Abstract
The cellular pharmacology of zidovudine (ZDV) and lamivudine (3TC) in vivo is not completely understood. This prospective longitudinal study investigated the relationship between HIV-1 serostatus, sex, race, and time on therapy with intracellular and plasma ZDV and 3TC concentrations. Of 20 HIV-seronegative and 23 HIV-seropositive volunteers enrolled, 16 (8 women) and 21 (5 women) completed all 12 study days, respectively. Volunteers began ZDV-3TC therapy (plus a third active drug in HIV-seropositive volunteers), and steady-state concentrations (Css) were determined after days 1, 3, 7, and 12. A repeated-measures mixed model was utilized. HIV-seronegative status was associated with 22% (95% confidence interval [CI], 0%, 50%) and 37% (15%, 67%) higher Css estimates compared to those of HIV-seropositive individuals for intracellular ZDV-TP and 3TC-TP levels, respectively. African-Americans had 36% (8%, 72%) higher ZDV-TP estimates than non-African-Americans. Sex was not associated with ZDV-TP or 3TC-TP (P > 0.19). Women had 36% (4%, 78%) higher plasma ZDV, but the effect was lessened when normalized by lean body weight (5% [−19%, 38%]; P = 0.68). Plasma 3TC was 19% (0%, 41%) higher in HIV-seropositive volunteers and 22% (0%, 48%) higher in African American volunteers, but these effects were not significant when corrected for creatinine clearance (7% [−9%, 20%] and −5% [−26%, 12%] for HIV serostatus and race, respectively; P > 0.35). These results suggest that HIV-seropositive status decreases and African American race elevates the cellular triphosphates of ZDV and 3TC. This information extends knowledge of ZDV and 3TC cellular pharmacology in vivo and provides new leads for future cellular pharmacology studies aimed at optimizing HIV prevention/treatment with these agents.
INTRODUCTION
Zidovudine (ZDV) and lamivudine (3TC) are nucleoside analog reverse transcriptase inhibitors (NRTI) with a long history of use in HIV-seropositive persons for treatment and at-risk HIV-seronegative persons for prevention (7, 11). NRTI efficacy and toxicity is dependent on the concentration of the triphosphate (TP) moiety of the drug inside cells (16, 25). NRTI are sequentially phosphorylated to the TP moiety, with the resulting NRTI phosphates being ion trapped inside the cell, which confers a unique pharmacokinetic (PK) profile for the TP versus the parent NRTI (5, 7). Drug-TP blocks human immunodeficiency virus 1 (HIV) replication by competing with endogenous nucleotides for incorporation into viral DNA chains and terminating chain elongation when incorporated (16). The TP is hypothesized to inhibit mitochondrial DNA (mtDNA) replication in the same manner, although other mechanisms of mitochondrial toxicity are possible (25, 27). As such, it is crucial to understand the disposition of NRTI-TP in vivo.
ZDV, a thymidine analog, is phosphorylated to the monophosphate (MP) anabolite by thymidine kinase 1 (TK1) and then to the diphosphate (DP) by thymidylate kinase 1, and finally by nucleotide diphosphate kinase to the TP moiety (9, 16). 3TC, a deoxycytidine analog, is anabolized to the MP by deoxycytidine kinase, to the DP by uridylate-cytidylate kinase, and to the active TP by 3′-phosphoglycerate kinase or nucleotide diphosphate kinase (9, 21, 40). Membrane transporters contribute to eventual TP concentrations through the influx of the parent NRTI and/or the efflux of the NRTI-MP (34). Nucleotidases and phosphatases degrade nucleotides, decreasing the efficiency of TP production (13, 22). Despite knowledge of these processes and clinical experience with the drugs, the patient-specific factors that affect cellular pharmacology in vivo are not clear.
In vitro results demonstrate up to 200-fold more ZDV-TP in mitogen-stimulated cells than in resting cells (17, 18). Similarly, 3TC-phosphates were increased 4- to 6-fold in phytohemagglutinin (PHA)-stimulated cells (18, 28). These in vitro findings may be important in the clinical setting, because HIV infection increases cellular activation directly and/or through microbial translocation (14, 24). However, it is unclear how these in vitro results with PHA extrapolate to the in vivo setting of HIV infection.
Lactic acidosis, a rare but life-threatening mitochondrial toxicity associated with long-term NRTI use, occurs approximately 20-fold more frequently in women (31, 41), suggesting possible sex-based differences in NRTI cellular pharmacology. Current data regarding associations between the cellular pharmacology of these drugs and sex are variable and inconclusive. One study of ZDV-3TC-indinavir among 29 men and 4 women demonstrated approximately 2-fold higher ZDV-TP and 1.4-fold higher 3TC-TP levels in women (4). Other studies have shown similar results for ZDV-phosphates (10, 38), but still others have shown opposite results for ZDV-phosphates (8). Given possible differences in NRTI response, there is an urgent need for additional comparisons of NRTI cellular pharmacology by sex.
The objective of the present study was to evaluate the effect of HIV infection and sex on the cellular pharmacology of the antiretroviral medications ZDV and 3TC.
MATERIALS AND METHODS
Study design and protocol.
This was an open-label, prospective, longitudinal, observational PK study with the specific aim of determining the effects of HIV serostatus and sex on ZDV and 3TC cellular pharmacology. HIV-seronegative and HIV-seropositive volunteers were recruited with the goal of enrolling equal numbers of men and women in each group. HIV-seronegative participants received 300 mg ZDV/150 mg 3TC (coformulated as Combivir) twice daily for approximately 12 days. HIV-infected subjects were either antiretroviral naïve or without therapy in the preceding 6 months. Their clinician provider must have prescribed a regimen that included 300 mg ZDV/150 mg 3TC (coformulated as Combivir) with another active antiretroviral component not from the NRTI class. This study was not interventional with respect to therapy for participants with HIV infection. General eligibility requirements included the ability to give informed consent; age of 18 to 55 years; no medical conditions that would interfere with the study conditions; no recent investigational medications or concomitant drugs that might alter renal drug clearance or cellular activation, including probenecid, chemotherapy, glucocorticoids, hematopoetic growth factors, or sex hormones (subjects were instructed to use two forms of nonhormonal birth control); no daily warfarin or aspirin; no lidocaine allergy; no hepatitis B virus infection in HIV-seronegative volunteers; no pregnancy or plan to become pregnant; estimated creatinine clearance of >60 ml/min; and body mass index (BMI) of ≤34. The study was approved by the Colorado Multiple Institution Review Board, and all subjects gave written consent.
Intensive PK studies were conducted on the first dose of therapy and then on days ∼3, ∼7, and ∼12. HIV-infected participants had additional visits at days ∼30 and ∼60 and months 6, 12, 18, and 24, if available. This report describes the PK visits shared by HIV-seronegative and HIV-seropositive volunteers, which were up to and including day 12. Each PK dose was given after an overnight fast and with a standardized meal (∼640 kcal; 15% protein, 40% fat, 45% carbohydrates). The PK sampling consisted of paired plasma and peripheral blood mononuclear cells (PBMCs) at 2, 5, and 8 h after the observed dose. During all visits, adverse events were monitored by safety laboratories and subjective well-being assessments. Adherence was quantified with medication counts and a questionnaire for HIV-seronegative participants and a questionnaire and viral load for the HIV-seropositive participants.
PBMC isolation.
Blood-draw cell preparation tubes (two 8-ml samples) were centrifuged at 1,800 × g for 30 min to harvest the PBMCs. PBMCs were suspended in phosphate-buffered saline (PBS) (ThermoScientific HyClone, Logan, UT), and then the cells were counted using a hemocytometer. PBMCs were pelleted, lysed with 500 μl of 70:30 methanol-water, and stored at −80°C until analysis.
Analytical determination of intracellular NRTI.
Intracellular samples were analyzed by a previously validated high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) method (12). Briefly, two million cells from the cellular extract (70:30 methanol-water lysate) were applied to a strong anion exchange solid-phase extraction (SPE) column (Waters QMA). The TP were isolated using a potassium chloride salt gradient. The TP fraction was then incubated with an acid phosphatase enzyme solution in a warm water bath to dephosphorylate the fraction to the parent drug.
The total volume of each dephosphorylated TP fraction was applied to a second SPE cartridge (Phenomenex Strata-X) to desalt and concentrate the fraction. The samples were eluted from the cartridge with methanol, dried, and reconstituted. Samples were analyzed by HPLC-MS/MS, utilizing a TSQ Vantage triple-quadrupole mass spectrometer (Thermo Scientific Fisher, San Jose, CA). The assay was linear from 5 to 2,000 fmol/sample for ZDV-TP and 0.1 to 100 pmol/sample for 3TC-TP. Concentrations were normalized to fmol/106 cells for ZDV and pmol/106 cells for 3TC.
Analytical determination of plasma drug levels.
Plasma samples were analyzed by a previously validated HPLC-MS/MS method (37) with a linear range of 1 to 3,000 ng/ml when 100 μl of plasma was assayed.
Pharmacokinetic methods.
The average concentration (Css) from 2 to 8 h after dose was calculated as the area under the concentration-time curve (AUC) from 2 to 8 h by the trapezoidal rule divided by the time interval (6 h). The Css was determined for each moiety (plasma and TP) for each study day (first dose and days 3, 7, and 12). The half-life was estimated by the accumulation factor [AF = 1/(1 − e−kelτ), where τ is the dosing interval] between the first-dose Css and steady-state Css (days 0 to 12, as appropriate) and solving for the elimination rate constant (kel). The half-life was calculated as ln(2)/kel.
Statistical analyses.
The mixed procedure in SAS (SAS Institute Inc., Cary, NC) was used with a repeated statement to determine appropriate models for the Css of each drug. This approach utilized all PK data generated by all subjects, including those who did not complete all 12 days of the study. Model selections were based on Akaike's information criterion (AIC) (3). A full model (including HIV serostatus, sex, race, day of therapy [categorical], and all possible interactions) was used to select the variance-covariance structure for each drug. Variance-covariance structures tested include unstructured (the least parsimonious model; 10 parameters, 1 for every possible variance or covariance), compound symmetry (the most parsimonious; 2 parameters, 1 for the variance and one for the covariance), and others (30), including spatial and heterogeneous models. After the final determination of the covariates to be included (as described below), each model was tested against a model with the same covariates but with the unstructured variance-covariance structure. This ensured that the chosen variance-covariance structure remained the best structure despite a less than full covariate model. Covariate model determination started with a full model that included HIV serostatus, sex, race, day of therapy (categorical), and all interactions, and it continued through all possible combinations of two- and three-way interactions before reduction to a model consisting of any one of the single covariates (HIV serostatus, sex, race, or day of therapy), encompassing all possible covariate models. The covariate model resulting in the minimum AIC was chosen as the most parsimonious and was used for subsequent analyses. Maximum likelihood estimation was used until the final model was chosen, after which restricted maximum likelihood estimation was utilized to determine the effect of HIV serostatus, sex, race, and day of therapy on drug levels. For each outcome, estimates of percent difference, along with the corresponding confidence intervals (CI) and P values, are reported for all covariates included in the final AIC model.
The study was designed to enroll 16 HIV-seronegative volunteers (8 women) and 32 HIV-seropositive volunteers (16 women) who completed at least the day 12 visit. The power analysis was based on 8 men and 8 women and an effect size of 125% for ZDV-TP concentrations in women compared to those in men. These estimates were founded on a ZDV-TP coefficient of variation of 50% and a 130% higher ZDV-TP in women than men from a previous study (4). The power to detect 3TC-TP differences was higher because variability was about 20% versus 50% for ZDV-TP (4). Additional HIV-seropositive subject numbers were sought to determine if sex differences were disease dependent and to provide data for different disease strata (advanced versus less-advanced disease), if possible.
RESULTS
Summary results. (i) Demographics and safety.
Twenty HIV-seronegative and 23 HIV-seropositive individuals started the study, and all PK data from these individuals were analyzed. The HIV-seronegative group (n = 20) consisted of 12 men and 8 women, while the HIV-seropositive group (n = 23) consisted of 17 men and 6 women, as shown in Table 1. The goal of enrolling half women in the HIV-seropositive group was not achieved. The median ages for HIV-seropositive subjects were 43.0 years for men and 39.5 years for women and were 30.5 years and 28.5 years for HIV-seronegative volunteers, respectively. The HIV-seronegative group contained two male and two female African-Americans; the HIV-seropositive group contained three male and four female African-Americans. The median CD4+ cell count for HIV-seropositive individuals was 270 cells/μl, while the median viral load was 4.5 log10 copies/ml. CD4+ cell counts and viral loads are separated by sex in Table 1. The third active antiretroviral drug (HIV-seropositive participants) was efavirenz (n = 9), lopinavir/ritonavir (n = 7), or atazanavir/ritonavir (n = 7). Creatinine clearance determined using lean body weight and the Cockcroft-Gault method is summarized in Table 1.
Table 1.
Subject demographics
| Subject demographics | Result |
|
|---|---|---|
| Men | Women | |
| HIV seronegative | ||
| Sex (no.) | 12 | 8 |
| Race | ||
| African-American (no.) | 2 | 2 |
| Non-African-American (no.) | 10 | 6 |
| Age (yr; median [IQR]) | 30.5 (28, 39) | 28.5 (24, 35) |
| Weight (kg; median [IQR]) | 86 (77, 91) | 68 (61, 73) |
| Creatinine clearance (ml/min; median [IQR]) | 128 (112, 140) | 109 (95, 121) |
| HIV seropositive | ||
| Sex (no.) | 17 | 6 |
| Race | ||
| African-American (no.) | 3 | 4 |
| Non-African-American (no.) | 14 | 2 |
| Age (yr; median [IQR]) | 43.0 (40, 47) | 39.5 (36, 46) |
| CD4+ (cells/μl; median [IQR]) | 329 (241, 459) | 219 (160, 318) |
| Plasma HIV-RNA (log10; median [IQR]) | 4.53 (4.26, 4.68) | 4.90 (4.61, 5.04) |
| Weight (kg; median [IQR]) | 80 (71, 83) | 74 (63, 85) |
| Creatinine clearance (ml/min; median [IQR]) | 118 (89, 131) | 85 (74, 99) |
Sixteen HIV-seronegative (8 women) and 21 HIV-seropositive volunteers (5 women) completed 12 days. Four HIV-seronegative (all men, 1 African-American) and two HIV-seropositive volunteers (1 African-American woman, 1 non-African-American man) did not complete all 12 days of the study. The four HIV-seronegative noncompleters withdrew due to moderate (grade II) nausea, vomiting, and/or diarrhea associated with the drug regimen. One HIV-seropositive subject withdrew due to personal reasons, the other due to nausea and vomiting. Typical adverse events in all subjects were mild (grade I) nausea, headache, and/or fatigue/malaise. The most common abnormal laboratory result in HIV-seronegative subjects was a mild (grade I) increase in bilirubin (n = 7). Adverse laboratory results for HIV-seropositive subjects included mild (grade I) decreases in albumin (n = 6) and moderate (grade II) increases in total bilirubin (n = 8), alanine aminotransferase (ALT) (n = 7), aspartate transaminase (AST) (n = 7), and amylase (n = 7).
(ii) Analytical results.
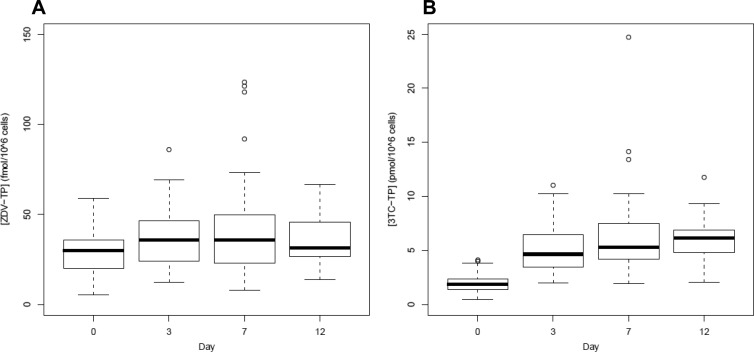
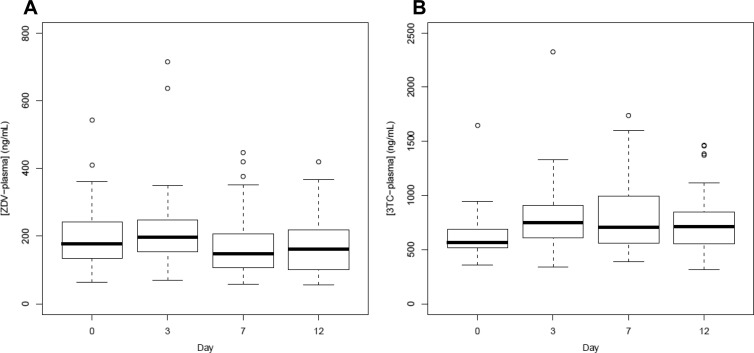
Median ZDV-TP Css levels were 30, 36, 36, and 31 fmol/106 cells at first dose and on days 3, 7, and 12, respectively. The same values for 3TC-TP were 1.8, 4.6, 5.2, and 6.1 pmol/106 cells, respectively. Css ranged from 5.4 to 123 fmol/106 cells for ZDV-TP and 0.48 to 25 pmol/106 cells for 3TC-TP. Similarly, median plasma Css levels were 180, 198, 149, and 164 ng/ml for ZDV and 565, 769, 706, and 737 ng/ml for 3TC, respectively. ZDV plasma Css ranged from 57 to 715 ng/ml, while 3TC plasma Css were between 315 and 2,327 ng/ml. Intersubject variability as judged by percent coefficient of variation ranged from 39% (day 12) to 68% (day 7) for ZDV-TP and from 31% (day 12) to 63% (day 7) for 3TC-TP. Variability in ZDV plasma levels ranged from 49% (first dose) to 59% (day 3) and 34% (day 0) and 42% (day 3) for 3TC plasma. Five of 20 HIV-seronegative individuals and four of 23 HIV-seropositive subjects were not fully adherent to the regimen by patient report. Of those who missed doses, none missed more than three during the 12 days of study. Average adherence during the course of the study was 99%.
Model development. (i) Variance-covariance structure.
A model consisting of HIV serostatus (positive [HIV = 1] or negative [HIV = 0]), sex (men [sex = 1] or women [sex = 0]), race (African-American [race = 1] or non-African-American [race = 0]), day of therapy (0, 3, 7, or 12), and the associated two-, three-, and four-way interactions were used to determine the optimal variance-covariance structure.
As shown in Fig. 1, the variance on day 7 was greater than the variances on days 0, 3, and 12 for both ZDV- and 3TC-TP, suggesting the need for a model which allowed the variances for each study day to be distinct. A heterogeneous compound symmetry (CSH) model yielded the best AIC for both ZDV- and 3TC-TP.
Fig 1.
Boxplots of TP levels by day. Boxplots show medians and IQR of ZDV-TP (A) and 3TC-TP (B) Css. Unfilled circles represent data outside 1.5 IQR (whiskers).
ZDV and 3TC plasma levels demonstrated less variability within and between study days (Fig. 2). A spatial linear logarithm [SP(LINL)] variance-covariance structure was chosen for both plasma models. In all cases, the chosen structure [CSH for TP and SP(LINL) for plasma] performed better by AIC than the unstructured variance-covariance structure for the determined covariate model.
Fig 2.
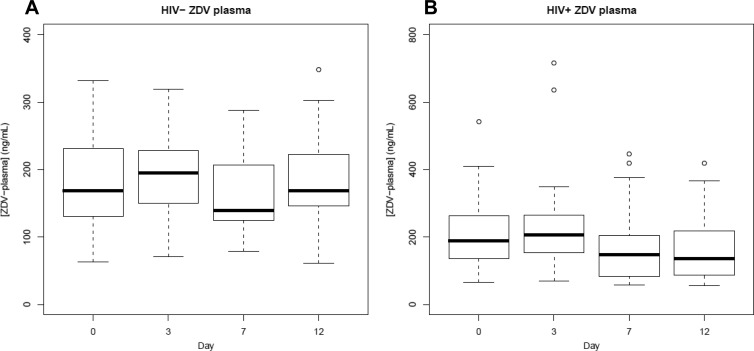
Boxplots of plasma levels by day. Boxplots show medians and IQR of ZDV (A) and 3TC (B) plasma Css. Unfilled circles represent data outside 1.5 IQR (whiskers).
(ii) Determination of significant covariates.
For each analysis described below (ZDV-TP, 3TC-TP, ZDV plasma, and 3TC plasma), models with interaction terms (two, three, or four way) were tested and resulted in poorer AIC values than models not containing the interaction terms. For all comparisons, two-sided tests were utilized with a significance level of α = 0.05.
(iii) ZDV-TP.
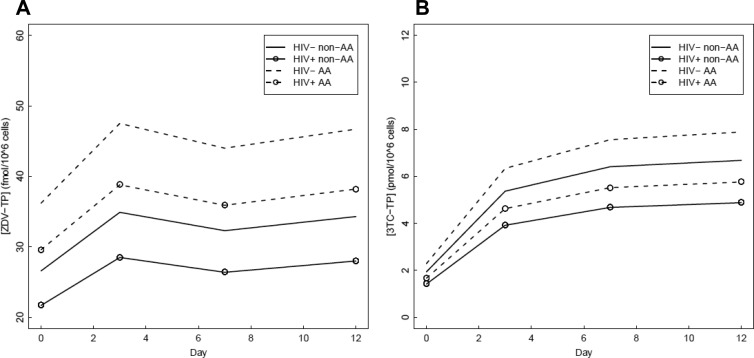
The optimal ZDV-TP model consisted of the HIV serostatus, race, and categorical study day covariates (Fig. 3A). This model predicted no significant ZDV-TP difference between men and women (difference [95% CI], 16% [−7%, 46%]; P = 0.19). HIV-seronegative subjects were estimated to have higher ZDV-TP levels than HIV-seropositive volunteers (22% [0%, 50%]; P = 0.05). Raw data showed the direction of this effect to be the same on all study days. ZDV-TP was significantly lower at day 0 (the first dose) than at days 3, 7, and 12 (P < 0.0008). The model predicted an accumulation of ZDV-TP from 28 fmol/106 cells at day 0 to 36 fmol/106 cells at day 12 (29% [12%, 48%]). Days 3, 7, and 12 were all predicted to be approximately the same, 36 fmol/106 cells. The predicted accumulation of ZDV-TP suggested an 8-h intracellular half-life. Finally, the model predicted African-Americans to have 36% higher ZDV-TP concentrations than non-African-Americans (36% [8%, 72%]; P = 0.01). Final model estimates of percent difference, with corresponding 95% confidence intervals and P values, are reported for all covariates in Table 2 and for drug accumulation in Table 3.
Fig 3.
Covariate effects on TP levels. Parameter estimates by day for ZDV-TP (A) and 3TC-TP (B) Css. AA, African-American.
Table 2.
Covariate results in final model
| Compound | Covariate ratio (95% CI) results by: |
||
|---|---|---|---|
| HIV serostatus | Sex | Racea | |
| ZDV-TP | HIV−, 22% increase (0%, 50%); P = 0.05 | AA, 36% increase (8%, 72%); P = 0.01 | |
| 3TC-TP | HIV−, 37% increase (15%, 62%); P = 0.0006 | AA, 18% increase (−3%, 43%); P = 0.09 | |
| ZDV plasma | Women, 36% increase (4%, 78%); P = 0.03 | ||
| 3TC plasma | HIV+, 19% increase (0%, 41%); P = 0.05 | AA, 22% increase (0%, 48%); P = 0.05 | |
AA, African-American.
Table 3.
Drug accumulation in final model
| Compound | Accumulation (95% CI) from day 0 to day: |
||
|---|---|---|---|
| 3 | 7 | 12 | |
| ZDV-TP | 31% increase (14%, 51%); P = 0.0002 | 37% increase (14%, 64%); P = 0.0009 | 29% increase (12%, 48%); P = 0.0004 |
| 3TC-TP | 177% increase (139%, 220%); P < 0.0001 | 231% increase (179%, 291%); P < 0.0001 | 245% increase (199%, 297%); P < 0.0001 |
| ZDV plasma | 4% increase (−9%, 19%); P = 0.57 | 17% decrease (−27%, −5%); P = 0.0077 | 18% decrease (−29%, −5%); P = 0.0057 |
| 3TC plasma | 26% increase (17%, 36%); P < 0.0001 | 22% increase (12%, 32%); P < 0.0001 | 20% increase (12%, 30%); P < 0.0001 |
(iv) 3TC-TP.
The chosen model for 3TC-TP included the covariates HIV serostatus, race, and study day without any interactions (Fig. 3B). 3TC-TP was not different between women and men (3% [−15%, 24%]; P = 0.79). HIV infection was a highly significant predictor of 3TC-TP levels (P = 0.0006). HIV-seronegative volunteers had higher 3TC-TP estimates than HIV-seropositive volunteers (37% [15%, 62%]). Raw data showed the direction of this effect to be the same on all study days. 3TC-TP accumulated during the duration of the study. Both day 0 and day 3 3TC-TP levels were significantly lower than those of the other study days (P < 0.0001 and 0.03, respectively). The model estimate of the first-dose 3TC-TP Css was 1.8 pmol/106 cells, day 3 was approximately 3-fold higher (4.99 pmol/106 cells), and concentrations at days 7 and 12 were similar and 20% higher than that on day 3 (5.95 and 6.20 pmol/106 cells, respectively). The accumulation in Css during the study predicted a half-life of 27 h. Estimates of 3TC-TP levels were higher in African-Americans than non-African-Americans (18% [−3%, 43%]; P = 0.09), but the covariate failed to reach significance at the α = 0.05 level, despite being included in the model based on AIC. Estimates of percent difference, with corresponding 95% confidence intervals and P values, are reported for all covariates included in the final AIC model in Table 2, while drug accumulation in the final AIC model is in Table 3.
(v) ZDV plasma.
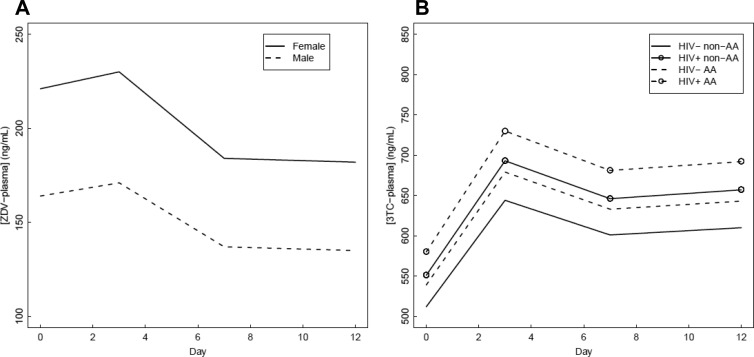
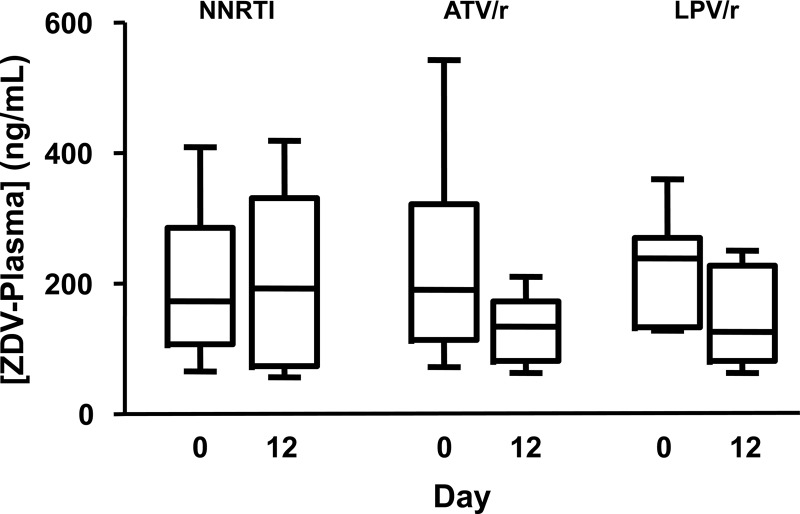
The final ZDV plasma model included only study day and sex as covariates (Fig. 4A), as the inclusion of neither HIV infection nor African-American race lowered the model AIC. HIV serostatus did not affect plasma ZDV levels (95% CI, −24%, 29%; P = 0.95), with both groups of subjects having predicted concentrations near 175 ng/ml. ZDV plasma levels were not different between non-African-Americans and African-Americans (18% [−14%, 59%]; P = 0.29). In contrast, sex was a significant covariate. Women were predicted to have ZDV plasma concentrations that were 36% higher than those of men (95% CI, 4%, 78%; P = 0.03), but this effect failed to achieve significance in subsequent analyses with body weight-normalized plasma ZDV levels (5%, [−19%, 38%]; P = 0.68). ZDV plasma concentrations decreased by approximately 20% during the duration of the study. Higher concentrations were predicted on days 0 and 3 (191 and 198 ng/ml, respectively) than on days 7 and 12 (158 and 155 ng/ml, respectively) (P < 0.01). Further investigation demonstrated that this effect occurred in HIV-seropositive but not HIV-seronegative volunteers (Fig. 5) and may be explained by the inductive properties of the third drug in the individual's ART regimen (Fig. 6). Estimates of percent difference, with corresponding 95% confidence intervals and P values, are reported for all covariates included in the final AIC model in Table 2, while drug accumulation in the final AIC model is in Table 3.
Fig 4.
Covariate effects on plasma levels. Parameter estimates by day for ZDV (A) and 3TC (B) plasma Css. AA, African-American.
Fig 5.
Boxplots of ZDV plasma levels by HIV serostatus. Boxplot showing medians and IQR of HIV-seronegative (A) and HIV-seropositive (B) Css. Unfilled circles represent data outside 1.5 IQR (whiskers).
Fig 6.
Influence of ritonavir (/r) on ZDV plasma levels. The boxplot shows medians and IQR and whiskers (5th to 95th percentile) of ZDV plasma Css in HIV-seropositive subjects on days 0 and 12 of the study, separated by the third drug used in their highly active antiretroviral therapy regimen. NNRTI, nonnucleoside reverse transcriptase inhibitor; ATV/r, atazanavir/ritonavir; LPV/r, lopinavir/ritonavir.
(vi) 3TC plasma.
The 3TC plasma model consisted of HIV serostatus, race, and study day (Fig. 4B). Sex was not a significant covariate (11% [−8%, 34%]; P = 0.28 for women versus men). Higher 3TC plasma levels were predicted for HIV-seropositive volunteers (19% [0%, 41%]; P = 0.05). African-American race was predictive of 22% higher 3TC plasma concentrations (95% CI, 0%, 48%; P = 0.05). However, these effects failed to achieve significance (P > 0.35) in subsequent analyses with creatinine clearance-normalized 3TC levels, with predicted changes of 7% (−9%, 20%) and −5% (−26%, 12%), respectively. Unlike ZDV in plasma, 3TC plasma levels increased during the duration of the study by approximately 20% (8%, 28%) (P < 0.0001). In contrast to 3TC-TP levels, 3TC plasma did not continue to accumulate to higher levels on each day of the study, as levels on days 3, 7, and 12 were similar (790, 762, and 751 ng/ml, respectively). The 3TC plasma half-life was 6.5 h based upon the accumulation factor. Estimates of percent difference, with corresponding 95% confidence intervals and P values, are reported for all covariates included in the final AIC model in Table 2, while drug accumulation in the final AIC model is in Table 3.
DISCUSSION
This study investigated the relationship between HIV serostatus, sex, race, and day of therapy and the in vivo pharmacology of plasma and intracellular ZDV and 3TC.
Two main aims of this study were to determine the effect of HIV serostatus and sex on the pharmacology of ZDV-TP and 3TC-TP. Previous results and in vitro literature led us to hypothesize that female sex and cell activation associated with HIV infection would increase intracellular drug levels (4, 17, 18, 38). However, the results from this study indicate that HIV-seropositive volunteers were predicted to have diminished ZDV-TP levels and significantly lower 3TC-TP levels than HIV-seronegative volunteers. Furthermore, no significant sex differences were observed in intracellular drug concentrations.
ZDV phosphorylation is well known to be activation state dependent, resulting in up to 200-fold higher intracellular triphosphate levels when cells were stimulated with PHA in vitro (17, 18). By extrapolation, it was expected that HIV infection would increase TP levels relative to those of HIV-seronegative volunteers because of higher cellular activation associated with the infection (14, 24). This discrepancy between in vitro and in vivo systems likely arises from the relative complexity of chronic HIV infection in vivo compared to PHA stimulation in vitro. Chronic cellular activation in vivo may, over time, downregulate enzymes that phosphorylate or influx drug or upregulate enzymes that dephosphorylate or efflux drug. Jacobsson et al. showed a 10-fold decrease in thymidylate kinase activity in PHA-stimulated cell extracts from HIV-seropositive versus HIV-seronegative subjects, suggesting that the continuous cellular activation with HIV infection causes an anergic effect (23). The present study demonstrates that in vivo studies are ultimately needed to best understand the effects of HIV infection and cell activation on cellular pharmacology in patients.
3TC has been labeled an activation-independent or resting cell-dependent phosphorylated drug by virtue of the smaller effect of PHA on 3TC phosphorylation in vitro, as well as a more favorable 3TC-TP/dCTP ratio in resting cells (18). The results from this study show that HIV infection was associated with significantly lower 3TC-TP concentrations than those found in HIV-seronegative volunteers (dCTP was not measured in the current study). This finding suggests that 3TC-TP production or elimination favors higher TP in the HIV-seronegative individual. One in vivo study showed lower enzymatic activity of deoxycytidine kinase in HIV-seropositive than in HIV-seronegative volunteers, which would be consistent with the findings in this study (39). The phosphorylation of 3TC in HIV-seronegative individuals suggests that 3TC is well suited to the HIV prevention field, similarly to emtricitabine (FTC), a structurally similar deoxycytidine analog (19).
HIV infection was associated with increased 3TC, but not ZDV, plasma concentrations. The likely explanation for the increase in 3TC plasma levels was HIV-associated decrease in renal function, as 3TC is excreted primarily renally and the effect was attenuated when 3TC plasma levels were normalized to creatinine clearance (20). Of interest, despite the increased systemic plasma 3TC concentration in HIV-seropositive individuals, the 3TC-TP levels were still significantly lower than those of HIV-seronegative volunteers.
Sex did not significantly influence intracellular levels of either drug. This is contrary to the anticipated increase in intracellular concentrations in women from work done previously (4). Other studies have also shown higher levels of ZDV-phosphates in women than men (38) and higher ZDV-TP (10) and 3TC-TP (35) in women. Another study showed the opposite relationship, namely, higher levels of intracellular ZDV-phosphates in men (8). Finally, a recent ex vivo study showed no significant effect of sex on intracellular metabolite levels (32). When the entirety of the literature is considered, no definitive statement on the effect of sex on intracellular levels of ZDV and 3TC in PBMC can be made at this time. Additional pharmacology studies with large sample sizes are warranted to help explain the 20-fold higher risk for lactic acidosis in women than in men (31, 41). Sex differences in phosphorylation in tissues other than PBMC, such as liver, would be one avenue to explore. Additionally, women may have a lower threshold of drug exposure that leads to lactic acidosis than men.
The lack of sex effect on intracellular levels was despite higher plasma ZDV levels in women than in men. The differences in ZDV plasma levels appeared to be explained by the lower body weight of women, as the effect was attenuated when ZDV levels were lean body weight normalized. The influence of glucuronidation on the metabolism of ZDV likely explains this finding. A review by Liston et al. suggests that male sex and increased body weight (correlated to increased liver size) result in higher rates of clearance for numerous drugs which are metabolized primarily through glucuronidation (29).
Although it was not a specific aim at the beginning of the study, African-American race was found to be associated with increased intracellular levels of ZDV-TP (P = 0.01) and 3TC-TP (P = 0.09) and plasma 3TC levels (P = 0.05). While the difference for 3TC plasma appeared to be explained by renal function, the differences in intracellular levels suggest genetic differences within the enzyme system pathways for ZDV- and 3TC-TP disposition. As one example, African-Americans and non-African-Americans carry genetic variants in the MRP4 enzyme with different frequencies (2, 6). Further analyses of race differences in larger sample sizes are needed to verify and extend these findings.
Finally, the effect of study day on intracellular levels was examined to determine the accumulation patterns and half-lives of the drugs in vivo. ZDV-TP accumulated to steady state by day 3. The half-life was estimated to be 8 h based on the accumulation factor. This value is similar to previous estimates of 7 to 11 h (4, 36). In contrast, 3TC-TP accumulated during the duration of the study. Intracellular 3TC levels increased at each study day through day 12, although the difference between days 7 and 12 was not statistically significant. The half-life was estimated to be 27 h by accumulation factor, which is consistent with previous estimates of 22 to 32 h (4, 36).
The decrease in ZDV plasma concentrations during the duration of the study was likely due to inductive drug-drug interactions with the ritonavir-boosted protease inhibitor used by the HIV-seropositive participants. The further examination of ZDV plasma levels, separated by HIV serostatus, showed that the decrease was apparent only in the HIV-seropositive group (Fig. 5). Additionally, Fig. 6 suggests that regimens that included either atazanavir/ritonavir or lopinavir/ritonavir resulted in decreased ZDV plasma concentrations between days 0 and 12. This is consistent with the known glucuronidation induction properties of lopinavir with ritonavir (1). Ritonavir by itself is also known to induce glucuronidation in a dose-dependent fashion (15). Nevertheless, ritonavir-boosted protease inhibitor-zidovudine combinations are clinically effective (33), despite the induction of ZDV glucuronidation by ritonavir.
3TC plasma levels increased from day 0 to day 3 before leveling off and staying consistent for the duration of the study. The accumulation factor predicted a half-life of 6.5 h, which is consistent with previous work, which showed a plasma half-life of between 5 and 7 h for 3TC (26).
In summary, results from this study show that HIV serostatus, but not sex, was associated with differences in intracellular ZDV-TP and 3TC-TP. Additional work is needed to further characterize cellular pharmacology in African-Americans versus non-African-Americans, elucidate female susceptibility to lactic acidosis, and to extend these findings with similar results in a larger sample size.
ACKNOWLEDGMENTS
We thank the NIH AIDS Research and Reference Reagent Program for the antiretroviral drugs used for assays; the study personnel and nurses who assisted with the clinical protocol; Courtney Fletcher, Mariana Gerschenson, Thomas Campbell, John Gerber, Martin Risk, and Liz Connick for assistance with the design or conduct of the study; and the volunteers who participated.
This work was supported by grants from the NIH, R01 AI64029 (P.L.A.) and TL1 RR025778 (J.E.R.), and NCRR Colorado CTSI grant number UL1 RR025780.
We declare no conflict of interest. The contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Footnotes
Published ahead of print 5 March 2012
REFERENCES
- 1. Abbott Laboratories 2011. Kaletra (lopinavir/ritonavir) package insert. Abbott Laboratories, North Chicago, IL [Google Scholar]
- 2. Abla N, et al. 2008. The human multidrug resistance protein 4 (MRP4, ABCC4): functional analysis of a highly polymorphic gene. J. Pharmacol. Exp. Ther. 325:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akaike H. 1974. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19:716–723 [Google Scholar]
- 4. Anderson PL, Kakuda TN, Kawle S, Fletcher CV. 2003. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 17:2159–2168 [DOI] [PubMed] [Google Scholar]
- 5. Anderson PL, et al. 2011. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J. Antimicrob. Chemother. 66:240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson PL, Lamba J, Aquilante CL, Schuetz E, Fletcher CV. 2006. Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-Infected adults: a pilot study. J. Acquir. Immune Defic. Syndr. 42:441–449 [DOI] [PubMed] [Google Scholar]
- 7. Anderson PL, Rower JE. 2010. Zidovudine and lamivudine for HIV Infection. Clin. Med. Rev. Ther. 2:115–127 [PMC free article] [PubMed] [Google Scholar]
- 8. Aweeka FT, et al. 2006. The impact of sex and contraceptive therapy on the plasma and intracellular pharmacokinetics of zidovudine. AIDS 20:1833–1841 [DOI] [PubMed] [Google Scholar]
- 9. Balzarini J. 1994. The metabolism and mechanism of antiretroviral action of purine and pyrimidine derivatives. Pharm. World Sci. 16:113–126 [DOI] [PubMed] [Google Scholar]
- 10. Bazzoli C, et al. 2011. Joint population pharmacokinetic analysis of zidovudine, lamivudine, and their active intracellular metabolites in HIV patients. Antimicrob. Agents Chemother. 55:3423–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broder S. 2010. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 85:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bushman LR, et al. 2011. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J. Pharm. Biomed. Anal. 56:390–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cihlar T, Ray AS. 2010. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 85:39–58 [DOI] [PubMed] [Google Scholar]
- 14. Deeks SG, et al. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947 [DOI] [PubMed] [Google Scholar]
- 15. Foisy MM, Yakiwchuk EM, Hughes CA. 2008. Induction effects of ritonavir: implications for drug interactions. Ann. Pharmacother. 42:1048–1059 [DOI] [PubMed] [Google Scholar]
- 16. Furman PA, et al. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 83:8333–8337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao W-Y, Shirasaka T, Johns DG, Broder S, Mitsuya H. 1993. Differential phosphorylation of azidothymidine, dideoxycytidine and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J. Clin. Investig. 91:2326–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao WY, Agbaria R, Driscoll JS, Mitsuya H. 1994. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J. Biol. Chem. 269:12633–12638 [PubMed] [Google Scholar]
- 19. Grant RM, et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heald A, et al. 1996. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob. Agents Chemother. 40:1514–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu C-H, et al. 2007. Comparison of the phosphorylation of 4′-ethynyl 2′,3′-dihydro-3′-deoxythymidine with that of other anti-human immunodeficiency virus thymidine analogs. Antimicrob. Agents Chemother. 51:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunsucker SA, Mitchell BS, Spychala J. 2005. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol. Ther. 107:1–30 [DOI] [PubMed] [Google Scholar]
- 23. Jacobsson B, Britton S, Törnevik Y, Eriksson S. 1998. Decrease in thymidylate kinase activity in peripheral blood mononuclear cells from HIV-infected individuals. Biochem. Pharmacol. 56:389–395 [DOI] [PubMed] [Google Scholar]
- 24. Jiang W, et al. 2009. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 199:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson AA, et al. 2001. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J. Biol. Chem. 276:40847–40857 [DOI] [PubMed] [Google Scholar]
- 26. Johnson MA, Moore KHP, Yuen GJ, Bye A, Pakes GE. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41–66 [DOI] [PubMed] [Google Scholar]
- 27. Kakuda TN. 2000. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22:685–708 [DOI] [PubMed] [Google Scholar]
- 28. Kewn S, Veal GJ, Hoggard PG, Barry MG, Back DJ. 1997. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem. Pharmacol. 54:589–595 [DOI] [PubMed] [Google Scholar]
- 29. Liston HL, Markowitz JS, DeVane CL. 2001. Drug glucuronidation in clinical psychopharmacology. J. Clin. Psychopharmacol. 21:500–515 [DOI] [PubMed] [Google Scholar]
- 30. Littell RC, Milliken GA, Stroup WW, Wolfinger RD. 1996. SAS system for mixed models. SAS Institute, Inc., Cary, NC [Google Scholar]
- 31. Osler M, Stead D, Rebe K, Meintjes G, Boulle A. 2010. Risk factors for and clinical characteristics of severe hyperlactataemia in patients receiving antiretroviral therapy: a case-control study. HIV Med. 11:121–129 [DOI] [PubMed] [Google Scholar]
- 32. Paintsil E, et al. 2011. Determinants of individual variation in intracellular accumulation of anti-HIV nucleoside analog metabolites. Antimicrob. Agents Chemother. 55:895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Panel on Antiretroviral Guidelines for Adults and Adolescents 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services, Washington, DC [Google Scholar]
- 34. Podgorska M, Kocbuch K, Pawelczy T. 2005. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochim. Polonica 52:749–758 [PubMed] [Google Scholar]
- 35. Pruvost A, et al. 2009. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob. Agents Chemother. 53:1937–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez JF, Rodriguez JL, Santana J, Garcia H, Rosario O. 2000. Simultaneous quantitation of intracellular zidovudine and lamivudine triphosphates in human immunodeficiency virus-infected individuals. Antimicrob. Agents Chemother. 44:3097–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rower JE, Klein B, Bushman LR, Anderson PL. 2011. Validation of a sensitive LC/MS/MS method for the determination of zidovudine and lamivudine in human plasma. Biomed. Chromatogr. 26:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stretcher BN, Pesce AJ, Frame PT, Stein DS. 1994. Pharmacokinetics of zidovudine phosphorylation in peripheral blood mononuclear cells from patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 38:1541–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Turriziani O, et al. 2005. Thymidine kinase and deoxycytidine kinase activity in mononuclear cells from antiretroviral-naive HIV-infected patients. AIDS 19:473–479 [DOI] [PubMed] [Google Scholar]
- 40. Van Rompay AR, Johansson M, Karlsson A. 2000. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol. Ther. 87:189–198 [DOI] [PubMed] [Google Scholar]
- 41. Wester CW, et al. 2007. Higher-than-expected rates of lactic acidosis among highly active antiretroviral therapy-treated women in Botswana: preliminary results from a large randomized clinical trial. J. Acquir. Immune Defic. Syndr. 46:318–322 [DOI] [PubMed] [Google Scholar]