Abstract
Antimicrobial peptides (AMPs) have been considered alternatives to conventional antibiotics for drug-resistant bacterial infections. However, their comparatively high toxicity toward eukaryotic cells and poor efficacy in vivo hamper their clinical application. OH-CATH30, a novel cathelicidin peptide deduced from the king cobra, possesses potent antibacterial activity in vitro. The objective of this study is to evaluate the efficacy of OH-CATH30 and its analog OH-CM6 against drug-resistant bacteria in vitro and in vivo. The MICs of OH-CATH30 and OH-CM6 ranged from 1.56 to 12.5 μg/ml against drug-resistant clinical isolates of several pathogenic species, including Escherichia coli, Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus. The MICs of OH-CATH30 and OH-CM6 were slightly altered in the presence of 25% human serum. OH-CATH30 and OH-CM6 killed E. coli quickly (within 60 min) by disrupting the bacterial cytoplasmic membrane. Importantly, the 50% lethal doses (LD50) of OH-CATH30 and OH-CM6 in mice following intraperitoneal (i.p.) injection were 120 mg/kg of body weight and 100 mg/kg, respectively, and no death was observed at any dose up to 160 mg/kg following subcutaneous (s.c.) injection. Moreover, 10 mg/kg OH-CATH30 or OH-CM6 significantly decreased the bacterial counts as well as the inflammatory response in a mouse thigh infection model and rescued infected mice in a bacteremia model induced by drug-resistant E. coli. Taken together, our findings demonstrate that the natural cathelicidin peptide OH-CATH30 and its analogs exhibit relatively low toxicity and potent efficacy in mouse models, indicating that they may have therapeutic potential against the systemic infections caused by drug-resistant bacteria.
INTRODUCTION
Traditional antibiotics have been widely used, resulting in the emergence of many antibiotic-resistant strains worldwide (12, 29, 40). Three classes of antibiotic-resistant pathogens are emerging as major threats to public health: (i) methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant S. aureus (VRSA), (ii) multidrug-resistant (MDR) and pan-drug-resistant Gram-negative bacteria, and (iii) MDR and extensively drug-resistant strains of Mycobacterium tuberculosis (12). Thus, there is a vital need for new effective therapeutics to conquer infections caused by drug-resistant bacteria.
Cationic antimicrobial peptides (AMPs) have become important potential candidates for therapeutic agents and have been considered viable alternatives to conventional antibiotics (5, 42). AMPs are an abundant and diverse group of antibacterial molecules that have been identified in a variety of invertebrate, plant, and animal species (6). Although the exact mechanism of action of AMPs has not been elucidated, it is generally proposed that the cytoplasmic membrane is the main target of most of these peptides. The increased permeability and loss of the barrier function as a result of damage to the membrane are primarily responsible for the bactericidal activity of AMPs (12, 35). The development of resistance to AMPs would be difficult because substantial changes in the lipid composition of the cellular membranes of microorganisms would be required (41). Although AMPs have been actively studied for many years, widespread clinical use has not yet occurred (16). The main challenge to the use of AMPs in systemic therapy is their high toxicity and poor efficacy in vivo. Another disadvantage is the cost associated with their synthesis (32). Most AMPs under development were approved for topical application only (25, 35). Thus, the discovery and development of smaller AMPs with low toxicity is important for the clinical application of antimicrobial drugs.
The cathelicidin family of AMPs is a large and diverse group of peptides. Cathelicidins contain a conserved N-terminal domain, the cathelin domain, in the inactive precursor peptide (39). Many different cathelicidin peptides have been isolated from a variety of species, including cow, pig, rabbit, sheep, human, and mouse (3). They are primarily produced by neutrophils and epithelial cells and have potent antimicrobial activity against bacteria, fungi, and enveloped viruses in vitro (38). However, these cathelicidin peptides are highly toxic to eukaryotic cells and red blood cells. For example, at concentrations 3 to 5 times its MIC against Escherichia coli D21, the peptide LL-37 also exhibits cytotoxic activity toward eukaryotic cells (19). Although a few cathelicidin peptides have been shown to be effective in some in vivo studies, the efficacy dose is close to their toxicity dose (1, 27).
Recently, we reported the first cloning of three cathelicidins from the elapidae snakes Naja atra, Bungarus fasciatus, and Ophiophagus hannah. In contrast to most cathelicidin peptides, the cathelicidin derived from the king cobra (OH-CATH30) exhibited potent, broad-spectrum, salt-independent antimicrobial activities and no obvious hemolytic activity (43, 44). These features suggest that OH-CATH30 could be developed as a novel systemically administered antimicrobial agent. However, the efficacy and toxicity of OH-CATH30 in vivo remain unclear. In this study, to optimize the size of OH-CATH30 while maintaining its potent antibacterial activity, a novel peptide, OH-CM6, was designed based on the sequence of OH-CATH30. In addition, we investigated the efficacy of OH-CATH30 and its analogs against drug-resistant, clinically isolated pathogens in vitro and in two mouse models of infection.
MATERIALS AND METHODS
Materials and microorganisms.
Microorganisms were obtained from the First Affiliated Hospital of Kunming Medical College (China) and belonged to eight different species as follows: (i) E. coli ATCC 25922, E. coli ML-35P, and clinically isolated MDR E. coli strains 1 to 6; (ii) P. aeruginosa ATCC 27853, P. aeruginosa PA 01, and clinically isolated MDR P. aeruginosa strains 1 and 2; (iii) Haemophilus influenzae ATCC 49247 and ATCC 49766; (iv) Klebsiella pneumoniae ATCC 13883 and ATCC 700603; (v) Enterobacter cloacae ATCC 13047, an E. cloacae clinical strain, and an Enterobacter aerogenes clinical strain; (vi) S. aureus ATCC 25923, S. aureus ATCC 43300 (MRSA), and clinically isolated S. aureus strains 1 to 3; (vii) Enterococcus faecalis ATCC 29212; and (viii) Candida albicans ATCC 2002 and a C. albicans clinical strain. The identification of species of clinical isolates was confirmed with the Vitek 2 system (bioMérieux, France), and the susceptibility of clinical isolates was determined with the Kirby-Bauer disk diffusion method, in accordance with the Clinical and Laboratory Standards Institute (CLSI) 2009 guidelines (10). All bacteria were cultured in LB medium (10 g/liter tryptone, 5 g/liter yeast extract, and 5 g/liter NaCl, pH 7.4) at 37°C, and fungi were cultured in YPD broth (1% yeast extract, 2% peptone, 2% d-glucose) at 30°C unless otherwise indicated. Human red blood cells and serum were provided by the Yunnan Blood Center (Kunming, China). Cefoperazone sodium (CFP) was a product of the General Pharmaceutical Factory of Harbin Pharmaceutical Group (Harbin, China). Polymyxin B (PMB) and vancomycin (VAN) were purchased from Amresco. All other reagents were analytical grade and were obtained from commercial sources.
Peptide synthesis.
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES, the only cathelicidin peptide in humans), pexiganan (GIGKFLKKAKKFGKAFVKILKK-NH2, an AMP developed for the treatment of diabetic foot infection), and OH-CATH30 and its analogs were synthesized by solid-phase synthesis on an Applied Biosystems Model 433A peptide synthesizer according to the manufacturer's standard protocols as reported previously (44). Briefly, after cleavage and side chain deprotection, the crude synthetic peptide was purified by reverse-phase high-performance lipid chromatography (RP-HPLC). The purity of each synthetic peptide was greater than 95%. The identity and purity of each peptide were further confirmed by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Voyager).
Antimicrobial assay.
A microdilution assay was used to determine the MICs of the various agents as previously described with minimal modifications (43). In each well of a 96-well plate, 100 μl LB broth and bacteria (5 × 105 CFU/ml) were incubated with various concentrations of antimicrobials for 18 h at 37°C. In the antifungal activity assay, fungi with an initial concentration of 1 × 105 CFU/ml were cultured with various concentrations of antimicrobials in YPD broth for 18 h at 30°C. The viability of the bacteria and fungi was determined by plating dilutions on LB and YPD agar plates, respectively. The concentrations of the antimicrobials were the same for each of the tested bacterial strains in three independent experiments, and the MIC values were obtained without interexperiment variations and expressed as μg/ml.
Hemolytic assay.
A hemolytic assay was performed with human red blood cells in liquid medium as reported previously (4). Cells were washed three times with phosphate-buffered saline (PBS) by centrifuging for 5 min at 1,000 × g and resuspending in PBS. Serial dilutions of the peptides were added to the cell suspensions and incubated at 37°C for 4 h. Then, the cells were centrifuged, and the absorbance of the supernatant was measured at 540 nm. Minimum hemolysis and maximum hemolysis were determined by adding PBS or 1% Triton X-100 to the cell samples, respectively. All experiments were performed in triplicate. The percentage of hemolysis was calculated according to the following equation:
Cytotoxic activity.
The cytotoxic activity was determined with the MTT [3-(4-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma] assay (23). The human keratinocyte cell line HaCaT was obtained from the Kunming Cell Bank of Type Culture Collection at the Chinese Academy of Sciences. Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (Gibco) under an atmosphere of 5% CO2 at 37°C. Approximately 2 × 104 cells were seeded in 96-well tissue culture plates in 100 μl medium per well. Various concentrations of different samples were added and incubated with the cells for 48 h. At the end of the exposure, 10 μl of MTT solution (5 mg/ml) was added, and the cells were incubated for another 4 h at 37°C. The MTT-containing medium was removed, and 150 μl of dimethyl sulfoxide was added. Plates were read on a multiwell scanning spectrophotometer with a wavelength of 570 nm. Cell viability was expressed as a percentage of the control group, which was assumed to be 100%. All experiments were performed in triplicate.
Bacterial killing kinetics.
The bacterial killing kinetics were determined by measuring the changes in the viable bacterial counts after peptide treatment (20). Briefly, E. coli 25922 and E. coli clinical strain 1 were grown at 37°C in LB broth. Aliquots of exponentially growing bacteria were resuspended in fresh LB broth at approximately 5 × 105 CFU/ml and separately exposed to OH-CATH30 and OH-CM6 at 2× the MIC for 0, 5, 10, 30, 45, 50, and 60 min at 37°C. At the indicated time intervals, 10-μl aliquots were serially diluted, plated on LB agar plates, and incubated overnight at 37°C. The viable colonies were counted the next day.
Effect of OH-CATH on the bacterial cytoplasmic membrane.
The interaction of peptides with the bacterial membrane was determined with the membrane potential-sensitive cyanine dye 3,5-dipropylthiacarbocyanine (diSC3-5) (Sigma, USA) as described previously (36). Briefly, E. coli 25922 was grown at 37°C to midlogarithmic phase in LB broth. The cells were collected by centrifugation, washed once in 5 mM HEPES, 0.5 mM EDTA, and 20 mM glucose (pH 7.2), and resuspended in the same buffer to an optical density at 600 nm (OD600) of 0.05. The bacteria were incubated with diSC3-5 at a final concentration of 0.4 μM for 30 min. Then, 100 mM KCl was added to equilibrate the cytoplasmic and external K+ concentrations. OH-CATH30 or OH-CM6 was added at a final concentration of 0 to 100 μg/ml. The fluorescence intensity was monitored with a Perkin-Elmer fluorescence spectrophotometer (Perkin-Elmer Corp., Norwalk, CT) with excitation and emission wavelengths of 622 nm and 670 nm, respectively.
Effect of serum on the antibacterial activity of OH-CATH peptides.
The effect of serum on the antibacterial activity of OH-CATH30 and its analogs was evaluated with a microdilution assay and a radial diffusion assay. For the microdilution assay, the antimicrobial activity of peptides against E. coli 25922 and S. aureus 25923 was evaluated in the presence of 25% human serum, and the inhibition of bacterial growth was detected at OD600. The radial diffusion assay was performed as described previously (22). Briefly, E. coli 25922 was grown to midlogarithmic phase in LB broth. Then, 106 CFU of bacteria were added to 10 ml of underlay agarose gel consisting of 0.03% (wt/vol) tryptic soy broth (TSB) and 1% (wt/vol) low-electroendosmosis-type (low-EEO) agarose (Sigma). The underlay was poured into a petri dish. After the agarose solidified, 4-mm sample wells were punched in the underlay gel. Different peptides at a final concentration of 100 μg/ml were incubated with 100% human serum for 0 to 12 h. Aliquots were taken at different time intervals, and a 10-μl sample was added to each well. The samples were allowed to diffuse into the gel for 3 h at 37°C, and the underlay gel was then covered with 10 ml overlay (6% TSB, 1% low-EEO agarose). The antibacterial activity was visualized as a clear zone around each well after incubation overnight at 37°C. The antibacterial activity was carefully measured to the nearest 0.1 mm of the clear zone after subtracting the diameter of the well.
Animals.
Kunming mice and ICR mice weighting 18 to 22 g were used in this study. Animals were provided by the Animal Center of the Kunming Medical College. All animals were housed in individual cages under a constant temperature (22°C) and humidity with a 12-h light/dark cycle and had access to food and water ad libitum throughout the study. The mice were euthanized by CO2 or ether inhalation at the end of the experiments. All procedures, care, and handling of the animals were approved by the Ethics Committee of the Kunming Institute of Zoology at the Chinese Academy of Sciences.
In vivo studies.
The in vivo acute toxicity of peptides in male Kunming mice was assessed by a single intraperitoneal (i.p.) or subcutaneous (s.c.) injection at various doses (n = 4, 0.3 ml per mouse). The peptides were dissolved in sterile PBS, and the controls received injections of vehicle alone. The animals were directly inspected for adverse effects for 6 h, and mortality was recorded for 7 days. The 50% lethal dose (LD50) was estimated according to the up-and-down method (24).
Murine thigh infection model.
ICR mice were rendered neutropenic by an i.p. injection of 150 and 100 mg/kg of body weight cyclophosphamide on days 0 and 3, respectively, and infection was induced on day 4 (11). The left thighs of mice were injected with E. coli 25922 (approximately 5 × 105 CFU/thigh) prepared as described above. Groups of mice (n = 6) were treated immediately with a dose of 1, 10, or 20 mg/kg peptides or 40 mg/kg CFP via a single s.c. injection. Mice in the negative group were left untreated. At 16 h postinoculation, mice were humanely sacrificed by CO2 asphyxiation, and the thighs were harvested. The thighs were weighed and homogenized with a microelectric homogenizer at 2,000 rpm for 5 min in 1 ml PBS. To determine the bacterial load in the thighs, the homogenate was serially diluted (10-fold dilutions) in LB broth, and aliquots (100 μl) of each dilution were plated on LB agar plates. The plates were incubated for 18 h at 37°C, and the bacterial colonies were counted. To measure the concentration of tumor necrosis factor alpha (TNF-α) in thigh tissue, the thigh homogenate was clarified by centrifugation at 1,000 × g at 4°C for 5 min, and the supernatant was collected and stored at −80°C. The level of TNF-α was measured with a sandwich enzyme-linked immunosorbent assay (ELISA) (R&D) according to the manufacturer's instructions.
Murine bacteremia models.
Lethal bacteremia was produced in Kunming mice with an i.p. injection of 200 μl of a bacterial suspension containing 2.5 × 108 CFU of E. coli clinical strain 3 (a blood-isolated MDR strain). Mice were randomly assigned to one of six groups: (i) OH-CATH30, (ii) d-OH-CATH30, (iii) OH-CM6, (iv) d-OH-CM6, (v) CFP, and (vi) untreated. After inoculation, the mice received peptide (1 to 20 mg/kg) or CFP (40 mg/kg) treatment via i.p. injection at 1 h, 4 h, and 8 h (n = 10) or at 4 h and 8 h (n = 7). The mice that survived 12 h, 24 h, 72 h, and 7 days after treatment were recorded, and the survival rate was calculated. Two hours after the last dose, 20-μl blood samples were taken from the tail veins of the mice to determine the bacterial counts by plating serial dilutions on LB agar plates. The plates were incubated overnight at 37°C, and the bacterial colonies were counted on the following day. The limit of bacterial counts detected by this method was 100 CFU/ml in blood.
Statistical analysis.
MIC values and 50% lethal concentrations (LC50) are presented as the geometric means of results from three separate experiments. The mortality rates between groups were compared using Fisher's exact test, and the Kaplan-Meier analysis was used to compare survival. The significance of the differences in the mean viable bacterial counts was assessed with the Kruskal-Wallis test. Significance was accepted when the P value was <0.05.
RESULTS
Sequence characteristics.
OH-CATH30 is a 30-residue peptide with excellent antimicrobial activity and weak hemolytic activity (43). To reduce the cost of peptide synthesis, we designed a smaller peptide, OH-CM6, by removing 10 amino acids from the C-terminal region of OH-CATH30 and substituting some amino acids (Table 1). To compare the efficacy and toxicity of l- and d-OH-CATH, the d-peptides were also synthesized. The sequences of OH-CATH30 and its derivatives are shown in Table 1. OH-CATH30 and OH-CM6 contain all l-amino acids, while d-OH-CATH30 and d-OH-CM6 contain all d-amino acids.
Table 1.
Sequences of peptides used in this study
| Peptide | Sequencea | Mass (Da) | Net charge |
|---|---|---|---|
| OH-CATH30 | KFFKKLKNSVKKRAKKFFKKPRVIGVSIPF | 3,595.14 | 12 |
| d-OH-CATH30 | KFFKKLKNSVKKRAKKFFKKPRVIGVSIPF | 3,595.14 | 12 |
| OH-CM6 | KFFKKLKKAVKKGFKKFAKV | 2,398.12 | 10 |
| d-OH-CM6 | KFFKKLKKAVKKGFKKFAKV | 2,398.12 | 10 |
Italics indicate d-amino acids.
In vitro efficacy.
The broth microdilution method was used to test the in vitro efficacy of OH-CATH30 and its analogs against pathogens and to compare OH-CATH30 and its analogs with pexiganan, CFP, and VAN. Table 2 lists the effects of the synthetic peptides against the 27 tested strains, which belong to eight different species, including MDR clinical strains. OH-CATH30 and its analogs exhibited potent antibacterial activities against Gram-negative and Gram-positive bacteria in LB broth, including drug-resistant clinically isolated strains, and no significant difference was observed between the standard strains and the clinical isolates. OH-CATH30 exhibited the highest antibacterial activities against the 25 tested bacteria, with MIC values of 1.56 to 25 μg/ml. The antibacterial activity of OH-CM6 and the d-peptides was similar to that of OH-CATH30 except for E. cloacae and E. aerogenes. As expected, CFP exerted strong activity against the standard strain; however, it failed to inhibit the growth of the clinically isolated strains, even at 100 μg/ml. It is exciting that l- and d-enantiomers of OH-CATH30 and OH-CM6 possessed potent antibacterial potency also against S. aureus clinical strains that were resistant to CFP and VAN. However, the MICs of OH-CATH30 and its analogs against C. albicans were above 200 μg/ml, indicating that OH-CATH30 and its analogs selectively inhibit the growth of bacteria and not C. albicans.
Table 2.
Antimicrobial activities of peptides
| Strain | MICa (μg/ml) |
||||||
|---|---|---|---|---|---|---|---|
| OH-CATH30 | d-OH-CATH30 | OH-CM6 | d-OH-CM6 | Pexi-ganan | CFP | VAN | |
| Gram-negative bacteria | |||||||
| E. coli ATCC 25922 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 | 0.4 | – |
| E. coli ML-35p | 3.125 | 6.25 | 6.25 | 6.25 | 6.25 | 25 | – |
| E. coli clinical strain 1b | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | >100 | – |
| E. coli clinical strain 2b | 3.125 | 6.25 | 3.125 | 6.25 | 6.25 | >100 | – |
| E. coli clinical strain 3b | 1.56 | 3.125 | 1.56 | 1.56 | 6.25 | 100 | – |
| E. coli clinical strain 4b | 12.5 | 12.5 | 12.5 | 12.5 | 25 | >100 | – |
| E. coli clinical strain 5b | 3.125 | 3.125 | 6.25 | 3.125 | 6.25 | >100 | – |
| E. coli clinical strain 6b | 3.125 | 3.125 | 3.125 | 6.25 | 6.25 | 100 | – |
| E. cloacae ATCC 13047 | 25 | 50 | >100 | >100 | 100 | 3.13 | – |
| E. cloacae clinical strainb | 3.125 | 25 | >100 | >100 | 25 | >100 | – |
| E. aerogenes clinical strainb | 6.25 | 12.5 | >100 | >100 | 12.5 | >100 | – |
| P. aeruginosa ATCC 27853 | 6.25 | 25 | 12.5 | 12.5 | 12.5 | 3.13 | – |
| P. aeruginosa PA 01 | 6.25 | 6.25 | 12.5 | 12.5 | 6.25 | 3.13 | – |
| P. aeruginosa clinical strain 1b | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | >100 | – |
| P. aeruginosa clinical strain 2b | 6.25 | 6.25 | 6.25 | 12.5 | 12.5 | >100 | – |
| H. influenzae ATCC 49247 | 3.125 | 6.25 | 3.125 | 6.25 | 6.25 | 0.8 | – |
| H. influenzae ATCC 49766 | 3.125 | 6.25 | 6.25 | 6.25 | 6.25 | 0.4 | – |
| K. pneumoniae ATCC 13883 | 6.25 | 3.125 | 6.25 | 6.25 | 3.125 | 3.13 | – |
| K. pneumoniae ATCC 700603 | 3.125 | 6.25 | 3.125 | 6.25 | 12.5 | 3.13 | – |
| Gram-positive bacteria | |||||||
| S. aureus ATCC 25923 | 3.125 | 3.125 | 12.5 | 6.25 | 6.25 | 0.4 | 0.8 |
| S. aureus ATCC 43300 MRSA | 6.25 | 6.25 | 3.125 | 6.25 | 6.25 | 3.13 | 1.56 |
| S. aureus clinical strain 1b | 3.125 | 3.125 | 6.25 | 6.25 | 6.25 | >100 | 12.5 |
| S. aureus clinical strain 2b | 6.25 | 3.125 | 3.125 | 3.125 | 3.125 | >100 | 6.25 |
| S. aureus clinical strain 3 | 6.25 | 6.25 | 12.5 | 6.25 | 12.5 | 50 | 1.56 |
| E. faecalis ATCC 29212 | 25 | 50 | 25 | 50 | 25 | 6.25 | 1.56 |
| Fungi | |||||||
| C. albicans ATCC 2002 | >200 | >200 | >200 | >200 | 25 | – | – |
| C. albicans clinical strain | >200 | >200 | >200 | >200 | 50 | – | – |
A minimal level of 99.9% inhibition is indicated. >, no activity detected at the concentration indicated. –, not assayed.
Resistant to three or more of the following antibiotics: gentamicin, amikacin, piperacillin, imipenem, ceftazidime, and levofloxacin.
Toxicity of OH-CATH30 and its analogs.
The l- and d-enantiomers of OH-CATH30 and OH-CM6 had low hemolytic activity against human red blood cells, even when 400 μg/ml peptides was incubated for 4 h. In contrast, pexiganan and LL-37 yielded 42% and 58% hemolysis at this concentration, respectively (Fig. 1A). The cytotoxic activities of OH-CATH30 and its derivatives were tested against the HaCaT cell line (Fig. 1B). OH-CATH30 and d-OH-CATH30 exhibited the weakest cytotoxic activities, with LC50 above 200 μg/ml. The viability of the cells was approximately 60% after exposure to OH-CM6 and d-OH-CM6 at 200 μg/ml. However, 200 μg/ml pexiganan resulted in only 23% cell viability.
Fig 1.
Toxicity of OH-CATH30 and its analogs in vitro and in vivo. (A) Hemolytic activity of peptides. Peptides at various concentrations were incubated with human red blood cells for 4 h at 37°C. (B) Survival of the HaCaT cells after being treated with peptides for 48 h. (C) Acute toxicity of peptides in Kunming mice via a single i.p or s.c injection.
The preliminary acute toxicity of OH-CATH30 and its analogs in vivo was determined with a single i.p. or s.c. injection of peptide at a range of doses in Kunming mice (Fig. 1C). For i.p. injection, the LD50 of OH-CATH30 was approximately 120 mg/kg, which was three and four times higher than those of pexiganan (40 mg/kg) and PMB (30 mg/kg), respectively. The LD50 of OH-CM6 was 100 mg/kg, which was not significantly different than that of OH-CATH30. The toxicity of d-OH-CATH30 (LD50, 80 mg/kg) was slightly higher than that of OH-CATH30. None of the OH-CATH peptides investigated in this study had any obvious toxicity in Kunming mice, and no mortality occurred within 7 days after a single s.c. injection of 160 mg/kg, whereas the LD50 of pexiganan and PMB were 90 and 60 mg/kg, respectively.
OH-CATH30 and OH-CM6 kill E. coli quickly by disrupting the cytoplasmic membrane.
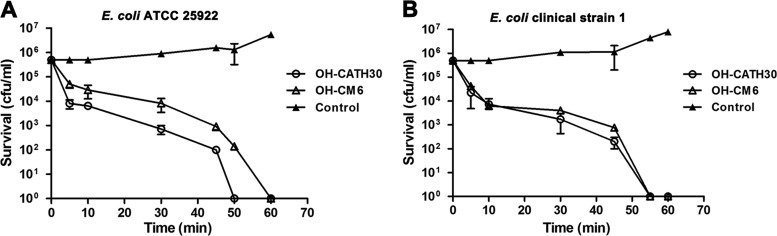
To investigate the preliminary mechanism of action of OH-CATH30, the bacterial killing kinetics of OH-CATH30 and OH-CM6 against E. coli 25922 and E. coli clinical strain 1 were investigated. Both peptides showed fast killing kinetics (Fig. 2A and B). At twice the MIC, the peptides quickly killed the bacteria and decreased the number of bacteria from 5 × 105 to approximately 1 × 104 CFU/ml within 10 min. The times needed for OH-CATH30 to induce 100% cell death were 50 and 55 min for E. coli 25922 and clinical strain 1, respectively, and OH-CM6 required 60 min to kill both strains.
Fig 2.
Killing kinetics of OH-CATH30 and OH-CM6. E. coli ATCC 25922 (A) and E. coli clinical strain 1 (B) (5 × 105 CFU/ml) were exposed to OH-CATH30 and OH-CM6 at 2× the MIC for 0, 5, 10, 30, 45, 50, and 60 min at 37°C.
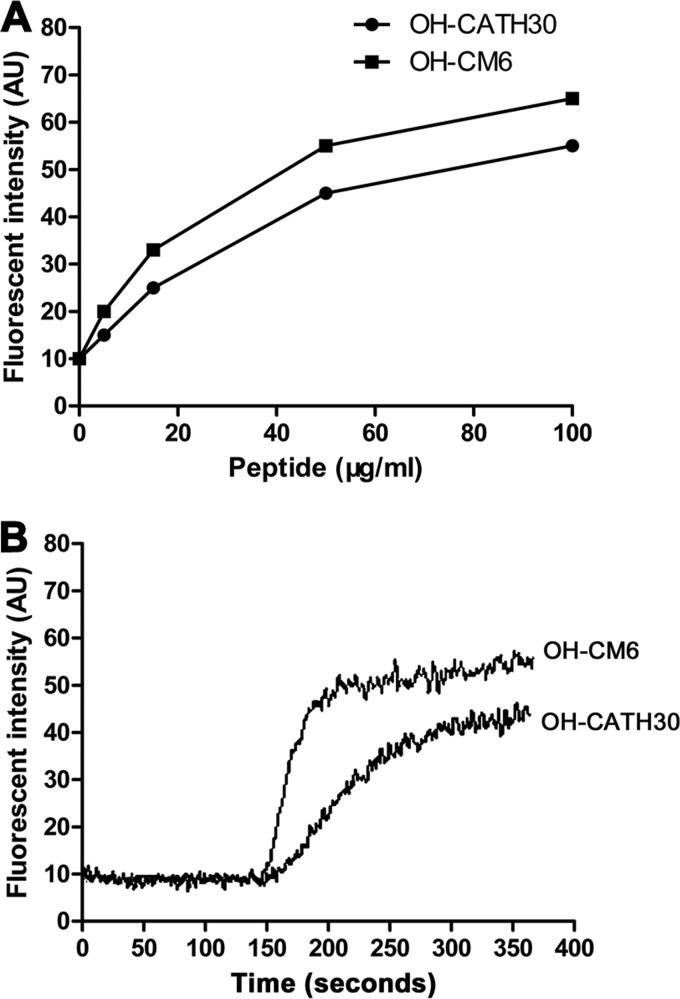
The fast killing kinetics of OH-CATH peptides were also supported by membrane disruption assays. The interaction of peptides with the bacterial cytoplasmic membrane was investigated by measuring the leakage of diSC3-5 dye from E. coli 25922. After incubation with E. coli (OD600, 0.05) at room temperature for 30 min, the dye entered the cytoplasmic membrane, and fluorescence decreased to 10% of its original levels. Both peptides caused leakage of the dye in a dose-dependent manner (Fig. 3A). OH-CATH30 at 50 μg/ml induced the release of 45% of the dye within 3 min, and OH-CM6 at 50 μg/ml induced the release of 55% of the dye within 1 min (Fig. 3B). These results demonstrate that the fast killing kinetics of OH-CATH30 and OH-CM6 may be due to permeabilization of the cytoplasmic membrane of E. coli.
Fig 3.
Effect of OH-CATH on the fluorescence intensity changes of E. coli 25922 incubated with diSC3-5. (A) OH-CATH30 and OH-CM6 result in the leakage of dye in a dose-dependent manner. (B) The kinetics of fluorescence intensity changes in the presence of OH-CATH30 and OH-CM6 at 50 μg/ml.
Effect of serum on the antibacterial activity of OH-CATH30 and its analogs.
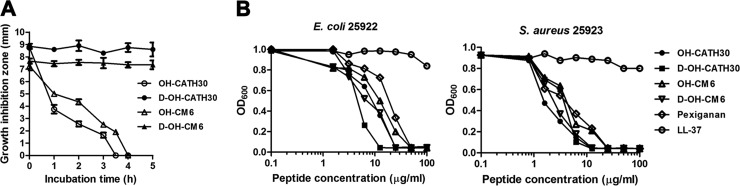
Peptide degradation and interaction with serum components, such as albumin, generally affect the antimicrobial activity of cationic AMPs. To determine the effect of serum on the antibacterial activity of OH-CATH30 and compare OH-CATH30 with OH-CM6 and their corresponding d-peptides, the antibacterial activities of OH-CATH30 and its analogs were tested by preincubating with 100% human serum with a radial diffusion assay and in the presence of 25% human serum with a microdilution assay. OH-CATH30 and OH-CM6 lost approximately 80% and 60% of their antibacterial activities against E. coli 25922, respectively, after incubation with 100% human serum for 3 h. However, the antibacterial activities of d-OH-CATH30 and d-OH-CM6 did not change, even after incubation for 12 h (Fig. 4A).
Fig 4.
Effect of serum on the antibacterial activity of OH-CATH30 and its analogs. (A) Peptides were preincubated with human serum for 0 to 12 h. Aliquots were taken at different time intervals, and the antibacterial activities of the aliquots against E. coli 25922 were determined using an agar radial diffusion method. (B) The antibacterial activity of the peptides was tested in the presence of 25% human serum using a microdilution assay. Each value shown is the mean ± standard error of results from three experiments.
The MICs of OH-CATH30 and its analogs against S. aureus 25923 in the presence of 25% human serum were two to four times higher than in the absence of serum (Fig. 4B). A slight change in the MICs of the d-peptides against E. coli 25922 was observed, whereas the MICs of the l-peptides were two to four times higher than in the absence of serum. LL-37, the human-derived cathelicidin peptide, did not inhibit the growth of either bacterial strain in the presence of 25% human serum (Fig. 4B). Thus, OH-CATH30 and OH-CM6 exerted antibacterial potency in the presence of 25% human serum. This may be due to their fast killing kinetics, which permits the peptides to exhibit their antibacterial effects before enzymatic degradation and protein binding in the serum. These findings indicate that OH-CATH30 and its derivatives may be effective when used systemically.
In vivo efficacy of OH-CATH.
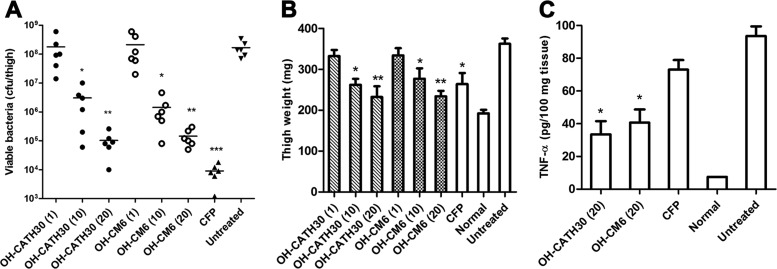
The efficacy of OH-CATH30 and its analogs was investigated in two mouse models. First, a mouse model of neutropenic thigh infection was employed to evaluate the antibacterial activity of OH-CATH30 and OH-CM6 in vivo. Mice were rendered neutropenic by the administration of cyclophosphamide. This procedure can decrease the influence of the host immune system and reduce the initial inoculation of bacteria (21). The left thigh was inoculated with E. coli 25922 (5 × 105 CFU). No deaths were observed within 24 h after inoculation. Treatments were initiated immediately via a single s.c. injection, and the bacterial load in the infected thighs was determined at 16 h postinoculation. Viable bacteria from the infected thighs after treatment with OH-CATH peptides and CFP are shown in Fig. 5A. OH-CATH30 and OH-CM6 markedly decreased the viable bacteria in a dose-dependent manner in treated mice compared to that in untreated mice (P < 0.05). CFP reduced the bacterial counts in infected thighs most potently. After infection with bacteria, the weight of the infected thighs of the untreated mice obviously increased. A slight increase in thigh weight was detected after a single dose of OH-CATH30 (10 mg/kg). OH-CM6 had an efficacy similar to that of OH-CATH30, and there was no significant difference between OH-CATH30 and OH-CM6 (Fig. 5B). The level of TNF-α in the thigh tissue was also determined by ELISA. OH-CATH30 and OH-CM6 (20 mg/kg) potently inhibited the production of TNF-α. In contrast, no marked difference was observed between the CFP-treated and the untreated groups (P = 0.066) (Fig. 5C).
Fig 5.
Efficacy of OH-CATH30 and OH-CM6 in the E. coli 25922-induced murine thigh infection model. (A) Thigh viable bacterial counts. (B) Thigh weight. (C) Level of TNF-α in thigh tissue. ICR mice (n = 6) were made neutropenic by the administration of cyclophosphamide and treated with OH-CATH peptides (1, 10, or 20 mg/kg) or CFP (40 mg/kg) immediately via a single s.c. injection after the bacterial challenge. *, P < 0.05 versus the untreated group.
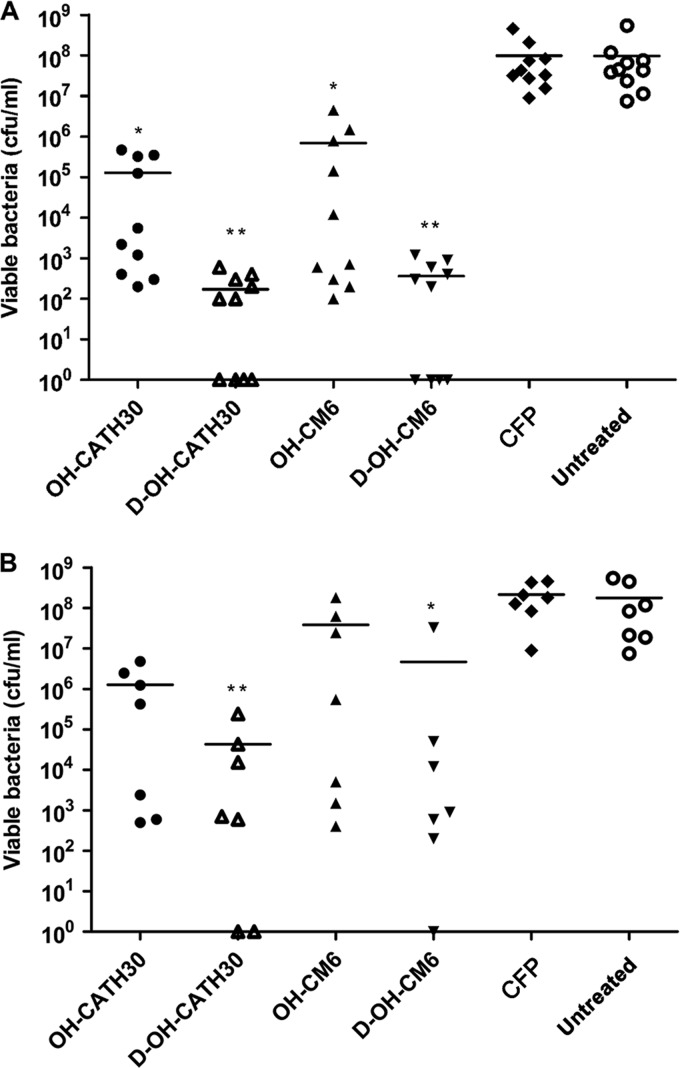
To further confirm the efficacy of OH-CATH30 and its analogs in vivo, the protective effects of the peptides against CFP-resistant E. coli were assessed in a murine bacteremia model. The minimum lethal dose of E. coli clinical strain 3 was 2.5 × 108 CFU/mouse via i.p. injection, and this dose resulted in a 100% mortality rate in Kunming mice within 24 h after inoculation. In the fast treatment condition, the treatment was administered 1 h after inoculation with bacteria. The mortality rates were significantly reduced in all peptide-treated groups (Fig. 6A) (P < 0.005). Specifically, the survival rates for OH-CATH30 and OH-CM6 treatment (80% and 70%, respectively) were significantly higher than those in both the untreated group and the CFP-treated group (P < 0.05). As a positive and negative control, all mice in the CFP-treated and untreated groups were dead within 24 h after inoculation with bacteria. Infected mice were rescued by OH-CATH30 treatment in a dose-dependent manner (Fig. 6B). As expected, d-OH-CATH30 and d-OH-CM6 rescued 100% of mice. No marked difference in protection was observed between the l-peptides and d-peptides. In the case of mortality, OH-CATH30 and OH-CM6 notably extended the life span of mice compared to that of the untreated group. In addition, all OH-CATH peptides also efficiently reduced blood bacterial counts compared to those of the untreated and CFP-treated groups (P < 0.05) (Fig. 7A). At 10 mg/kg, OH-CATH30 and OH-CM6 reduced the bacterial counts from 108 CFU/ml to approximately 105 CFU/ml.
Fig 6.
Survival of mice in a bacteremia model induced by the MDR E. coli clinical strain. Mice received treatment with OH-CATH peptides (1, 10, or 20 mg/kg) or CFP (40 mg/kg) via i.p. injection. (A and B) Treatment initiated at 1 h postinoculation (n = 10). (C and D) Treatment initiated at 4 h postinoculation (n = 7).
Fig 7.
Blood bacterial counts in a bacteremia model at 2 h after the last treatment. Mice received treatment with OH-CATH peptides (10 mg/kg) or CFP (40 mg/kg) via i.p. injection. (A) Treatment initiated at 1 h postinoculation (n = 10). (B) Treatment initiated at 4 h postinoculation (n = 7). Each point represents the determination from a single animal, and the line shows the mean value. *, P < 0.05 versus the untreated and CFP-treated groups.
In the delayed-treatment condition, treatments were initiated 4 h after inoculation to mimic the clinical situation. Severe bacteremia was successfully induced 4 h after inoculation under our experimental conditions. OH-CATH30 and OH-CM6 rescued 57% of mice, while d-OH-CATH30 and d-OH-CM6 resulted in a 70% survival rate for mice in this severe bacteremia model (Fig. 6C and D). In addition, d-OH-CATH30 and d-OH-CM6 significantly reduced the blood bacterial load to 104 and 106 CFU/ml, respectively (P < 0.05). However, the difference in the blood bacterial counts between the OH-CATH30-, OH-CM6-, and CFP-treated groups and the untreated group was not significant according to the Kruskal-Wallis test (Fig. 7B).
DISCUSSION
Bacterial resistance to current antibiotic drugs is spreading rapidly worldwide. The mechanisms of action of AMPs differ from those of conventional antibiotics, spurring interest in the development of AMPs as a novel therapeutic approach to treat infections (9, 12, 14, 29, 42). However, the comparatively higher toxicity and poor efficacy of AMPs in vivo have hampered their clinical application, and most therapeutic peptides are being developed for topical use only (30). In contrast to most AMPs, OH-CATH30, which is derived from the king cobra, possesses potent antibacterial activity and low hemolytic activity, indicating that it is a much better candidate for the development of a new antimicrobial agent (43, 44). Current AMP research usually focuses on the discovery of novel peptides and evaluating their activity in vitro. Little attention has been focused on further investigating their therapeutic potential in vivo. In this study, evaluating the efficacy in vivo is important for the development of OH-CATH30 as a therapeutic agent against bacterial infection.
Peptide drugs are usually made by chemical synthesis, which is very expensive. To reduce the cost of development required for therapeutic or industrial purposes, shorter peptide candidates are more desirable (17). The role of the C-terminal residues of cathelicidin peptides in antimicrobial activity has been studied. BMAP-18, a truncated analog of the bovine cathelicidin peptide BMAP-27, is comprised of only the 18 N-terminal residues and exhibits a slight reduction in antimicrobial activity (15, 33). In our study, we attempted to minimize the size of OH-CATH30 while maintaining its strong antimicrobial activity. We designed a novel smaller peptide, OH-CM6, by removing 10 amino acid residues from the C-terminal region of OH-CATH30 and substituting some amino acids. This modification maintained the potent antibacterial activity of OH-CATH30, indicating that the C-terminal domain has little influence on antibacterial activity.
OH-CATH30 and OH-CM6 exhibited in vitro strong antibacterial activity against the control strains and clinical drug-resistant strains, and their MICs were comparable to that of pexiganan, an AMP developed for the treatment of diabetic foot infection (13). Previous studies have demonstrated that cathelicidins such as LL-37 and SMAP29 can permeabilize the bacterial cytoplasmic membrane (2, 34). Analysis of the killing kinetics demonstrated that OH-CATH30 and OH-CM6 killed bacteria quickly, within 60 min, at 2× the MIC. The effect of both peptides on the bacterial cytoplasmic membrane was confirmed by assessing dye leakage, which demonstrated that OH-CATH30 and OH-CM6 disrupted the integrity of the bacterial cytoplasmic membrane. AMPs possess potent antibacterial activity by disrupting the bacterial cytoplasmic membrane but generally also have strong hemolytic and cytotoxic activities (26, 31). However, no obvious hemolytic activity was observed after treatment with OH-CATH30 or OH-CM6 at 200 μg/ml, a concentration that is approximately 50-fold higher than the MIC. OH-CATH30 exhibited potent antibacterial activity between 1.5 μg/ml and 25 μg/ml and only slight toxicity in mammalian cells and fungi, suggesting a specific discrimination between prokaryotic and eukaryotic cell membranes.
The first step in evaluating the efficacy of AMPs in vivo is the assessment of toxicity in vitro and in vivo. The acute toxicity of the OH-CATH peptides was investigated in Kunming mice. Importantly, OH-CATH30 and OH-CM6 exhibited lower toxicity following a single i.p. or s.c. injection than did pexiganan or PMB. A slight increase in toxicity was observed for the d-peptides. The low toxicity of OH-CATH30 and its analogs facilitated the evaluation of their efficacy in vivo. Although AMPs show superior activity in vitro, they may not be effective in vivo (18). Thus, we further investigated the efficacy of OH-CATH30 and OH-CM6 in the mouse models of neutropenic thigh infection and bacteremia induced by a clinically isolated drug-resistant E. coli strain.
In the neutropenic thigh infection model, OH-CATH30 significantly reduced the number of bacteria in the infected thighs as well as the inflammatory response in a dose-dependent manner. The effects of OH-CM6 were similar to those of OH-CATH30. Although CFP (40 mg/kg) eliminated E. coli 25922 in the infected thighs, it failed to inhibit the inflammatory response. Clinically used antibiotics can lead to the release of endotoxin that increases the inflammatory response (8). In addition to direct antibacterial activity, most AMPs have immunomodulatory activity (7, 37). Therefore, OH-CATH30 may play a role in the immunomodulatory activity against infections. We intend to investigate the immunomodulatory activity of OH-CATH30 in future work.
In the bacteremia model, 1 h postinoculation is sufficient to allow the spread of bacteria to every part of the mouse, and this murine model is typically used to evaluate the systemic efficacy of antibiotics in the pharmaceutical industry (28). In contrast to CFP, a first-line antibiotic used against infection, OH-CATH30 and OH-CM6 could protect mice against bacteremia induced by clinically isolated drug-resistant E. coli. Although OH-CATH30 and OH-CM6 lost their activity after incubation with 100% human serum for 4 h, they rescued mice in the bacteremia model following multiple i.p. injections. We also tested the efficacy of d-OH-CATH30 and d-OH-CM6 in the bacteremia model. d-OH-CATH30 and d-OH-CM6 exhibited potent antibacterial activity in vitro. Consistent with their in vitro effect, the d-peptides were also effective in vivo.
In conclusion, OH-CATH30 and its truncated peptide, OH-CM6, exhibit strong antibacterial activity against drug-resistant clinical isolates by disrupting the bacterial cytoplasmic membrane. In addition, OH-CATH30 and its analogs were effective in the mouse models of neutropenic thigh infection and bacteremia with a high therapeutic index. Taken together, these results indicate that OH-CATH30 and OH-CM6 are excellent templates for the development of novel therapeutic agents against systemic infections caused by MDR bacteria and are suitable for further investigation.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (NSFC-Yunnan joint funding U1132601, 31071926, 30960384), the National Basic Research Program of China (973 Program, 2010CB529800), and the National Science & Technology Major Project (2009ZX09103-147).
We acknowledge Yan Du for providing microbial strains and assistance with determining the susceptibility of clinical isolates.
Footnotes
Published ahead of print 9 March 2012
REFERENCES
- 1. Ahmad I, Perkins WR, Lupan DM, Selsted ME, Janoff AS. 1995. Liposomal entrapment of the neutrophil-derived peptide indolicidin endows it with in vivo antifungal activity. Biochim. Biophys. Acta 1237:109–114 [DOI] [PubMed] [Google Scholar]
- 2. Anderson RC, Hancock RE, Yu PL. 2004. Antimicrobial activity and bacterial-membrane interaction of ovine-derived cathelicidins. Antimicrob. Agents Chemother. 48:673–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bals R, Wilson JM. 2003. Cathelicidins—a family of multifunctional antimicrobial peptides. Cell. Mol. Life Sci. 60:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bommineni YR, et al. 2007. Fowlicidin-3 is an alpha-helical cationic host defense peptide with potent antibacterial and lipopolysaccharide-neutralizing activities. FEBS J. 274:418–428 [DOI] [PubMed] [Google Scholar]
- 5. Braunstein A, Papo N, Shai Y. 2004. In vitro activity and potency of an intravenously injected antimicrobial peptide and its dl amino acid analog in mice infected with bacteria. Antimicrob. Agents Chemother. 48:3127–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brogden KA. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238–250 [DOI] [PubMed] [Google Scholar]
- 7. Brown KL, et al. 2011. Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J. Immunol. 186:5497–5505 [DOI] [PubMed] [Google Scholar]
- 8. Byl B, et al. 2001. Ceftazidime- and imipenem-induced endotoxin release during treatment of gram-negative infections. Eur. J. Clin. Microbiol. Infect. Dis. 20:804–807 [DOI] [PubMed] [Google Scholar]
- 9. Cirioni O, et al. 2006. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 50:1672–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. M100–S19 CLSI, Wayne, PA [Google Scholar]
- 11. Dartois V, et al. 2005. Systemic antibacterial activity of novel synthetic cyclic peptides. Antimicrob. Agents Chemother. 49:3302–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischbach MA, Walsh CT. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ge Y, et al. 1999. In vitro antibacterial properties of pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 43:782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giacometti A, et al. 2004. Cathelicidin peptide sheep myeloid antimicrobial peptide-29 prevents endotoxin-induced mortality in rat models of septic shock. Am. J. Respir. Crit. Care Med. 169:187–194 [DOI] [PubMed] [Google Scholar]
- 15. Haines LR, et al. 2009. Killing of trypanosomatid parasites by a modified bovine host defense peptide, BMAP-18. PLoS Negl. Trop. Dis. 3:e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hancock RE, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 17. Hilpert K, Volkmer-Engert R, Walter T, Hancock RE. 2005. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 23:1008–1012 [DOI] [PubMed] [Google Scholar]
- 18. Hoffmann R, Bulet P, Urge L, Otvos L., Jr 1999. Range of activity and metabolic stability of synthetic antibacterial glycopeptides from insects. Biochim. Biophys. Acta 1426:459–467 [DOI] [PubMed] [Google Scholar]
- 19. Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. 1998. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273:3718–3724 [DOI] [PubMed] [Google Scholar]
- 20. Lee JY, et al. 2008. Salt-resistant homodimeric bactenecin, a cathelicidin-derived antimicrobial peptide. FEBS J. 275:3911–3920 [DOI] [PubMed] [Google Scholar]
- 21. Leendertse M, et al. 2009. Neutrophils are essential for rapid clearance of Enterococcus faecium in mice. Infect. Immun. 77:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lehrer RI, Rosenman M, Harwig SS, Jackson R, Eisenhauer P. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137:167–173 [DOI] [PubMed] [Google Scholar]
- 23. Lin QP, et al. 2008. Lipopolysaccharide neutralization by the antibacterial peptide CM4. Eur. J. Pharmacol. 596:160–165 [DOI] [PubMed] [Google Scholar]
- 24. Lipnick RL, et al. 1995. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem. Toxicol. 33:223–231 [DOI] [PubMed] [Google Scholar]
- 25. Lipsky BA, Holroyd KJ, Zasloff M. 2008. Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin. Infect. Dis. 47:1537–1545 [DOI] [PubMed] [Google Scholar]
- 26. Ludtke SJ, et al. 1996. Membrane pores induced by magainin. Biochemistry 35:13723–13728 [DOI] [PubMed] [Google Scholar]
- 27. McGwire BS, Olson CL, Tack BF, Engman DM. 2003. Killing of African trypanosomes by antimicrobial peptides. J. Infect. Dis. 188:146–152 [DOI] [PubMed] [Google Scholar]
- 28. Noto PB, et al. 2008. Alternative stabilities of a proline-rich antibacterial peptide in vitro and in vivo. Protein Sci. 17:1249–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oyston PC, Fox MA, Richards SJ, Clark GC. 2009. Novel peptide therapeutics for treatment of infections. J. Med. Microbiol. 58:977–987 [DOI] [PubMed] [Google Scholar]
- 30. Pacor S, Giangaspero A, Bacac M, Sava G, Tossi A. 2002. Analysis of the cytotoxicity of synthetic antimicrobial peptides on mouse leucocytes: implications for systemic use. J. Antimicrob. Chemother. 50:339–348 [DOI] [PubMed] [Google Scholar]
- 31. Rinaldi AC, et al. 2002. Temporin L: antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem. J. 368:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sarig H, et al. 2010. A miniature mimic of host defense peptides with systemic antibacterial efficacy. FASEB J. 24:1904–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skerlavaj B, et al. 1996. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J. Biol. Chem. 271:28375–28381 [DOI] [PubMed] [Google Scholar]
- 34. Thennarasu S, et al. 2010. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys. J. 98:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaara M. 2009. New approaches in peptide antibiotics. Curr. Opin. Pharmacol. 9:571–576 [DOI] [PubMed] [Google Scholar]
- 36. Wu M, Maier E, Benz R, Hancock RE. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235–7242 [DOI] [PubMed] [Google Scholar]
- 37. Yu PL, Cross ML, Haverkamp RG. 2010. Antimicrobial and immunomodulatory activities of an ovine proline/arginine-rich cathelicidin. Int. J. Antimicrob. Agents 35:288–291 [DOI] [PubMed] [Google Scholar]
- 38. Zanetti M. 2004. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75:39–48 [DOI] [PubMed] [Google Scholar]
- 39. Zanetti M, Gennaro R, Romeo D. 1995. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 374:1–5 [DOI] [PubMed] [Google Scholar]
- 40. Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395 [DOI] [PubMed] [Google Scholar]
- 41. Zasloff M. 2007. Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. J. Am. Soc. Nephrol. 18:2810–2816 [DOI] [PubMed] [Google Scholar]
- 42. Zhang L, et al. 2005. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 49:2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y, et al. 2010. Structure-function relationship of king cobra cathelicidin. Peptides 31:1488–1493 [DOI] [PubMed] [Google Scholar]
- 44. Zhao H, et al. 2008. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides 29:1685–1691 [DOI] [PubMed] [Google Scholar]