Abstract
We evaluated ceftobiprole against the well-characterized Enterococcus faecalis strain OG1RF (with and without the β-lactamase [Bla] plasmid pBEM10) in a murine urinary tract infection (UTI) model. Ceftobiprole was equally effective for Bla+ and Bla− OG1 strains, while ampicillin was moderately to markedly (depending on the inoculum) less effective against Bla+ than Bla− OG1 strains. These data illustrate an in vivo effect on ampicillin of Bla production by E. faecalis and the stability and efficacy of ceftobiprole in experimental UTI.
TEXT
Enterococci cause various infections, most commonly urinary tract infections (UTIs) (13, 16, 18, 20, 34). Ceftobiprole (BAL9141) is a new cephalosporin with broad in vitro activity against Gram-positive cocci, including Enterococcus faecalis (2, 4, 9, 15), and ceftobiprole medocaril (prodrug; BAL5788) has been shown to be active against vancomycin-resistant and β-lactamase-positive (Bla+) (penicillinase-producing) E. faecalis strains in a mouse peritonitis model and against staphylococci in endocarditis models (1, 7, 10, 11). Among pyrrolidinone-3-ylidenemethyl cephems, ceftobiprole exhibits good affinities for E. faecalis PBPs, which explains its in vivo and in vitro activity (1, 14). However, the efficacy of ceftobiprole against E. faecalis infection in a mouse UTI model has not been evaluated. The major goal of the present study was to evaluate the efficacy of ceftobiprole compared to that of ampicillin against strains of E. faecalis with and without a Bla-encoding plasmid and to assess a possible in vivo inoculum effect with ampicillin, which would suggest lower efficacy of ampicillin in a high-bacterial-density infection sites against a Bla+ strain and large amounts of Bla at the same infection sites. We also sought to determine if ceftobiprole would suffer an effect from large amounts of Bla at the same site(s).
OG1RF (referred to herein as Bla− OG1) (6, 26) is a rifampin- and fusidic acid-resistant strain of E. faecalis, and Bla+ OG1 contains the plasmid pBEM10 (25), encoding Bla and high-level gentamicin resistance. These strains were used in order to compare effect of Bla in the same E. faecalis host background. Ceftobiprole (BAL 9141), used for in vitro MICs, and ceftobiprole medocaril, used for in vivo experiments, were obtained from Johnson & Johnson (Raritan, NJ), and vancomycin and ampicillin were obtained from Sigma (St. Louis, MO). MICs were determined by following CLSI guidelines (8), with E. faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213 as controls. MICs of ampicillin and ceftobiprole for a standard inoculum (105 CFU/ml) and a high inoculum (107 CFU/ml) were also determined. All animal manipulations and 50% infective dose (ID50) determinations were done by our previously described methods (32, 33). For in vivo antibiotic testing, our standard inoculum of 105 CFU/mouse (≥100 times the calculated ID50) was used for Bla− OG1 and Bla+ OG1, and in the case of Bla+ OG1, a high inoculum of 107 CFU/mouse (10,000 times the calculated ID50) was also used to determine an in vivo “inoculum effect” against the beta-lactam antibiotics, i.e., ampicillin and ceftobiprole. Subcutaneous (s.c.) therapy commenced at 1 h postinoculation (1 hpi) based on reports showing that 1 h postinoculation is sufficient for kidney colonization and intracellular bacterial community formation in mouse bladders (19). Single doses of ceftobiprole medocaril and vancomycin (2-fold range from 6.25 to 50 mg/kg of body weight) were given 1 hpi, i.e., equivalent to 4.3 to 34.2 mg/kg of ceftobiprole (parent drug); this is similar to doses previously used for s.c. ceftobiprole in mice (3, 12) and generates concentrations achievable in humans with standard human dosing (31). Two doses of ampicillin (2-fold range from 12.5 to 200 mg/kg, s.c., 1 hpi and 2 hpi) were used to avoid any potential bias for ceftobiprole; levels achieved with 80 mg/kg, s.c., 1-h dosing interval has previously been shown (with ampicillin-sulbactam) to simulate ampicillin human doses of 3 g (24). An untreated but infected group of animals served as controls for each test bacterium, and the numbers of CFU of bacteria in kidneys and bladders obtained 48 h postinfection were compared between untreated and treatment groups (5, 21). The minimum detection limit of bacteria in these experiments was 102 CFU/gm. The 50% protective doses (PD50) were determined by the method of Reed and Muench (29), and protection was defined as no recovery of bacteria from kidney or bladder homogenates. Randomly selected colonies recovered from organs were tested by nitrocefin and/or by pulsed-field gel electrophoresis to confirm that they were the inoculated strains. The log10 CFU per gram of bacteria in tissues (kidneys and bladders) were analyzed for significance by the unpaired t test using Graph Pad Prism version 4.0 (GraphPad Software, San Diego, CA). The guidelines stipulated by the animal welfare committee of the University of Texas Health Science Center at Houston were followed (protocol HSC-AWC-09-023).
The MICs of ceftobiprole against Bla− OG1 and Bla+ OG1 with 105 CFU/ml were 1 μg/ml and 0.5 μg/ml, while the ampicillin MICs were 1 and 4 μg/ml with 105 CFU/ml, respectively (Table 1). The MICs of vancomycin against Bla− OG1 and Bla+ OG1 with 105 CFU/ml were 1 μg/ml. At 107 CFU/ml, ampicillin MICs were 1 and >128 μg/ml against Bla− OG1 and Bla+ OG1, respectively, and the ceftobiprole MIC was 1 μg/ml against both strains (Table 1). Since vancomycin is not a substrate for Bla, we did not test it at the higher inoculum.
Table 1.
PD50s of ceftobiprole and other antibiotics against E. faecalis strains Βla− OG1 and Βla+ OG1 in a mouse UTI model
| Strain | Inoculum (CFU) | Antibiotic | MIC (μg/ml) | No. of doses (time [h] postinoculation) | PD50 (mg/kg body wt) |
|
|---|---|---|---|---|---|---|
| Kidney | Bladder | |||||
| Βla− OG1 | 105 | Ceftobiprolea | 1 | 1 (1) | 10 | 18 |
| Ampicillin | 1 | 2 (1, 2) | 7 | 19 | ||
| Vancomycin | 1 | 1 (1) | 31 | 36 | ||
| Βla+ OG1 | 105 | Ceftobiprole | 0.5 | 1 (1) | 7 | 15 |
| Ampicillin | 4 | 2 (1, 2) | 44 | 66 | ||
| Vancomycin | 1 | 1 (1) | 27 | 38 | ||
| Βla+ OG1 | 107 | Ceftobiprole | 1 | 1 (1) | 33 | 31 |
| Ampicillin | >128 | 2 (1, 2) | >200 | >200 | ||
| Vancomycinb | ||||||
Ceftobiprole (BAL 9141) was used for in vitro MIC determinations, and ceftobiprole medocaril (prodrug; BAL5788) was used for in vivo experiments.
Vancomycin was not tested at the higher inoculum, since it is not known to be affected by E. faecalis Bla and the purpose was to evaluate an in vivo effect of Bla on ampicillin and test the stability of ceftobiprole.
In mice inoculated with Bla− OG1 (105 CFU), ampicillin (two doses) and ceftobiprole (one dose) showed almost equal PD50s, while vancomycin showed 3- to 4-times-higher PD50s for kidneys (Table 1). In mice inoculated with Bla+ OG1 (105 CFU), PD50s of ampicillin (two doses) were 4 to 6 times higher than those of ceftobiprole (Table 1) and those for Bla− OG1, while the PD50 for vancomycin was the same. Data for bladder were generally in agreement with those from kidneys but are not shown further here, since we and others have observed greater variability in bladder colonization than kidney colonization (22, 23, 33). For mice inoculated with Bla+ OG1 (107 CFU), ampicillin (two doses) PD50s were >6 times higher than those of ceftobiprole (Table 1). While ceftobiprole and ampicillin were equally effective against Bla− OG1 at 105 CFU, there was a 2- to 3-fold decrease in PD50 with ampicillin against 105 CFU of Bla+ OG1 versus Bla− OG1 and a 10- to >20-fold difference with ampicillin against 107 CFU versus 105 CFU of Bla+ OG1.
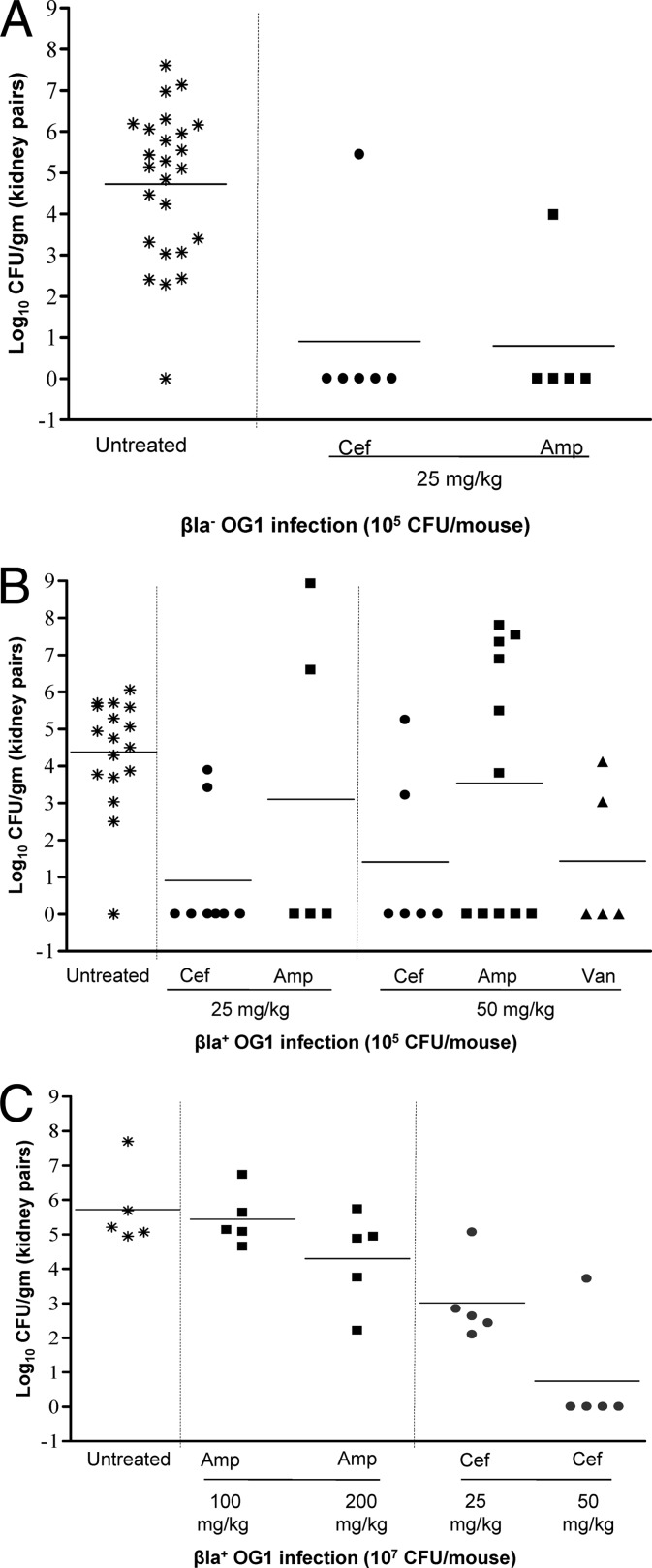
The reduction in CFU in kidneys with ceftobiprole and ampicillin is shown in Fig. 1A. In mice inoculated with Bla− OG1 (105 CFU), both ceftobiprole and ampicillin resulted in significantly reduced CFU in kidneys versus untreated animals at doses of 12.5 mg/kg (data not shown) and 25 mg/kg (P < 0.001 for ceftobiprole and ampicillin) (Fig. 1A) and were not significantly different from each other (P = 0.9). An in vivo effect on ampicillin was seen in Bla+ OG1-inoculated mice (Fig. 1B). In mice inoculated with 105 CFU of Bla+ OG1, ampicillin at 25 and 50 mg/kg showed nonsignificant differences in the number of CFU/g (P > 0.3) in kidneys versus untreated mice (Fig. 1B), while ceftobiprole showed a significant CFU/g reduction (P < 0.0001 and < 0.002) at both doses (Fig. 1B). Vancomycin showed significant CFU/g reduction (P < 0.002) in kidneys at 50 mg/kg versus untreated mice (Fig. 1B), even though this dose is lower than the dose reported to simulate concentrations achieved in humans (12, 30); data for 25 mg/kg vancomycin are not shown, since this is lower than the PD50. With 107 of Bla+ OG1, 100 and 200 mg/kg ampicillin showed nonsignificant differences in numbers of CFU/g (P = 0.1 and > 0.6, respectively) in kidneys versus untreated mice (Fig. 1C), while ceftobiprole showed significant CFU/g reduction versus ampicillin (P < 0.005 for 25 mg/kg ceftobiprole versus 100 mg/kg ampicillin; P < 0.005 and 0.006 for 50 mg/kg ceftobiprole versus 100 mg/kg and 200 mg/kg ampicillin, respectively) (Fig. 1C).
Fig 1.
Dose and inoculum effect in a mouse UTI model. (A) Bla− OG1 at an inoculum of 105 CFU. Bacterial counts from kidneys of mice treated with ceftobiprole (single 25-mg/kg dose) and ampicillin (two 25-mg/kg doses) and untreated controls are shown. Horizontal bars represent the geometric means (P < 0.001 for ceftobiprole and ampicillin at 25 mg/kg for all treated versus untreated control mice). (B) Bla+ OG1 at an inoculum of 105 CFU. Bacterial counts from kidneys of mice treated with ceftobiprole (single doses of 25 and 50 mg/kg), ampicillin (two doses of 25 and 50 mg/kg each), and vancomycin (single dose of 50 mg/kg) and untreated controls are shown. Horizontal bars represent the geometric means (P < 0.0001 and < 0.002 for ceftobiprole at 25 and 50 mg/kg, respectively, P > 0.3 for ampicillin at 25 and 50 mg/kg, and P < 0.002 for vancomycin at 50 mg/kg for all treated versus untreated control mice). (C) Bla+ OG1 at an inoculum of 107 CFU. Bacterial counts from kidneys of mice treated with ceftobiprole (single doses of 25 mg and 50 mg/kg) and ampicillin (two doses of 100 and 200 mg/kg each) and untreated controls are shown. Horizontal bars represent the geometric means (P < 0.005 for ceftobiprole at 25 mg/kg versus ampicillin at 100 mg/kg and P < 0.005 and 0.006 for ceftobiprole at 50 mg/kg versus ampicillin at 100 mg/kg and 200 mg/kg, respectively).
We previously showed that the β-lactamase enzyme in E. faecalis is identical to the type A staphylococcal enzyme (25, 35), and ceftobiprole has been reported to be a poor substrate for type A S. aureus enzyme (PC1) (28). Our recently published study using ceftobiprole and various cephalosporins against 98 clinical methicillin-susceptible S. aureus strains, representing four types of Bla, showed lower high- and standard-inoculum MICs of ceftobiprole than of other cephalosporins (27), reflective of the stability of ceftobiprole to staphylococcal β-lactamases, including type A. The failure of ampicillin against high inocula of Bla+ OG1 is similar to an observation made in a rat endocarditis model, where high Bla+ E. faecalis density in vegetations showed a biological effect with ampicillin therapy, even though the bacteria were susceptible in vitro at a standard inoculum (17).
In conclusion, we observed an in vivo effect of the E. faecalis β-lactamase and ampicillin treatment failure in the mouse UTI model, while ceftobiprole was efficacious in animals even when a high inoculum of Bla+ E. faecalis was used. Our findings suggest that ceftobiprole may have potential against urinary tract infections caused by antibiotic-resistant E. faecalis strains and support its further investigation against such infections.
ACKNOWLEDGMENTS
This work was supported by a grant from Johnson & Johnson to The University of Texas Health Science Center at Houston.
We thank Karen Jacques-Palaz and L. Charlene Thomson for their technical assistance.
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Arias CA, Singh KV, Panesso D, Murray BE. 2007. Evaluation of ceftobiprole medocaril against Enterococcus faecalis in a mouse peritonitis model. J. Antimicrob. Chemother. 60:594–598 [DOI] [PubMed] [Google Scholar]
- 2. Arias CA, Singh KV, Panesso D, Murray BE. 2007. Time-kill and synergism studies of ceftobiprole against Enterococcus faecalis, including beta-lactamase-producing and vancomycin-resistant isolates. Antimicrob. Agents Chemother. 51:2043–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azoulay-Dupuis E, et al. 2004. Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia. Antimicrob. Agents Chemother. 48:1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berenger R, Bourdon N, Auzou M, Leclercq R, Cattoir V. 2011. In vitro activity of new antimicrobial agents against glycopeptide-resistant Enterococcus faecium clinical isolates from France between 2006 and 2008. Med. Mal. Infect. 41:405–409 [DOI] [PubMed] [Google Scholar]
- 5. Blango MG, Mulvey MA. 2010. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob. Agents Chemother. 54:1855–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourgogne A, Hilsenbeck SG, Dunny GM, Murray BE. 2006. Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J. Bacteriol. 188:2875–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chambers HF. 2005. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:884–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. CLSI 2005. Performance standard for antimicrobial susceptibility testing; 15th informational supplement, M100–S15. Clinical and Laboratory Standards Institute, Wayne, Pa [Google Scholar]
- 9. Deshpande LM, Jones RN. 2003. Bactericidal activity and synergy studies of BAL9141, a novel pyrrolidinone-3-ylidenemethyl cephem, tested against streptococci, enterococci and methicillin-resistant staphylococci. Clin. Microbiol. Infect. 9:1120–1124 [DOI] [PubMed] [Google Scholar]
- 10. Entenza JM, Hohl P, Heinze-Krauss I, Glauser MP, Moreillon P. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Entenza JM, et al. 2011. In vivo synergism of ceftobiprole and vancomycin against experimental endocarditis due to vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 55:3977–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandez J, et al. 2010. In vivo activity of ceftobiprole in murine skin infections due to Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:116–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guiton PS, Hung CS, Hancock LE, Caparon MG, Hultgren SJ. 2010. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 78:4166–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hebeisen P, et al. 2001. In vitro and in vivo properties of Ro 63–9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henry X, Amoroso A, Coyette J, Joris B. 2010. Interaction of ceftobiprole with the low-affinity PBP 5 of Enterococcus faecium. Antimicrob. Agents Chemother. 54:953–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hidron AI, et al. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 17. Hindes RG, et al. 1989. Treatment of experimental endocarditis caused by a beta-lactamase-producing strain of Enterococcus faecalis with high-level resistance to gentamicin. Antimicrob. Agents Chemother. 33:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hooton TM, Stamm WE. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. North Am. 11:551–581 [DOI] [PubMed] [Google Scholar]
- 19. Hung CS, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat. Protoc. 4:1230–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huycke MM, Sahm DF, Gilmore MS. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4:239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hvidberg H, et al. 2000. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 44:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kau AL, et al. 2005. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73:2461–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kemp KD, Singh KV, Nallapareddy SR, Murray BE. 2007. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect. Immun. 75:5399–5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lister PD, Sanders CC. 1995. Comparison of ampicillin-sulbactam regimens simulating 1.5- and 3.0-gram doses to humans in treatment of Escherichia coli bacteremia in mice. Antimicrob. Agents Chemother. 39:930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murray BE, Mederski-Samaroj B. 1983. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J. Clin. Invest. 72:1168–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murray BE, et al. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nannini EC, et al. 2010. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 54:2206–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Queenan AM, Shang W, Kania M, Page MG, Bush K. 2007. Interactions of ceftobiprole with beta-lactamases from molecular classes A to D. Antimicrob. Agents Chemother. 51:3089–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reed LJ, Muench H. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493–497 [Google Scholar]
- 30. Reyes N, et al. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:4344–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitt-Hoffmann A, et al. 2004. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2576–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh KV, Lewis RJ, Murray BE. 2009. Importance of the epa locus of Enterococcus faecalis OG1RF in a mouse model of ascending urinary tract infection. J. Infect. Dis. 200:417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singh KV, Nallapareddy SR, Murray BE. 2007. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J. Infect. Dis. 195:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stickler DJ. 2008. Bacterial biofilms in patients with indwelling urinary catheters. Nat. Clin. Pract. Urol. 5:598–608 [DOI] [PubMed] [Google Scholar]
- 35. Zscheck KK, Murray BE. 1991. Nucleotide sequence of the beta-lactamase gene from Enterococcus faecalis HH22 and its similarity to staphylococcal beta-lactamase genes. Antimicrob. Agents Chemother. 35:1736–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]