such combination that has garnered a lot of preclinical attention. It is important to recognize that, to achieve the dual goals of shortening the duration of therapy and suppressing resistance, the combination therapy must increase the rate of kill as well as suppress resistance. Suppressing resistance but slowing the rate of bacterial kill is unlikely to achieve the goal of shortening therapy.
In this efficacy study, it was observed that rifampin exposure in mice was reduced after administration of multiple doses and of the drug in combination with moxifloxacin whereas moxifloxacin exposure levels remained unchanged. Rifampin is reported to be a substrate of CYP3A4, and P-glycoprotein mediated the efflux pathway in mice (10). It is also possible that, in similarity to the response in humans, there could be autoinduction of rifampin and that the autoinduction might not related to CYP3A4 induction. It is uncertain whether higher activity of one or both of these two elimination pathways or autoinduction could be responsible for higher metabolism or reduced absorption of rifampin upon multiple dosing. Rifampin was reported to reduce moxifloxacin exposure in humans due to induction of phase II metabolic pathways such as sulfation of moxifloxacin (12). But there was no significant change in moxifloxacin PK in the mouse in the presence of rifampin.
Importantly, there is also a pharmacodynamic interaction between moxifloxacin and rifampin, with the combination regimen demonstrating a level of cell kill that closely followed that seen with the mice administered moxifloxacin alone. Figure 1 demonstrates that, relative to the theoretical additive-effect time line, the lower end of the 95% confidence boundary for the combination therapy group shows no overlap for days 35, 42, 49, and 56. This indicates a definite antagonistic interaction, although it should be noted that the magnitude of the antagonism was modest.
The drug combination described here is a valuable addition to the therapeutic armamentarium, as it provides excellent resistance suppression (2) but with a cell kill rate that is almost identical to that achieved with moxifloxacin alone. These findings are consistent with those seen in our previous hollow-fiber infection model experiments.
In HFS studies using NRP-phase M.tuberculosis H37Ra, it was observed that the combination of rifampin (600 mg) and moxifloxacin (400 mg) at human-equivalent doses was antagonistic with respect to cell kill compared to the respective monotherapies. Similarly, efficacy in the chronic mouse model for this combination was not better than that seen with moxifloxacin alone. In the HFS, rifampin alone was more effective than moxifloxacin alone; however, the reverse was seen in the mouse model. Inferiority of rifampin therapy in mouse could be due to a reduction in exposure upon multiple dosing as well as to the combination with moxifloxacin. The difference between the two systems with respect to the reported results could also be due to the fact that the murine system focused only on the first 28 days of treatment; thus, it is possible that not all of the bacterial population was in the NRP phase as employed in the HFS study.
Also of major interest is that there was absolutely no resistance emergence seen in either of the monotherapy arms throughout the duration of the experiment. This is despite the fact that moxifloxacin initiates error-prone replication in M. tuberculosis (8). It is possible to select resistant organisms from the murine model, as has been previously demonstrated by Ginsburg et al. (4) and Almeida et al. (1). However, in both instances, a concentration step was necessary for the culture prior to the spray so that bacterial burdens could meet or exceed a level of approximately 8 log10 (CFU/g). However, even with that step incorporated into the experiments, not all animals demonstrated resistance emergence during the course of the experiment. This is in contrast to the HFS, where it is possible to select resistant organisms by the use of a starting inoculum of 8 log10 CFU/ml. This is because the HFS lacks the innate immune mechanism present in a murine model, which is likely to play a role in the selection of such mutants.
As new drugs are finally coming on line for treatment of tuberculosis, the ability to determine doses that suppress resistance is of great value. While it is important to recognize that combination therapy would help suppress resistance, it must also be recognized that there would be occasions of pharmacokinetic mismatching or pharmacokinetic siloing because of differences in penetration (3). Therefore, prudence dictates that individual drug doses and schedules be designed as much as possible to help suppress resistance. The lack of resistance emergence in the mouse model under the conditions tested is not surprising, since the bacterial burden was only around 107 at onset of treatment, a time at which few, if any, spontaneous drug-resistant mutants would have been present. Further, coincident with the onset of adaptive immunity, multiplication is slowed down, which reduces generation of new mutants. The bacterial burden in a human patient with TB is far greater than the load achieved in mice lungs. Thus, the number of spontaneously occurring resistant mutants at the onset of treatment in humans is far greater. Indeed, this is a well-known limitation of the mouse model. Therefore, while the murine system is immensely valuable, examination of alternative systems, such as the hollow-fiber infection model, represents additional value because of the ability to explore resistance suppression regimens alone and in combination. It is also pertinent that the findings reported here are based on the use of one laboratory strain of M. tuberculosis; subsequent investigations performed with more strains would further strengthen the findings.
In summary, we have demonstrated in the murine system that the combination of moxifloxacin plus rifampin is mildly antagonistic with respect to cell kill, which is directly in line with our prior observation in the hollow-fiber model. We have also demonstrated that, when the standard technique that results in a burden of circa 107 CFU/g of lung homogenate was used, monotherapy was unable to amplify a resistant subpopulation over 28 days even though moxifloxacin incites error-prone replication, which is different from the results observed with the hollow-fiber system.
ACKNOWLEDGMENTS
This work was supported by the Bill and Melinda Gates Foundation through their TB Drug Accelerator project.
We have no conflicts of interest to divulge.
Footnotes
Published ahead of print 2 April 2012
REFERENCES
- 1. Almeida D, Nuermberger E, Tyagi S, Bishai WR, Grosset J. 2007. In vivo validation of the mutant selection window hypothesis with moxifloxacin in a murine model of tuberculosis. Antimicrob. Agents Chemother. 51:4261–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drusano GL, et al. 10 August 2010. The combination of rifampin plus moxifloxacin is synergistic for resistance suppression but is antagonistic for cell kill for Mycobacterium tuberculosis as determined in a hollow-fiber infection model. mBio 1:3 e00139-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drusano GL, et al. 2011. July 12 Effect of administration of moxifloxacin plus rifampin against Mycobacterium tuberculosis for 7 of 7 days versus 5 of 7 days in an in vitro pharmacodynamic system. mBio 2:e00108–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ginsburg AS, et al. 2005. Emergence of fluoroquinolone resistance in Mycobacterium tuberculosis during continuously dosed moxifloxacin monotherapy in a mouse model. Antimicrob. Agents Chemother. 49:3977–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greco WR, Bravo G, Parsons JC. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331–385 [PubMed] [Google Scholar]
- 6. Gumbo T, et al. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reference deleted.
- 8. O'Sullivan DM, Hinds J, Butcher PD, Gillespie SH, McHugh TD. 2008. Mycobacterium tuberculosis DNA repair in response to subinhibitory concentrations of ciprofloxacin. J. Antimicrob. Chemother. 62:1199–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayaram R, et al. 2003. Pharmacokinetics-pharmacodynamics of rifampicin in aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schuetz EG, Schinkel AH, Relling MV, Scheutz JD. 1996. P-glycoprotein: a major determinant of rifampicin-inducible expression of cytochrome P4503A in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 93:4001–4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shandil RK, et al. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob. Agents Chemother. 51:576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weiner M, et al. 2007. Effects of rifampin and multidrug resistance gene polymorphism on concentrations of moxifloxacin. Antimicrob. Agents Chemother. 51:2861–2866 [DOI] [PMC free article] [PubMed] [Google Scholar]