Abstract
The aim of this open-label, fixed-sequence study was to investigate the potential of the botanical supplement milk thistle (silymarin) to interact with the boosted protease inhibitor combination darunavir-ritonavir. Fifteen HIV-infected patients receiving antiretroviral therapy with darunavir-ritonavir (600/100 mg twice daily) for at least 4 weeks were included. Silymarin (150 mg every 8 h) was added to the antiretroviral treatment from days 1 to 14. Darunavir concentrations in plasma were determined by high-performance liquid chromatography immediately before and 1, 2, 4, 6, 8, 10, and 12 h after a morning dose of darunavir-ritonavir on day 0 and darunavir-ritonavir plus silymarin on day 14. Individual darunavir pharmacokinetic parameters were calculated by noncompartmental analysis and compared between days 0 and 14 by means of the geometric mean ratio (GMR) and its 90% confidence interval (CI). The median age was 48 years (interquartile range, 44 to 50 years), and the median body weight was 70 kg (interquartile range, 65 to 84 kg). Silymarin was well tolerated, and all participants completed the study. The GMRs for darunavir coadministered with silymarin relative to darunavir alone were 0.86 (90% CI, 0.70 to 1.05) for the area under the concentration-time curve from 0 to 12 h, 0.83 (90% CI, 0.80 to 0.98) for the maximum concentration, and 0.94 (90% CI, 0.73 to 1.19) for the concentration at the end of the dosing interval. In summary, coadministration of silymarin with darunavir-ritonavir seems to be safe in HIV-infected patients; no dose adjustment for darunavir-ritonavir seems to be necessary.
INTRODUCTION
The use of alternative medicines, including botanical supplements, is common among HIV-infected patients. More than one-third of patients have been reported to take herbal remedies, usually as a self-prescribed treatment and without medical supervision (26, 30a, 38). Although botanical supplements are often perceived to be innocuous, in fact they can modulate various cytochrome P450 enzymes (e.g., CYP3A4) and drug transporters (e.g., P-glycoprotein [P-gp]), providing a reason to suspect potential herb-drug interactions (22, 24, 25).
The most widely recognized herb to interact with drugs is St. John's wort (Hypericum perforatum), which is a potent inducer of CYP3A4 and P-gp activity (18, 19) and which has been related to significant decreases in exposure to different drugs, including the antiretrovirals indinavir and nevirapine (5, 35). Additionally, numerous in vitro studies have shown that other botanical supplements are also capable of modulating CYP and P-gp activity and, thus, interact with drugs (3, 22, 25), although results from human in vivo studies have been less convincing (14, 15, 22, 25).
Silybum marianum, an herb commonly known as milk thistle, currently ranks among the top-selling botanical supplements (27). The main active components of milk thistle are flavonolignan complexes, collectively known as silymarin. Silymarin has been reported to exert hepatoprotective properties against a variety of xenobiotics (20, 31, 32, 42) and may be administered for the treatment of liver diseases (11, 39). Noteworthy, there is recent evidence of the inhibition of hepatitis C virus (HCV) RNA polymerase by silymarin (1, 28), and results from several small clinical trials suggest that silymarin could be used as an adjunctive therapy for HCV infection (10, 11, 29, 34). Therefore, a number of HIV-infected patients, particularly those coinfected with HCV, may be interested in taking milk thistle in addition to antiretroviral therapy (30a).
In vitro studies have shown that silymarin may function as a substrate and inhibitor of CYP3A4 and P-gp (2, 3, 41, 43, 44), and this may result in interactions with many drugs, including HIV protease inhibitors. However, clinical studies with milk thistle have produced discordant results regarding its ability to modulate these proteins in vivo (7, 12, 14, 15, 17, 37), and evidence on the interaction between milk thistle and antiretroviral drugs comes from clinical trials that have not used currently recommended treatment regimens (8, 30, 36).
The aim of the present study was, therefore, to evaluate the potential of silymarin to interact with a boosted protease inhibitor such as darunavir-ritonavir.
MATERIALS AND METHODS
Study design.
This open-label, fixed-sequence study enrolled 15 HIV-infected patients who were receiving antiretroviral therapy with darunavir-ritonavir at a dosage of 600/100 mg twice daily for at least 4 weeks and whose HIV-1 RNA load in plasma was <50 copies/ml. All patients gave written informed consent before enrollment, the protocol was approved by the ethics committee of the Hospital Universitari Germans Trias i Pujol, and the study was performed according to the stipulations of the Declaration of Helsinki and registered (Clinicaltrials.gov NCT01346982).
According to the package insert, patients received one capsule containing 150 mg of silymarin every 8 h from days 1 to 14 (Legalon; lot no. D0003; Rottapharm Madaus, Barcelona, Spain). All pills came from a single lot, which was externally controlled and certified to contain 100% of the labeled content of silymarin. Antiretroviral treatment remained unchanged. Serial blood samples to determine darunavir and ritonavir concentrations in plasma were collected immediately before and 1, 2, 4, 6, 8, 10, and 12 h after a witnessed morning dose of darunavir-ritonavir on day 0 and at the same times before and after darunavir-ritonavir plus silymarin on day 14. Darunavir and ritonavir were dosed with a standard breakfast consisting of 550 kcal (43% carbohydrate, 39% fat, 18% protein).
Demographic and clinical variables (including age, body weight and height, and use of concomitant drugs, including over-the-counter medications) were recorded. Safety was evaluated by clinical interview and physical examination as well as by laboratory assessment (blood counts, chemistry, CD4+ T cell count, and HIV-1 RNA load) on days 0, 14, and 28. To enhance adherence to scheduled clinical visits and the treatment protocol, patients were provided with a visit calendar. Apart from days 0 and 14, drug intake was not directly observed; adherence was assessed by means of a diary in which the patient recorded medication intake and by pill count on day 14.
Analytical and pharmacokinetic analysis.
Blood samples for darunavir and ritonavir determinations were collected into K-EDTA-containing 10-ml tubes. Plasma was isolated by centrifugation (3,200 × g for 15 min) and stored at −20°C until analysis. Darunavir and ritonavir concentrations were determined by high-performance liquid chromatography with a photo diode array detector (HPLC-PDA 2996; Waters, Barcelona, Spain), according to a validated method. The analytical column was a NovaPak C18 3.9- by 150-mm with a NovaPak C18 guard column (Waters). The method involved liquid-liquid extraction of drug from plasma with methyl tert-butyl ether. The mobile phase consisted of a gradient elution with phosphate buffer in acetonitrile (pH 6.70). The method was linear over the range of 0.05 to 10.0 mg/liter for both drugs (lower limit of quantification, 0.05 mg/liter; intra- and interday variation, <10%). Our laboratory subscribes to the external quality assurance program organized by the Association for Quality Assessment in Therapeutic Drug Monitoring and Clinical Toxicology of Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands (9).
Darunavir and ritonavir pharmacokinetic parameters were calculated for each individual using a noncompartmental approach by means of the WinNonlin software application (version 2.0; Pharsight, Mountain View, CA). The area under the concentration-time curve during the dose interval of 0 to 12 h (AUC0-12) was calculated by means of the linear trapezoidal rule. Maximum concentrations (Cmax) and the concentrations at the end of the 12-h dosing interval (C12) were obtained by inspection of the concentration data.
Statistical analysis.
Data analysis was carried out using SPSS (version 15.0) statistical software (Chicago, IL). Darunavir and ritonavir pharmacokinetic parameters were described by the geometric mean and compared between days 0 and 14 by the geometric mean ratio (GMR) and its 90% confidence interval (CI). Pharmacokinetic parameters were natural log transformed before analysis, and confidence intervals for means (and for the difference between two means) were constructed on the natural log scale on the basis of an analysis-of-variance model with treatment as a fixed effect. The results were exponentiated and reported with lower and upper limits of the 90% CIs.
A power calculation indicated that 15 patients would provide an 80% chance of detecting a 25% difference in the AUC0–12 for darunavir at a level of significance of P equal to 0.1.
RESULTS
A total of 15 Caucasian HIV-infected males were enrolled; 4 were coinfected with HCV. The median age was 48 years (interquartile range, 44 to 50) years, and the median body weight was 70 kg (interquartile range, 65 to 84 kg). Antiretroviral treatments in addition to darunavir-ritonavir included raltegravir in 12 patients, tenofovir in 10, emtricitabine in 8, etravirine in 7, and maraviroc in 1. The median CD4+ T cell count was 439 cells/mm3 (interquartile range, 360 to 614 cells/mm3).
Silymarin was well tolerated, and all participants completed the study. The only adverse event reported was mild heartburn in one patient. All patients maintained an HIV-1 RNA load of <50 copies/ml until the end of the study.
Darunavir and ritonavir pharmacokinetics.
Darunavir and ritonavir plasma concentration-time curves following administration of darunavir-ritonavir alone or in combination with multiple doses of silymarin are shown in Fig. 1. Darunavir and ritonavir pharmacokinetic parameters with and without silymarin coadministration are summarized in Tables 1 and 2, respectively. Coadministration of silymarin resulted in a slight, though not statistically significant, decrease in darunavir and ritonavir exposure, as both Cmax and AUC0-12 decreased by about 15%, on the average. There was no substantial effect on the darunavir C12 (Fig. 2), which remained above the protein-binding-corrected 50% inhibitory concentration (IC50) for viral strains with protease inhibitor resistance-associated mutations (0.55 mg/liter) (6) in all participants.
Fig 1.
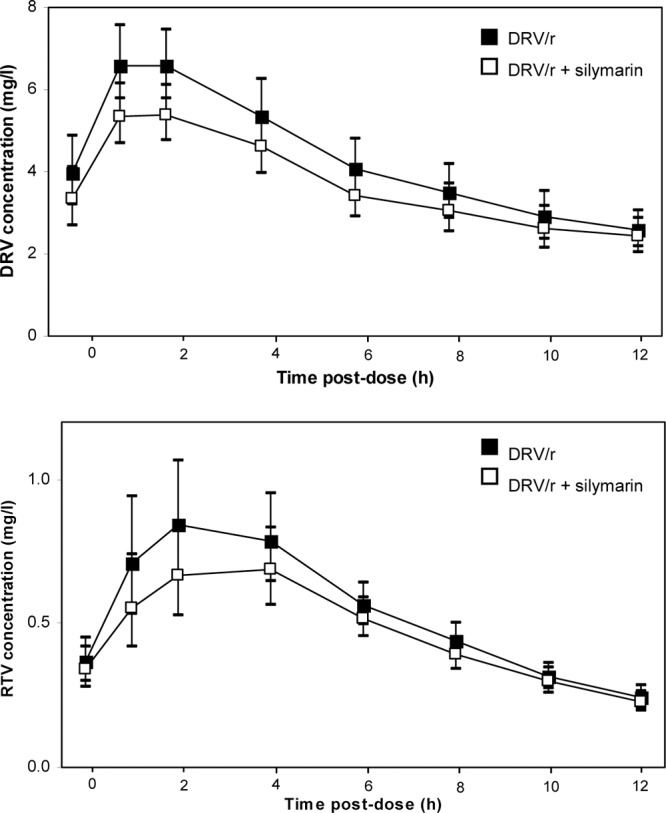
Geometric mean darunavir (DRV) and ritonavir (RTV) plasma concentration profiles of darunavir-ritonavir (DRV/r) with or without coadministration of multiple doses of silymarin. Error bars represent the 90% confidence interval.
Table 1.
Comparison of darunavir pharmacokinetic parameters with and without coadministration of multiple doses of silymarina
| Drugs or parameter | AUC0-12 (mg · h/liter) | Cmax (mg/liter) | C12 (mg/liter) |
|---|---|---|---|
| DRV/r | 53.21 (46.03–61.38) | 7.08 (6.28–7.98) | 2.58 (2.17–3.07) |
| DRV/r + silymarin | 45.60 (39.54–52.72) | 5.86 (5.20–6.61) | 2.42 (2.04–2.87) |
| GMR | 0.86 (0.70–1.05) | 0.83 (0.70–0.98) | 0.94 (0.73–1.19) |
| P | 0.215 | 0.07 | 0.654 |
Data are expressed as geometric mean (90% confidence interval). DRV/r, darunavir-ritonavir; GMR, geometric mean ratio; AUC0-12, area under the time-concentration curve from 0 to 12 h after dosing; Cmax, maximum concentration; C12, concentration at the end of the 12-h dosing interval.
Table 2.
Comparison of ritonavir pharmacokinetic parameters with and without coadministration of multiple doses of silymarina
| Drugs or parameter | AUC0-12 (mg · h/liter) | Cmax (mg/liter) | C12 (mg/liter) |
|---|---|---|---|
| DRV/r | 6.73 (5.82–7.80) | 0.95 (0.80–1.12) | 0.24 (80.21–0.28) |
| DRV/r + silymarin | 6.00 (5.19–6.93) | 0.86 (0.73–1.01) | 0.23 (0.19–0.26) |
| GMR | 0.89 (0.72–1.09) | 0.90 (0.72–1.14) | 0.94 (0.75–1.16) |
| P | 0.346 | 0.465 | 0.601 |
Data are expressed as geometric mean (90% confidence interval). DRV/r, darunavir-ritonavir; GMR, geometric mean ratio; AUC0-12, area under the time-concentration curve from 0 to 12 h after dosing; Cmax, maximum concentration; C12, concentration at the end of the 12-h dosing interval.
Fig 2.
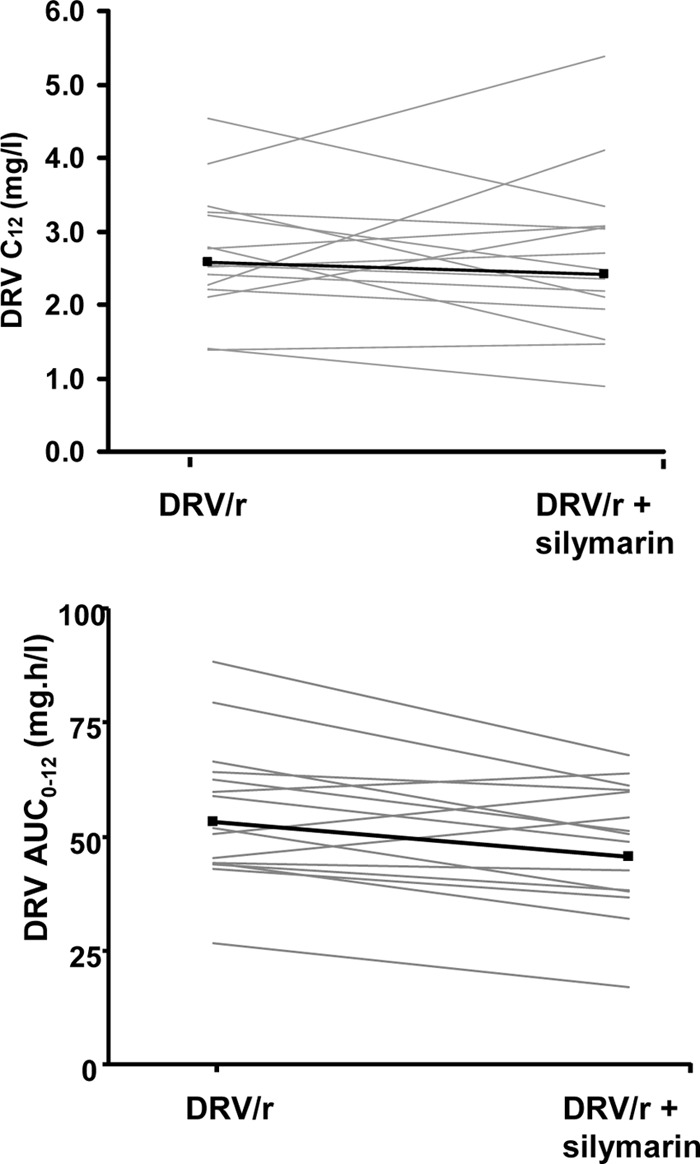
Darunavir (DRV) concentration at the end of the 12-h dosing interval (C12) and area under the time-concentration curve from 0 to 12 h (AUC0–12) values after administration of darunavir-ritonavir with or without multiple doses of silymarin. Gray lines represent individual values, and black lines represent the geometric mean.
DISCUSSION
Although in vitro inhibition of CYP3A4 and P-gP by silymarin (2, 3, 41, 43, 44) suggests that coadministration of this drug with HIV protease inhibitors such as darunavir could theoretically result in higher drug exposure, our findings show that the in vivo effect seems to be negligible. We found that there was a close correspondence of the pharmacokinetic profiles of darunavir in the presence and absence of coadministered silymarin.
Coadministration of silymarin with darunavir-ritonavir did result in a mean darunavir Cmax and an AUC0–12 that were nonsignificantly lower by 15%. The magnitude of this interaction is on the same order as that reported between efavirenz and darunavir in a study by Sekar and colleagues (40), who concluded that no darunavir dose adjustment was required. The potential impact of this interaction on the clinical outcome of HIV-infected patients seems to be quite limited in patients without darunavir resistance-associated mutations. Even when darunavir is dosed once daily, darunavir trough concentrations have been shown to be nearly 40 times above the protein binding-corrected IC50 for wild-type viral strains (39a). The only scenario where this tendency toward interaction might be clinically relevant could be in treatment-experienced patients harboring viral strains with decreased susceptibility to protease inhibitors. Even small decreases in drug concentration could put such patients at higher risk of treatment failure and further selection of resistance mutations. In the present study, however, using the darunavir-ritonavir dose of 600/100 mg twice daily, darunavir concentrations at the end of the dosing interval remained above the protein binding-corrected IC50 for viral strains resistant to other protease inhibitors (6).
The interaction between milk thistle and antiretroviral drugs was studied several years ago in healthy volunteers who received indinavir with or without multiple doses of silymarin (8, 30, 36). Administration of silymarin also failed to influence the indinavir AUC in these studies, although slight reductions in indinavir trough concentrations were observed. Indinavir was given at a dose of 800 mg every 8 h without ritonavir in these studies, however, reflecting dosing that is no longer recommended for routine clinical care of HIV-infected patients (33). Protease inhibitors are currently boosted by means of coadministered low doses of ritonavir, which acts as a potent CYP3A4 inhibitor (21, 33). This strategy helps maintain drug concentrations far above the concentration needed to inhibit viral replication. Additionally, strong inhibition of CYP3A4 by ritonavir may also limit the potential of other agents to interact with protease inhibitors, as seems to have been the case in our study.
Our results are in line with those reported by Gurley and various coauthors (14–17), who did not observe any significant interaction between silymarin and midazolam, debrisoquine, or digoxin, used as probes for CYP3A4, CYP2D6, and P-gp, respectively, in humans. Various factors may help explain discrepancies between results from in vitro experiments (2, 3, 41, 43, 44) and the above-mentioned in vivo studies. First, there may be great variability in silymarin content among different milk thistle brands available on the market. Furthermore, discrepancies between the labeled and the actual content of active constituents have been reported for many botanical preparations (13). For our study, we purchased a single lot of silymarin from a sole vendor that used an external laboratory to certify that pills contained 100% of the labeled content of the drug. A second, more plausible explanation for discrepancies between in vitro and in vivo findings may be related to the poor and erratic oral bioavailability of silymarin, given that milk thistle flavonolignans exhibit low solubility in water (4, 23). Since we did not measure silymarin concentrations in plasma, we cannot confirm its solubility and bioavailability in our study. For the same reason, it remains to be determined whether our findings may be extrapolated to other milk thistle formulations.
In summary, our results suggest that coadministration of silymarin with darunavir-ritonavir and probably with other HIV boosted protease inhibitors is safe and well tolerated and does not result in significant pharmacokinetic interactions. We therefore believe that no dose adjustment for darunavir-ritonavir seems to be necessary.
ACKNOWLEDGMENTS
We thank the staff at the clinical site where data for this study were gathered and the patients who participated. We also acknowledge the contribution of Mary Ellen Kerans, who gave her advice on English language expression in the final version of the manuscript.
We have no conflicts of interest that are directly relevant to the context of this study.
This study was funded by a grant from the Spanish Health Department (Ministerio de Sanidad, Política Social e Igualdad; EC10-092) and by the Lluita contra la SIDA Foundation—Gala contra la Sida, Barcelona, Spain, 2011. M.V. is supported by FIS trough grant CP04/00121 from the Spanish Health Department in collaboration with the Institut de Recerca de l'Hospital de la Santa Creu i Sant Pau, Barcelona, Spain, and she is a member of CIBERSAM (funded by the Spanish Health Department, Instituto de Salud Carlos III).
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Ahmed-Belkacem A, et al. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology 138:1112–1122 [DOI] [PubMed] [Google Scholar]
- 2. Beckmann-Knopp S, et al. 2000. Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacol. Toxicol. 86:250–256 [DOI] [PubMed] [Google Scholar]
- 3. Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. 2000. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine 7:273–282 [DOI] [PubMed] [Google Scholar]
- 4. Das S, Roy P, Auddy RG, Mukherjee A. Silymarin nanoparticle prevents paracetamol-induced hepatotoxicity. Int. J. Nanomed. 6:1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Maat MM, et al. 2001. Drug interaction between St John's wort and nevirapine. AIDS 15:420–421 [DOI] [PubMed] [Google Scholar]
- 6. De Meyer S, et al. 2005. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 49:2314–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deng JW, et al. 2008. Effect of silymarin supplement on the pharmacokinetics of rosuvastatin. Pharm. Res. 25:1807–1814 [DOI] [PubMed] [Google Scholar]
- 8. DiCenzo R, et al. 2003. Coadministration of milk thistle and indinavir in healthy subjects. Pharmacotherapy 23:866–870 [DOI] [PubMed] [Google Scholar]
- 9. Droste JA, Aarnoutse RE, Koopmans PP, Hekster YA, Burger DM. 2003. Evaluation of antiretroviral drug measurements by an interlaboratory quality control program. J. Acquir. Immune Defic. Syndr. 32:287–291 [DOI] [PubMed] [Google Scholar]
- 10. Eurich D, et al. Treatment of hepatitis C-virus-reinfection after liver transplant with silibinin in nonresponders to pegylated interferon-based therapy. Exp. Clin. Transplant.9::1–6 [PubMed] [Google Scholar]
- 11. Ferenci P, et al. 2008. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology 135:1561–1567 [DOI] [PubMed] [Google Scholar]
- 12. Fuhr U, Beckmann-Knopp S, Jetter A, Luck H, Mengs U. 2007. The effect of silymarin on oral nifedipine pharmacokinetics. Planta Med. 73:1429–1435 [DOI] [PubMed] [Google Scholar]
- 13. Garrard J, Harms S, Eberly LE, Matiak A. 2003. Variations in product choices of frequently purchased herbs: caveat emptor. Arch. Intern. Med. 163:2290–2295 [DOI] [PubMed] [Google Scholar]
- 14. Gurley B, et al. 2006. Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J. Clin. Pharmacol. 46:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gurley BJ, et al. 2006. Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab. Dispos. 34:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gurley BJ, et al. 2005. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John's wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging 22:525–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gurley BJ, et al. 2008. Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John's wort, and echinacea. Mol. Nutr. Food Res. 52:755–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutmann H, et al. 2006. Hypericum perforatum: which constituents may induce intestinal MDR1 and CYP3A4 mRNA expression? Planta Med. 72:685–690 [DOI] [PubMed] [Google Scholar]
- 19. Hennessy M, et al. 2002. St Johns wort increases expression of P-glycoprotein: implications for drug interactions. Br. J. Clin. Pharmacol. 53:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hruby K, Csomos G, Fuhrmann M, Thaler H. 1983. Chemotherapy of Amanita phalloides poisoning with intravenous silibinin. Hum. Toxicol. 2:183–195 [DOI] [PubMed] [Google Scholar]
- 21. Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Ann. Med. 43:375–388 [DOI] [PubMed] [Google Scholar]
- 22. Izzo AA, Ernst E. 2009. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 69:1777–1798 [DOI] [PubMed] [Google Scholar]
- 23. Kim YC, et al. 2003. Comparative bioavailability of silibinin in healthy male volunteers. Int. J. Clin. Pharmacol. Ther. 41:593–596 [DOI] [PubMed] [Google Scholar]
- 24. Ladenheim D, et al. 2008. Potential health risks of complementary alternative medicines in HIV patients. HIV Med. 9:653–659 [DOI] [PubMed] [Google Scholar]
- 25. Lee LS, Andrade AS, Flexner C. 2006. Interactions between natural health products and antiretroviral drugs: pharmacokinetic and pharmacodynamic effects. Clin. Infect. Dis. 43:1052–1059 [DOI] [PubMed] [Google Scholar]
- 26. Littlewood RA, Vanable PA. 2008. Complementary and alternative medicine use among HIV-positive people: research synthesis and implications for HIV care. AIDS Care 20:1002–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loguercio C, Festi D. 2011. Silybin and the liver: from basic research to clinical practice. World J. Gastroenterol. 17:2288–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehrab-Mohseni M, et al. Legalon-SIL downregulates HCV core and NS5A in human hepatocytes expressing full-length HCV. World J. Gastroenterol. 17:1694–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Melhem A, et al. 2005. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J. Clin. Gastroenterol. 39:737–742 [DOI] [PubMed] [Google Scholar]
- 30. Mills E, et al. 2005. Milk thistle and indinavir: a randomized controlled pharmacokinetics study and meta-analysis. Eur. J. Clin. Pharmacol. 61:1–7 [DOI] [PubMed] [Google Scholar]
- 30a. Moltó J, et al. 2012. Use of herbal remedies among HIV-infected patients: patterns and correlates. Med. Clin. (Barc.) 138:93–98 [DOI] [PubMed] [Google Scholar]
- 31. Muriel P, Garciapina T, Perez-Alvarez V, Mourelle M. 1992. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J. Appl. Toxicol. 12:439–442 [DOI] [PubMed] [Google Scholar]
- 32. Muriel P, Moreno MG, Hernandez Mdel C, Chavez E, Alcantar LK. 2005. Resolution of liver fibrosis in chronic CCl4 administration in the rat after discontinuation of treatment: effect of silymarin, silibinin, colchicine and trimethylcolchicinic acid. Basic Clin. Pharmacol. Toxicol. 96:375–380 [DOI] [PubMed] [Google Scholar]
- 33. Panel on Antiretroviral Guidelines for Adults and Adolescents 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. U.S. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf [Google Scholar]
- 34. Payer BA, et al. Successful HCV eradication and inhibition of HIV replication by intravenous silibinin in an HIV-HCV coinfected patient. J. Clin. Virol. 49:131–133 [DOI] [PubMed] [Google Scholar]
- 35. Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. 2000. Indinavir concentrations and St John's wort. Lancet 355:547–548 [DOI] [PubMed] [Google Scholar]
- 36. Piscitelli SC, et al. 2002. Effect of milk thistle on the pharmacokinetics of indinavir in healthy volunteers. Pharmacotherapy 22:551–556 [DOI] [PubMed] [Google Scholar]
- 37. Rajnarayana K, Reddy MS, Vidyasagar J, Krishna DR. 2004. Study on the influence of silymarin pretreatment on metabolism and disposition of metronidazole. Arzneimittelforschung 54:109–113 [DOI] [PubMed] [Google Scholar]
- 38. Risa KJ, et al. 2002. Alternative therapy use in HIV-infected patients receiving highly active antiretroviral therapy. Int. J. STD AIDS 13:706–713 [DOI] [PubMed] [Google Scholar]
- 39. Salmi HA, Sarna S. 1982. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand. J. Gastroenterol. 17:517–521 [DOI] [PubMed] [Google Scholar]
- 39a.Sekar V, et al. Abstr. 15th Conf. Retrovir. Opportunist. Infect., abstr. 769.2008. [Google Scholar]
- 40. Sekar VJ, et al. 2007. Pharmacokinetic interaction between TMC114/r and efavirenz in healthy volunteers. Antivir. Ther. 12:509–514 [PubMed] [Google Scholar]
- 41. Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. 2004. Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab. Dispos. 32:587–594 [DOI] [PubMed] [Google Scholar]
- 42. Valenzuela A, Lagos C, Schmidt K, Videla LA. 1985. Silymarin protection against hepatic lipid peroxidation induced by acute ethanol intoxication in the rat. Biochem. Pharmacol. 34:2209–2212 [DOI] [PubMed] [Google Scholar]
- 43. Venkataramanan R, et al. 2000. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metab. Dispos. 28:1270–1273 [PubMed] [Google Scholar]
- 44. Zhang S, Morris ME. 2003. Effect of the flavonoids biochanin A and silymarin on the P-glycoprotein-mediated transport of digoxin and vinblastine in human intestinal Caco-2 cells. Pharm. Res. 20:1184–1191 [DOI] [PubMed] [Google Scholar]


