Abstract
There is little information about the effectiveness of ciprofloxacin in regions where ciprofloxacin-resistant Escherichia coli is prevalent. This study was conducted to evaluate whether ciprofloxacin is effective as the initial empirical antibiotic for treatment of uncomplicated acute pyelonephritis (APN) due to ciprofloxacin-resistant E. coli. A total of 255 women with clinical diagnoses of uncomplicated APN due to E. coli were enrolled in the emergency department between March 2005 and December 2008. All enrolled patients were initially treated with ciprofloxacin. Patients were followed up 4 to 7 days after the start of therapy and 14 to 21 days after its completion. At the first follow-up visit, ciprofloxacin was changed to the appropriate antibiotic when necessary, depending on the antibiotic susceptibility results. Not only improvement of symptoms and signs but also microbiologic eradication was assessed at each visit. Fifteen percent (39/255) of the E. coli isolates were resistant to ciprofloxacin. There was no statistically significant difference between the clinical cure rates of the ciprofloxacin-susceptible group and the ciprofloxacin-resistant group at the first follow-up (87.0% versus 76.9%, P = 0.135) or the second follow-up (98.6% versus 94.9%, P = 0.177). However, there was a lower microbiologic cure rate in the ciprofloxacin-resistant group than in the ciprofloxacin-susceptible group (92.4% versus 41.7%, P = 0.000) at the first follow-up visit. No complications occurred in the ciprofloxacin-resistant group during the follow-up period. Our findings indicate that ciprofloxacin is an appropriate choice for empirical therapy of uncomplicated APN and has no serious adverse outcomes, if it is tailored appropriately, even for women infected with ciprofloxacin-resistant E. coli.
INTRODUCTION
Urinary tract infections (UTI), including acute pyelonephritis (APN), are among the most common bacterial infections in women. Escherichia coli is the most common pathogen, but the antimicrobial susceptibility patterns of E. coli differ considerably in different countries (3, 15). The Infectious Diseases Society of America (IDSA) recommended fluoroquinolones as initial empirical therapy for APN in areas where the frequency of resistance of community uropathogens to trimethoprim-sulfamethoxazole (TMP-SMX) exceeds 20% and that to fluoroquinolones is less than 10% (4).
Unfortunately the reported frequency of ciprofloxacin-resistant E. coli isolates causing UTI in the community is around 20% in Korea (12); hence, concern about inappropriate empirical therapy for APN has arisen. While there is evidence that discordant treatment has a negative impact on clinical outcome (6, 10, 14), its impact is known to be variable depending on the site of infection (7). APN in particular is reported to have a lower severity index as well as a lower mortality rate than non-urinary tract infections (1), so it is unclear what strategies should be used for employing fluoroquinolones at the outset of therapy in areas of high ciprofloxacin resistance and for modifying regimens based on the results of susceptibility tests, because no previous study has focused specifically on ciprofloxacin or on uncomplicated APN regardless of the presence of bacteremia (13). We therefore designed a prospective observational study to evaluate the clinical effectiveness of ciprofloxacin as empirical treatment for uncomplicated APN in Korea, where the prevalence of resistance of uropathogenic E. coli to fluoroquinolones exceeds 10%.
MATERIALS AND METHODS
Study populations.
Female patients aged 15 years or over who first visited the emergency clinic in Seoul National University Bundang Hospital from 1 March 2005 to 31 December 2008 were prospectively evaluated. This study was approved by our institutional review board. As it was an observational study, patients were not randomized. A standardized case report form was used to collect patient information, and we focused on patients initially considered to have uncomplicated APN specifically caused by E. coli.
The inclusion criteria for diagnosis of suspected uncomplicated APN consisted of the simultaneous presence of (i) fever of over 38°C as an axillary temperature; (ii) pyuria, defined as the presence of 5 or more leukocytes per high-power field of urinary sediment; (iii) dysuria syndrome (painful urination, sense of residual urine, or urinary frequency); and (iv) tenderness in the flank. In all cases, an E. coli-positive urine or blood culture was necessary for inclusion.
Exclusion criteria were (i) the presence of septic shock, (ii) previously known urinary tract abnormality, (iii) APN proven to be complicated in another hospital, (iv) pregnancy or lactation, and (v) use of a systemic antimicrobial within the previous 72 h. Patients proven to have the following conditions were excluded during workup: those who had newly proven complications (urinary tract obstruction or malformation, or renal abscess); or those who were not prescribed ciprofloxacin as an initial empirical antibiotic treatment.
Study procedures.
Patients received an initial 400-mg dose of intravenous ciprofloxacin followed by oral ciprofloxacin, 500 mg twice daily. Patients who tolerated the oral medication were discharged at the clinician's discretion, while those who were unable to tolerate it were hospitalized for parenteral treatment. Ciprofloxacin was prescribed for 7 to 14 days but was replaced by a more appropriate antibiotic (usually oral cephalosporin) on the basis of susceptibility data if the organism was resistant to ciprofloxacin. Clinical and microbiological follow-up was done at 4 to 7 days after the start of therapy and at 14 to 21 days after its completion. The first follow-up day from initial treatment was the day when, in addition to recording clinical assessments, follow-up microbiologic studies were ordered for the patients admitted to the hospital. For patients not admitted to the hospital, the first follow-up day was the first day they visited the outpatient clinic after discharge from the emergency department. The presence or absence of symptoms and signs suggestive of urinary tract infection, including positive findings recorded when the patients visited the emergency clinic, was reassessed. If particular symptoms were not specifically commented on in the records, they were presumed to be absent when we collected and analyzed the data. Microbiological tests were not conducted if patients did not give consent at follow-up visits. Adverse events were recorded.
Microbiologic studies.
A semiquantitative urine culture and blood cultures were collected from all patients before the start of therapy, as well as a urine culture on days 4 to 7 of therapy, and 14 to 21 days after its completion, or if symptoms of UTI appeared at any time during follow-up. Organisms present at ≥103 CFU/ml in a urine sample were identified by standard microbiological techniques. All blood samples were cultured using an automated culturing system (Vitek, bioMérieux, Marcy l'Etoile, France). Antibiotic sensitivity testing was performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines (2). For stored blood isolates, the MIC for ciprofloxacin was determined by the Etest (AB Biodisk, Solna, Sweden). In addition, multiplex PCR testing was done to verify the presence of six plasmid-mediated quinolone resistance determinants (PMQR), i.e., qnrA, qnrB, qnrC, qnrS, aac(6′)-Ib-cr, and qepA, and PCR amplification of the quinolone resistance-determining regions (QRDRs) was carried out to determine the mutations of gyrA and parC genes as previously described (9).
Outcome measures.
Clinical cure was defined as the absence of all signs and symptoms of illness. Clinical failure was defined as deterioration, persistence of signs and symptoms, or recurrence after initial cure or improvement. Microbiological cure was defined as pathogen growth of less than 103 CFU/ml from the urine, as well as no growth from blood cultures if bacteremia was initially documented. Microbiological failure at follow-up visits was defined as superinfection with a new uropathogen or persistence of the original pathogen.
Statistical analysis.
Data were analyzed with SPSS version 17.0 (SPSS, Inc., Chicago, IL). Binomial data were analyzed by the chi-square (χ2) test or Fisher's exact test, and continuous data by Student's t test. Results below a 5% significance level were considered meaningful.
RESULTS
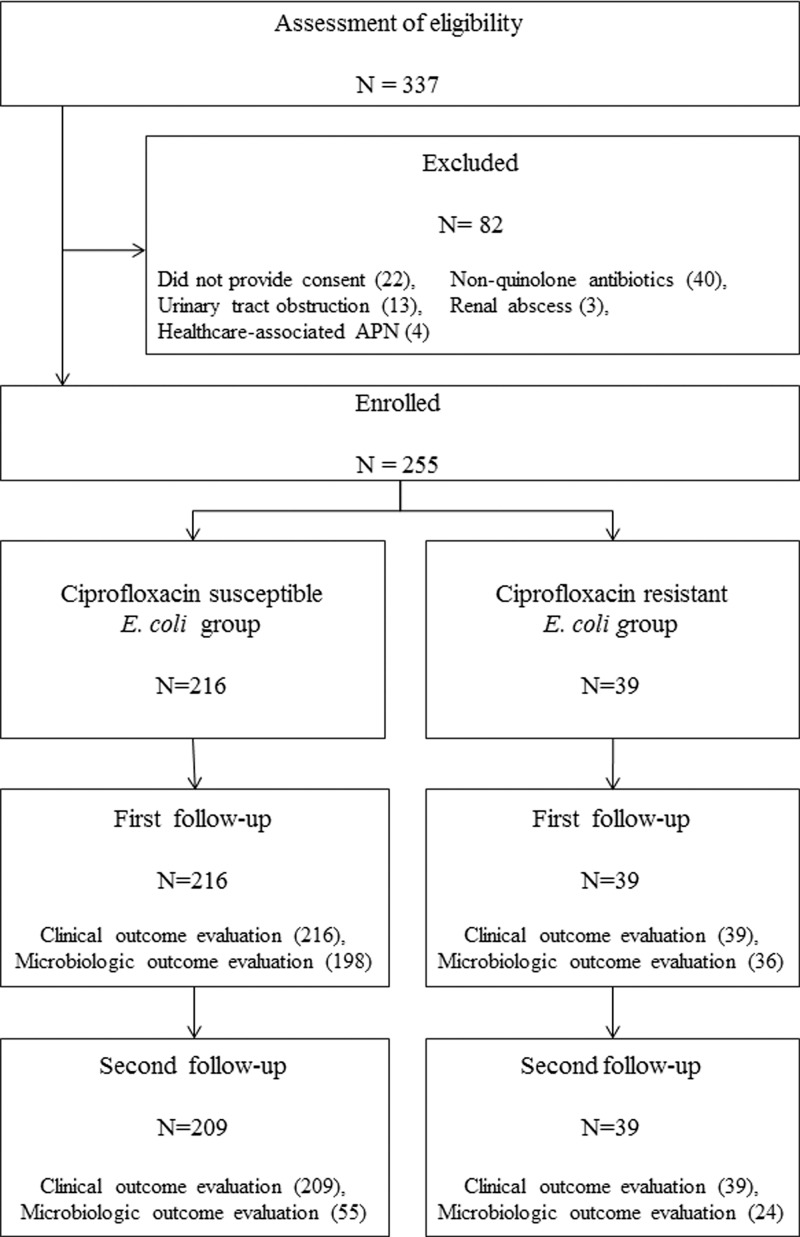
Of the 337 uncomplicated-APN patients, 255 were enrolled and 82 were excluded (Fig. 1). There were no significant differences in demographic characteristics between the patients excluded and those enrolled, except that histories of urinary tract infection were more common in the excluded group (40.2% versus 26.3%, P = 0.016). All the enrolled subjects were followed for 4 to 7 days after the start of therapy. Two hundred forty-eight patients were also evaluated on a second visit after the end of treatment, but about two-thirds (169) of them did not submit urine specimens because most did not have any symptoms and did not want to visit the hospital twice, first to submit a sample and then to check the result. In addition, the physician did not insist on taking urine samples because routine follow-up urine culture after completion of therapy is not recommended in most nonpregnant adults who remain asymptomatic (8). The median age of the enrolled patients was 54 (15 to 88) years. Of the E. coli isolates from urine or blood cultures, 39 (15.3%) were resistant to ciprofloxacin, all of which were susceptible to expanded-spectrum cephalosporins. There was no significant difference in demographic and clinical characteristics, except for mean age, between the patients with ciprofloxacin-resistant and those with ciprofloxacin-susceptible infections (Table 1).
Fig 1.
Patient enrollment and follow-up assessment.
Table 1.
Demographic and clinical characteristicsa
| Variables | Ciprofloxacin-susceptible E. coli (n = 216) | Ciprofloxacin-resistant E. coli (n = 39) | P value |
|---|---|---|---|
| Initial assessment in emergency room | |||
| Age (yr) (mean ± SD) | 51.9 (±17.8) | 61.4 (±16.5) | 0.002 |
| Diabetes | 40 (18.5) | 7 (17.9) | 1.000 |
| Previous UTI history (>3 months) | 53 (24.5) | 14 (35.9) | 0.176 |
| Acute pyelonephritis | 12 (5.6) | 5 (12.8) | |
| Cystitis | 41 (19.0) | 9 (23.1) | |
| Duration of prehospital illness (days) (mean ± SD) | 4.9 (±4.8) | 4.1 (±3.1) | 0.289 |
| Bacteremia | 73 (33.8) | 11 (28.2) | 0.581 |
| During follow-up assessment | |||
| Days to first follow-up from initial treatment | |||
| For patients initially not admitted | 5.5 | 5.1 | 0.485 |
| For patients initially admitted | 3.1 | 3.9 | 0.145 |
| Hospitalization | 46 (21.3) | 13 (33.3) | 0.147 |
| Duration (days) (mean ± SD) | 7.8 (±3.1) | 8.2 (±5.0) | 0.692 |
| Duration of therapy (days) (mean ± SD) | 13.2 (±4.0) | 14.9 (±5.0) | 0.018 |
| Adverse drug events | 10 (4.6) | 2 (5.1) | 1.000 |
Data represent numbers of participants (% of total) unless otherwise indicated.
The overall clinical cure rate was 85.5% (218/255) and the microbiological cure rate was 84.6% (198/234) at the first follow-up visit. While the clinical cure rates at the first follow-up were not significantly different in the two groups, 87.0% (188/216) versus 76.9% (30/39) (P = 0.135), the microbiological cure rate was significantly lower among the women infected with ciprofloxacin-resistant E. coli: 92.4% (183/198) versus 41.7% (15/36) (P < 0.001). Infection was microbiologically proven by urine culture for 237 patients and by blood culture for 18 patients. E. coli grew to a concentration of ≥105 CFU/ml in 214 (90.3%) of the 237 pretreatment urine cultures, 104 to 105 CFU/ml in 22 (9.3%), and 103 to 104 CFU/ml in only 1 culture. In contrast, among 36 patients with positive urine cultures at the first follow-up, E. coli was present at ≥105 CFU/ml in 16 (44.4%) cultures, 104 to 105 CFU/ml in 12, and 103 to 104 CFU/ml in 8 cultures. At the second follow-up visit, the overall clinical and microbiological cure rates were 98.0% (243/248) and 78.5% (62/79), respectively. Clinical cure rates were 98.6% (206/209) in the susceptible group and 94.9% (37/39) in the resistant group (P = 0.177) (Table 2). No complications, including renal or perirenal abscess, metastatic infection, progression to septic shock, or death, occurred in the discordant treatment group during the follow-up period. Although ciprofloxacin was administered for 5.8 days on average before the switch to an appropriate antibiotic in the discordant treatment group, the duration of therapy was comparable for the two groups (Table 1). Although the average age was significantly higher among the women infected with ciprofloxacin-resistant E. coli, neither the clinical cure rate nor the microbiological cure rate was significantly different in the two groups after adjustment for this variable.
Table 2.
Clinical and microbiologic cure rates
| Variable | No./total no. (%) of patients with: |
OR (95% CI)a | P value | |
|---|---|---|---|---|
| Ciprofloxacin-susceptible E. coli | Ciprofloxacin-resistant E. coli | |||
| First follow-up (4–7 days after initial treatment) | ||||
| Clinical cure | 188/216 (87.0) | 30/39 (76.9) | 2.014 (0.866–4.685) | 0.135 |
| Microbiologic cure | 183/198 (92.4) | 15/36 (41.7) | 17.08 (7.328–39.811) | 0.000 |
| Second follow-up (14–21 days after completion of treatment) | ||||
| Clinical cure | 206/209 (98.6) | 37/39 (94.9) | 3.712 (0.600–22.979) | 0.177 |
| Microbiologic cure | 45/55 (81.8) | 17/24 (70.8) | 1.853 (0.607–5.653) | 0.372 |
OR, odds ratio; CI, confidence interval.
Of 41 blood isolates available, 35 were susceptible to ciprofloxacin. However, 12 of these strains had a high MIC (more than 0.125 μg/ml) below the breakpoint for susceptibility. All 12 isolates had mutations in gyrA, parC, or both, but only 1 isolate harbored aac(6′)-Ib-cr. At the first follow-up visit, the clinical cure rates were 87.0% (20/23) for the patients with bacteremic APN caused by E. coli isolates with a MIC of <0.125 μg/ml and 91.7% (11/12) for those infected by E. coli with a high MIC (P = 1.000). The microbiological cure rates were 95.2% (20/21) and 91.7% (11/12), respectively (P = 1.000). Neither difference in outcome was statistically significant.
DISCUSSION
Although fluoroquinolones are considered the drugs of choice for treating community-acquired APN in women, there are insufficient data on the clinical impact of empirical treatment with fluoroquinolones in communities where the prevalence of resistance to it exceeds 10%. Ciprofloxacin is used frequently as the empirical antimicrobial agent for community-acquired APN in many countries despite increasing rates of resistance of E. coli to fluoroquinolones.
Even though the microbiological cure rate at the first follow-up was significantly lower among women infected with ciprofloxacin-resistant E. coli, there was no meaningful difference in clinical cure rates or admission rates between the two groups, and the clinical outcomes after completion of therapy were also comparable. This finding may be explained in several ways: the low severity of APN compared to infections at other sites (7); the appropriateness of treatment, even if delayed, after microbiological diagnosis; the clinical efficacy attributable to high concentrations of ciprofloxacin in the urine (5); or the fitness costs of antibiotic resistance in E. coli (18, 19). The inclusion of only uncomplicated APN in this study may also have contributed to this finding. Because empirical TMP-SMX showed lower efficacy and higher relapse rates than fluoroquinolones (16) and UTI has a lower risk of mortality than do infections at other sites (7), our results suggest that fluoroquinolones may be used for the initial empirical antibiotic regimen, after which one would switch to a therapy concordant with microbiological results.
The ciprofloxacin resistance rate (15.2%) of E. coli isolates in women with uncomplicated community-acquired infection was lower than previously reported in Korea (11, 12). Since local resistance rates reported in hospital antibiograms are often skewed by using cultures obtained from inpatients or those with complex infections (17), the resistance rates in previous studies may have been overestimated. However, our data are unable to establish the highest fluoroquinolone resistance level that would not degrade the effectiveness of fluoroquinolones as an empirical antibiotic for uncomplicated APN.
The study has a few limitations. First, the sample of cases infected with ciprofloxacin-resistant E. coli may have been too small to yield a significant difference of clinical cure rate between the two groups. If the significance levels of alpha and beta are set at 5% and 20%, the ciprofloxacin resistance rate is 15%, and the clinical cure rate in the ciprofloxacin-susceptible group is 87%, more than 800 subjects are needed to detect a 10% difference. Therefore, the possibility of a type II error should be acknowledged. Second, since our results pertained only to women with uncomplicated APN, these findings should not be extrapolated to men, patients with complicated infections, or those with severe sepsis. Finally, because we evaluated the microbiological cure rate at the second visit for only about one-third of the enrolled patients, there may have been a selection bias such that we could not accurately determine the impact of initial discordant treatments on the microbiological relapse rate.
In conclusion, fluoroquinolones may be an appropriate choice for initial empirical therapy of acute uncomplicated pyelonephritis without serious adverse outcomes if it is tailored appropriately on the basis of susceptibility data, even in areas where the fluoroquinolone resistance rate of the uropathogens, notably E. coli, exceeds 10%.
ACKNOWLEDGMENTS
This work was supported by grant no. 03-2009-007 from the Seoul National University Bundang Hospital Research Fund.
We thank the patients, physicians, and nurses in the emergency clinic and laboratory staff members for their cooperation.
Footnotes
Published ahead of print 5 March 2012
REFERENCES
- 1. Blot S, Vandewoude K, De Bacquer D, Colardyn F. 2002. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin. Infect. Dis. 34:1600–1606 [DOI] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2005. Performance standards for antimicrobial disk susceptibility testing: 15th informational supplement, vol 25 Approved standard M100-S15. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Fihn SD. 2003. Clinical practice. Acute uncomplicated urinary tract infection in women. N. Engl. J. Med. 349:259–266 [DOI] [PubMed] [Google Scholar]
- 4. Gupta K, et al. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 52:e103–e120 [DOI] [PubMed] [Google Scholar]
- 5. Gupta K, Hooton TM, Stamm WE. 2001. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Intern. Med. 135:41–50 [DOI] [PubMed] [Google Scholar]
- 6. Harbarth S, et al. 2003. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 115:529–535 [DOI] [PubMed] [Google Scholar]
- 7. Hyle EP, et al. 2005. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae: variability by site of infection. Arch. Intern. Med. 165:1375–1380 [DOI] [PubMed] [Google Scholar]
- 8. Jack DS, Kaye D. 2010. Urinary tract infection, p 957–985 In Mandell GL, Bennett JE, Dolin R. (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 7th ed Elsevier/Churchill Livingstone, Philadelphia, PA [Google Scholar]
- 9. Kim HB, et al. 2009. Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob. Agents Chemother. 53:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kollef MH. 2008. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin. Infect. Dis. 47(Suppl 1):S3–S13 [DOI] [PubMed] [Google Scholar]
- 11. Lee MY, et al. 2010. Dissemination of ST131 and ST393 community-onset, ciprofloxacin-resistant Escherichia coli clones causing urinary tract infections in Korea. J. Infect. 60:146–153 [DOI] [PubMed] [Google Scholar]
- 12. Lee SJ, et al. 2011. Antimicrobial resistance in community-acquired urinary tract infections: results from the Korean Antimicrobial Resistance Monitoring System. J. Infect. Chemother. 17:440–446 [DOI] [PubMed] [Google Scholar]
- 13. Lee SS, Kim Y, Chung DR. 2011. Impact of discordant empirical therapy on outcome of community-acquired bacteremic acute pyelonephritis. J. Infect. 62:159–164 [DOI] [PubMed] [Google Scholar]
- 14. Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. 2003. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin. Infect. Dis. 36:1418–1423 [DOI] [PubMed] [Google Scholar]
- 15. Ronald A. 2002. The etiology of urinary tract infection: traditional and emerging pathogens. Am. J. Med. 113(Suppl 1A):14S–19S [DOI] [PubMed] [Google Scholar]
- 16. Talan DA, et al. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women: a randomized trial. JAMA 283:1583–1590 [DOI] [PubMed] [Google Scholar]
- 17. Ti TY, et al. 2003. What is true community-acquired urinary tract infection? Comparison of pathogens identified in urine from routine outpatient specimens and from community clinics in a prospective study. Eur. J. Clin. Microbiol. Infect. Dis. 22:242–245 [DOI] [PubMed] [Google Scholar]
- 18. Velasco M, et al. 2001. Decreased invasive capacity of quinolone-resistant Escherichia coli in patients with urinary tract infections. Clin. Infect. Dis. 33:1682–1686 [DOI] [PubMed] [Google Scholar]
- 19. Vila J, et al. 2002. Are quinolone-resistant uropathogenic Escherichia coli less virulent? J. Infect. Dis. 186:1039–1042 [DOI] [PubMed] [Google Scholar]