Abstract
Raltegravir shows marked pharmacokinetic variability in patients, with gastrointestinal pH and divalent-metal binding being potential factors. We investigated raltegravir solubility, lipophilicity, pKa, and permeativity in vitro to elucidate known interactions with omeprazole, antacids, and food, all of which increase gastric pH. Solubility of raltegravir was determined at pH 1 to 8. Lipophilicity of raltegravir was determined using octanol-water partition. Raltegravir pKa was determined using UV spectroscopy. The effects of pH, metal salts, and omeprazole on the cellular permeativity of raltegravir were determined using Caco-2 monolayers. Cellular accumulation studies were used to determine the effect of interplay between pH and ABCB1 transport on raltegravir accumulation. Samples were analyzed using liquid chromatography-tandem mass spectroscopy (LC-MS/MS) or scintillation counting. Raltegravir at 10 mM was partly insoluble at pH 6.6 and below. Raltegravir lipophilicity was pH dependent and was reduced as pH was increased from 5 to 9. The pKa of raltegravir was 6.7. Raltegravir cellular permeativity was heavily influenced by changes in extracellular pH, where apical-to-basolateral permeativity was reduced 9-fold (P < 0.05) when apical pH was increased from 5 to 8.5. Raltegravir cellular permeativity was also reduced in the presence of magnesium and calcium. Omeprazole did not alter raltegravir cellular permeativity. Cellular accumulation of raltegravir was increased independently by inhibiting ABCB1 and by lowering extracellular pH from pH 8 to 5. Gastrointestinal pH and polyvalent metals can potentially alter the pharmacokinetic properties of raltegravir, and these data provide an explanation for the variability in raltegravir exposure in patients. The evaluation of how divalent-metal-containing products, such as multivitamins, that do not affect gastric pH alter raltegravir pharmacokinetics in patients is now justified.
INTRODUCTION
Raltegravir, the first licensed integrase inhibitor for treatment of HIV, has potent in vitro and clinical activity (22). The primary route of raltegravir metabolism is glucuronidation via UDP glucuronosyltransferase 1A1 (14), and the drug is not a substrate or inhibitor of the major cytochrome P450 enzymes (12). However, there are known drug interactions involving UGT1A1 which may alter raltegravir plasma exposure. Atazanavir inhibits UGT1A1, causing an increase in raltegravir exposure (4), and rifampin mediates induction of UGT1A1, causing a decrease in raltegravir exposure (27). In addition, raltegravir is a weak substrate for the drug transporters ABCB1, SLC22A6, and SLC15A1 and also inhibits SLC22A6 in vitro (18). Marked inter- and intrapatient variability in raltegravir plasma exposure exists, and the relationship between the pharmacokinetics (PK) and pharmacodynamics (PD) of the drug remains poorly understood (7).
The pH of the gastrointestinal (GI) tract is an important factor in the absorption of many drugs, potentially altering drug release, solubility, chemical stability, charge state, and/or intestinal permeativity (5). Ionic drugs with a pKa within the physiological pH range are susceptible to alterations in GI pH because drugs with an ionic charge are less able to permeate the intestine wall without active transport. In healthy volunteers, raltegravir area under the curve (AUC), maximum plasma concentration (Cmax) and plasma concentration 12 h after dosing (C12) increased 3.1-, 4.2-, and 1.5-fold, respectively, following 5 days of 20 mg omeprazole once daily (13); higher solubility at increasing pH was postulated as the mechanism of interaction (2). However, GI pH was not directly measured in that study, and there are no published data showing the effect of pH on raltegravir solubility in vitro. In a similar study using HIV-infected patients, raltegravir geometric mean ratios (GMR) for AUC, Cmax, and C12 were 1.4-, 1.5-, and 1.2-fold higher, respectively, following 5 days of 20 mg omeprazole once daily and 1.5-, 1.6-, and 1.1-fold higher, respectively, following a single dose of 20 mg famotidine (24). HIV-infected patients, particularly those with advanced disease progression, have higher gastric pHs than uninfected individuals (25), and this may explain why acid-reducing agents showed less impact on raltegravir PK in HIV-infected patients in the study by Rhame et al. (24).
After ingestion of a meal, gastric pH is briefly elevated due to the buffering and diluting effect of the food (5). The extent of gastric pH increase and the rate at which pH is lowered to fasting-state levels depend on the volume of the food, the ability of the food to stimulate gastric acid secretion, and the rate of gastric emptying. Other factors, such as the fat and protein content of food, are also important. In a steady-state food effect study using healthy subjects, alterations in raltegravir exposure from a fasting state were shown for low-fat (GMR of AUC, Cmax, and C12 of 0.54, 0.48, and 0.86, respectively), medium-fat (GMR of AUC, Cmax, and C12 of 1.13, 1.05, and 1.66, respectively), and high-fat (GMR of AUC, Cmax, and C12 of 2.11, 1.96, and 4.13, respectively) meals (1). However, that study concluded that the effect of food was not clinically significant and recommended that raltegravir can be taken without regard to food.
Integrase strand transfer inhibitors work by chelating magnesium ions at the integrase enzyme active site, thus preventing the insertion of viral DNA into the host cell's DNA (8). Therefore, binding with free magnesium (and possibly other polyvalent cationic metals) in the GI tract is likely to alter raltegravir absorption. Indeed, interactions between integrase inhibitors and metal-containing products have been investigated. Raltegravir C12 and time to Cmax (Tmax) were reduced by 67% and 1.75 h, respectively, when raltegravir was taken with an antacid containing magnesium and aluminum, although the AUC and Cmax were not significantly altered (15). Importantly, 75% of subjects taking antacids had a raltegravir C12 lower than 15 ng/ml (i.e., the 95% inhibitory concentration [IC95] of raltegravir in 50% human serum). We hypothesized that this interaction may be mediated by an increase in raltegravir solubility with increased pH (decreasing the Tmax) and binding of raltegravir to the metal ions in the antacid (decreasing C12).
This study aimed to establish in vitro the solubility, lipophilicity, and pKa of raltegravir using a range of buffered pH solutions and solvents. The impact of pH, metal cations, and omeprazole on raltegravir transcellular permeativity across an in vitro model of absorption were also determined. Raltegravir is a substrate of ABCB1 in vitro (18), and the interplay between pH and ABCB1 transport was investigated in cellular accumulation assays to ascertain whether active transport of raltegravir by ABCB1 was influenced by pH.
MATERIALS AND METHODS
Chemical reagents and materials.
Caco-2 cells were purchased from the European Collection of Cell Cultures (Salisbury, United Kingdom). Raltegravir potassium salt and [3H]raltegravir were gifts from Merck (Whitehouse Station, NJ). Lopinavir was a gift from Abbott (Chicago, IL). [3H]lopinavir was purchased from Moravek Biochemicals (Brea, CA). Tariquidar was purchased from Xenova (Sloane, United Kingdom). Acetonitrile was purchased from J. T. Baker (Deventer, Holland). [14C]mannitol was purchased from American Radiolabeled Chemicals (St. Louis, MO). All other reagents were obtained from Sigma (Poole, United Kingdom).
Creation of buffered pH solutions for solubility, lipophilicity, and pKa experiments.
Stock aqueous solutions were buffered to pH 1 (50 mM potassium chloride plus 134 mM hydrogen chloride), 2 (50 mM potassium chloride plus 13 mM hydrogen chloride), 3 (50 mM potassium hydrogen phthalate plus 22.3 mM hydrogen chloride), 4 (50 mM potassium hydrogen phthalate plus 0.1 mM hydrochloric acid), 5 (50 mM potassium hydrogen phthalate plus 22.6 mM sodium hydroxide), 6 (50 mM monopotassium phosphate plus 5.6 mM sodium hydroxide), 7 (50 mM monopotassium phosphate plus 29.1 mM sodium hydroxide), 8 (50 mM Tris hydroxymethyl aminomethane plus 29.2 mM hydrogen chloride), and 9 (50 mM Tris hydroxymethyl aminomethane plus 5.7 mM hydrogen chloride). These stock solutions were used in experiments or were adjusted using 1 M hydrochloric acid or 1 M sodium hydroxide to create the required pH.
Determination of raltegravir solubility.
Raltegravir solubility was determined at 1 mM and 10 mM (estimated range of drug concentrations in the stomach and duodenum of the gut following raltegravir dissolution) in a pH range of 1 to 8. Solutions were mixed on a mechanical shaker (120 rpm, 37°C, 2 h) and vortexed to allow maximum dissolution of raltegravir. Samples were then centrifuged to pellet undissolved drug (3,000 × g, 10 min, 22°C), and the supernatant was carefully removed. Raltegravir concentrations in the supernatant were determined using liquid chromatography-tandem mass spectroscopy (LC-MS/MS).
Determination of raltegravir lipophilicity.
Raltegravir lipophilicity was determined across a physiologically relevant pH range of 1 to 9 using the octanol-water shake flask method. The organic solvent used was either 1-octanol, which allows hydrogen bonding, or cyclohexane, which does not allow hydrogen bonding. The aqueous and organic solvents were mutually saturated on a mechanical shaker (240 rpm, 24 h, 22°C) before use. Raltegravir was added to the aqueous solvent at 10 μM (a concentration ensuring complete solubility in all pH solutions) before the aqueous and organic solvents were combined in a 1:1 ratio and the mixtures were shaken for 30 min. Mixtures were centrifuged (800 × g, 30 min, 22°C), and a sample was taken from the aqueous compartment for LC-MS/MS analysis. The apparent log(P) was calculated as log(Corganic/Caqueous), where Caqueous is the concentration of raltegravir in the aqueous solvent and Corganic is the concentration of raltegravir in the organic solvent (estimated by deducting raltegravir in the aqueous compartment from the total amount in the mixture).
Determination of raltegravir pKa.
Raltegravir pKa was determined using UV spectroscopy. Raltegravir was prepared in buffered pH solutions to a final concentration of 8 μM (a concentration giving an adequate signal for quantification). The buffered pH solutions ranged between pH 3.5 and 9.5. Drug solutions were added to UV cells, which were then placed in a UV spectrophotometer (Perkin Elmer Lambda 25 UV-Vis) and allowed to acclimatize (25°C, 5 min). Following this, the UV-Vis spectra were measured between 200 nm and 500 nm. The absorbance values were recorded at 300 nm (absorbance peak predominant at lower pH) and 333 nm (absorbance peak predominant at higher pH). For each pH, the absorbance at 333 nm was divided by the absorbance at 300 nm and plotted to calculate the pKa.
Cell culture.
Caco-2 cells were maintained in cell culture (37°C, 5% CO2) by passaging at 70% confluence using cell culture medium (Dulbecco's modified Eagle medium [DMEM], 15% FCS). The passage number of the cells used in this study was between 25 and 35. Caco-2 monolayers were cultured as previously described (18) and were subsequently used to determine the effects of pH, metal salts, and omeprazole on raltegravir monolayer permeativity. Briefly, confluent Caco-2 cells were seeded onto polycarbonate membrane Transwells at a density of 5 × 105 cells/cm2. Medium was replaced initially after 24 h and then every 48 h. Plates were used in the experiments 21 days after seeding. Monolayer integrity was checked on the day of the experiment using a Millicell-ERS (Millipore) to determine the transepithelial electrical resistance (TEER) across the monolayer. A TEER of >600 was deemed acceptable. In addition, radiolabeled [14C]mannitol was added to separate wells on each plate (n = 3) to confirm monolayer integrity. [14C]mannitol samples were analyzed by liquid scintillation counting (Tri-Carb; Beckman), and plates were used only if the average apparent permeativity of mannitol was less than 1 × 10−6 cm s−1.
Impact of pH on raltegravir permeativity of cell monolayers.
On the day of study, the TEER was assessed and the medium in each plate was replaced with the appropriate pH-buffered incubation solutions. The pH in the basolateral compartments was maintained at pH 7.4 (Hanks balanced salt solution [HBSS] containing 25 mM HEPES), and the pH in the apical compartments was maintained at pH 5, 6, or 6.5 (HBSS containing 10 mM morpholineethanesulfonic acid [MES]), pH 7, 7.4, or 8 (HBSS containing 25 mM HEPES), or pH 8.5 (HBSS containing 10 mM Tricine). Compartments were allowed to equilibrate (37°C, 30 min). The incubation buffer in the apical (for transport in the apical-to-basolateral [A-to-B] direction) and basolateral (for transport in the basolateral-to-apical transport [B-to-A] direction) compartments was replaced with the appropriate incubation buffer containing raltegravir (50 μM), and plates were incubated (37°C, 5% CO2). A concentration of 50 μM raltegravir, which has been shown to be nontoxic for Caco-2 cells in previous studies (18), was used to ensure complete dissolution of drug at all pHs (as predicted by the solubility data) and to ensure that drug concentrations in receiver compartments were sufficiently high to be quantifiable by LC-MS/MS. Samples were taken from the receiver compartments at 0, 30, 60, 90, and 120 min and replaced with fresh incubation buffer of the correct pH. Samples were analyzed using LC-MS/MS. Results were used to determine apparent permeativity (Papp; units are 10−6 cm s−1) for each direction and to determine the efflux ratio, which is the ratio of B-to-A Papp to A-to-B Papp. Papp was calculated as [(dQ/dt) × v]/(A × C0) (6), where dQ/dt is the change in drug concentration in the receiver compartment over time (nM/s), v is the volume in the receiver compartment (ml), A is the total surface area of the Transwell membrane (cm2), C0 is the initial drug concentration in the donor compartment (nM), and Papp is the apparent permeativity (cm/s).
Impact of divalent and monovalent metals on raltegravir cell monolayer permeativity.
On the day of study, the TEER was assessed, and the medium in each plate was replaced with the appropriate pH-buffered incubation solution. The pH in the basolateral compartments was maintained at pH 7.4 (HBSS without calcium chloride or magnesium sulfate [H6648; Sigma] containing 25 mM HEPES), and the pH in the apical compartments was maintained at pH 5 (HBSS without calcium chloride or magnesium sulfate and containing 10 mM MES) or pH 7.4 (HBSS without calcium chloride or magnesium sulfate and containing 25 mM HEPES). In the apical compartments at pH 5, magnesium chloride (1 mM, 10 mM, or 25 mM), magnesium sulfate (25 mM), calcium chloride (25 mM), or potassium chloride (25 mM) was added. In the apical compartments at pH 7.4, magnesium chloride (1, 10, or 25 mM) was added. A maximum concentration of 25 mM was used for magnesium chloride, as higher concentrations showed Caco-2 cell toxicity (data not shown). Control apical compartments at pH 5 and pH 7.4 which had no added metal salts other than those in the original buffer were also used. The basolateral compartments were maintained at pH 7.4 and had no added metal salts other than those in the original buffer. A concentration of 1 μM was used for all test compounds in order to maximize the metal-to-drug ratio. Lopinavir was used as a negative control, as it is not believed to bind to metals. The incubation buffer in the apical (for A-to-B transport) and basolateral (for B-to-A transport) compartments was replaced with the appropriate incubation buffer containing radiolabeled [3H]raltegravir (1 μM, 0.5 μCi/ml) or lopinavir (1 μM, 0.5 μCi/ml), and plates were incubated (37°C, 5% CO2). Samples were taken from the receiver compartments at 0 and 30 min and were analyzed by liquid scintillation counting. The Papp and efflux ratios were calculated as described above.
Impact of omeprazole on raltegravir cell monolayer permeativity.
For induction studies using Transwells, the cell monolayers were incubated with omeprazole (10 μM) for the last 3 days of the 21-day monolayer maturation. On the day of the experiment, the TEER was assessed, and the medium in each plate was replaced with warm incubation buffer (HBSS containing 25 mM HEPES, pH 7.4) and allowed to equilibrate (37°C, 30 min).
For inhibition studies, the incubation buffer contained omeprazole at 20 μM, as lower concentrations are known to inhibit CYP2C9 and CYP2C19 in vitro (16). The incubation buffer in the apical (for A-to-B transport) and basolateral (for B-to-A transport) compartments was replaced with incubation buffer containing the test substrate raltegravir (1 μM) with or without 20 μM omeprazole, and plates were incubated (37°C, 5% CO2). A concentration of 1 μM raltegravir was used to avoid saturation of drug transporters (11). Samples were taken from the receiver compartments at 0 and 60 min and were analyzed by liquid scintillation counting (Tri-Carb; Beckman). The Papp and efflux ratios were calculated as described above.
Cellular accumulation.
Caco-2 cells were seeded (5 × 104 cells/ml) on 6-well plates and grown for 5 days to allow plate surface coverage (DMEM with 15% fetal bovine serum [FBS], 37°C, 5% CO2). Medium was removed, cells were washed with warm HBSS, and the medium was replaced with the appropriate pH-buffered incubation solution and allowed to equilibrate (37°C, 15 min). The pH in the wells was fixed at pH 5 or 6 (HBSS containing 10 mM MES) or pH 7 or 8 (HBSS containing 25 mM HEPES). The test substrate raltegravir (1 μM) was added to the wells, and plates were incubated (37°C, 5% CO2, 10 min). A concentration of 1 μM raltegravir was used to avoid saturation of drug transporters (11). A separate incubation was undertaken in which cells were preincubated (DMEM with 15% FBS, 37°C, 5% CO2) prior to raltegravir addition with the potent noncompetitive ABCB1 inhibitor tariquidar (300 nM, 30 min), which was also included during the 10 min of raltegravir incubation. Following incubation, an extracellular sample was removed for analysis, wells were washed three times with ice-cold HBSS, and 500 μl tap water was added to each empty well to lyse cells. Plates were frozen at −20°C overnight to aid removal of cells. Plates were thawed, and 500 μl acetonitrile was added to each well to release drug from protein. The well contents were transferred to separate 1.5-ml tubes for centrifugation (10 min, 3,000 × g, 22°C), and supernatant was removed. Supernatant was vacuum dried and reconstituted in high-performance liquid chromatography (HPLC)-grade water for LC-MS/MS analysis.
LC-MS/MS analysis.
Samples from all studies other than cell accumulation studies could be directly injected into the LC-MS/MS system after dilution with HPLC-grade water. Samples from cell accumulation studies were prepared as described above for cellular accumulation method. The LC-MS/MS system used for sample analysis consisted of a Surveyor autosampler and an LCQ DecaXP ion trap detector (Thermo, Hemel Hempstead, United Kingdom). Chromatographic separation was performed at 30°C on a Fortis C18 3-μm column (50 by 2.1 mm [inside diameter]; Fortis Technologies, Neston, United Kingdom). Mobile phases were solution A (95% HPLC-grade water, 5% acetonitrile, 0.05% formic acid) and solution B (10% HPLC grade water, 90% acetonitrile, 0.05% formic acid), and the flow rate was 0.4 ml/min. Separation was achieved with a gradient elution beginning with 80% solution A and 20% solution B. Solution B was gradually increased to 100% over 0.5 min and maintained for a further 2 min. Solution A was increased to 80% over 0.1 min and maintained for 3.9 min, giving a total run time of 6.5 min. The retention time of raltegravir was 4.78 min, and the lowest limit of quantification was 31.25 ng/ml. High quality control (QC) (2,000 ng/ml) had an interday and intraday accuracy of 98.2% and 97.1%, respectively, and an interday and intraday precision of 97.0% and 93.1%, respectively. Medium QC (200 ng/ml) had an interday and intraday accuracy of 96.8% and 99.1%, respectively, and an interday and intraday precision of 89.1% and 89.2%, respectively. Low QC (100 ng/ml) had an interday and intraday accuracy of 96.5% and 92.8%, respectively, and an interday and intraday precision of 90.0% and 95.3%, respectively. All QCs gave accuracy and precision higher than 85%, in accordance with U.S. Food and Drug Administration (FDA) guidelines (23), and the linear range for raltegravir quantification was 31.25 to 1,000 ng/ml.
Statistical analysis.
Data were analyzed using PASW 18 for Windows. All data were tested for normality using the Shapiro-Wilk test. An independent t test was used to determine significance of normally distributed data. The Mann-Whitney U test was used for all other data. A two-tailed P value above 0.05 was accepted as being statistically significant.
RESULTS
Raltegravir solubility.
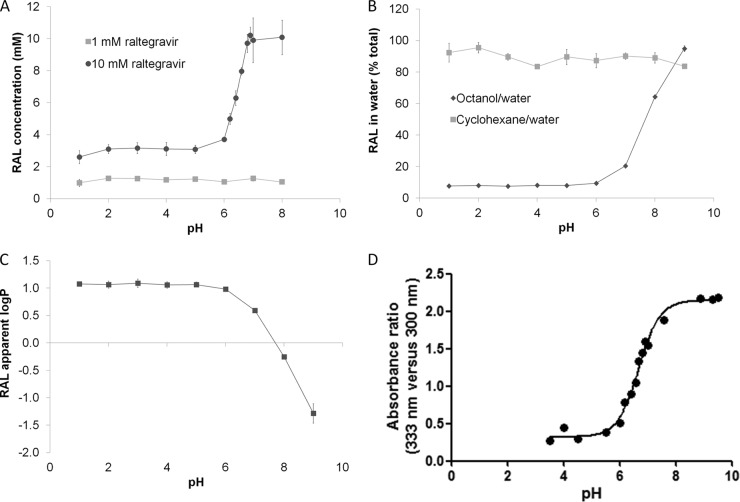
Figure 1A shows raltegravir solubility over a range of buffers with pHs from 1 to 8. A concentration of 1 mM raltegravir was fully soluble across this pH range. However, 10 mM raltegravir was fully soluble only at pH 6.8, 6.9, 7, and 8 and achieved mean supernatant concentrations of 8.0 mM at pH 6.6, 6.3 mM at pH 6.4, 5.0 mM at pH 6.2, 3.7 mM at pH 6, and around 3 mM at pH 5 and below.
Fig 1.
(A) Raltegravir (RAL) solubility at 1 mM and 10 mM in a range of pH solutions. Data are means ± standard deviations (SD) (≥3 experimental replicates). (B) Partition of raltegravir (10 μM) between cyclohexane and water and octanol and water at different pHs. Data are means ± SD (4 experimental replicates; 3 biological replicates). (C) Apparent log(P) of raltegravir (10 μM) using octanol and water at different pHs. Data are means ± SD (4 experimental replicates; 3 biological replicates). (D) Determination of raltegravir pKa using UV spectroscopy. Data are expressed as relative absorbance at two wavelengths as a function of the pH of the buffer solution (1 experimental replicate).
Raltegravir lipophilicity.
When cyclohexane was used as the organic solvent, raltegravir remained predominantly in the aqueous compartment (∼90%) in all pH solutions, whereas using octanol resulted in low raltegravir concentrations (∼7%) in the aqueous compartments of pH 5 or less (Fig. 1B). Therefore, when octanol was used as the organic solvent, the apparent log(P) of raltegravir was stable between aqueous pH 1 and 5 but decreased from 1.06 to −1.29 between pH 5 and 9 (P < 0.05) (Fig. 1C). A negative log(P) is usually associated with poor cell membrane permeativity (10).
Raltegravir pKa.
Figure 1D shows the relative absorbance at two wavelengths (333 nm versus 300 nm) as a function of the pH of the buffer solution. The most substantial change in the curve occurs between pH 6 and 8. If it is assumed that this change in peak ratios is due to an alteration of the charge state of raltegravir, the pKa is calculated as pH 6.7.
Impact of pH on raltegravir cell monolayer permeativity.
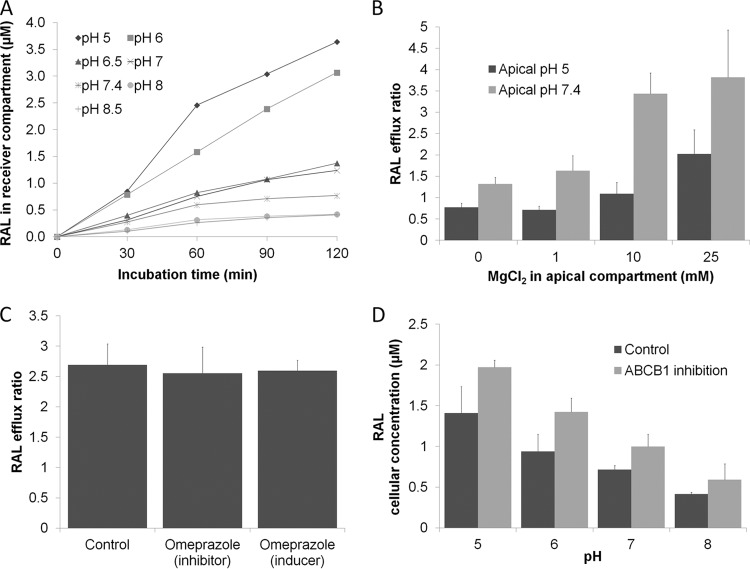
Figure 2A depicts the apical-to-basolateral raltegravir permeativity through Caco-2 cell monolayers at various apical pH values. Increasing the apical pH from 5 to 8.5 decreased the rate and extent of raltegravir monolayer permeativity in the apical-to-basolateral direction (P < 0.05). The Papp and efflux ratio of raltegravir at 60 min incubation are shown in Table 1. Raltegravir efflux ratio increased 12-fold as apical pH was increased from 5 to 8.5 (P < 0.05). When both apical and basolateral pHs were 7.4, the efflux ratio was 2.5, suggesting active transport in the basolateral-to-apical direction. However, reducing apical pH to 6 or below caused raltegravir permeativity to predominate in the apical-to-basolateral direction, suggesting that pH has the potential to overcome the effect of active drug transport.
Fig 2.
(A) Time course experiment showing A-to-B permeation of raltegravir (RAL; 50 μM) obtained with apical buffers with the indicated range of pHs in Caco-2 monolayers over 120 min. The basolateral pH was maintained at 7.4. Data are means of raltegravir accumulation in the basolateral (receiver) compartment (3 experimental replicates). (B) Efflux ratio of raltegravir (1 μM) in Caco-2 monolayers using a range of magnesium chloride concentrations in the apical compartment. The apical compartment was maintained at pH 5 or pH 7.4, and the basolateral compartment was maintained at pH 7.4. (C) Efflux ratio of raltegravir (1 μM) in the presence of omeprazole (inhibitor study; 20 μM) or with cells preincubated with omeprazole for 3 days (induction study; 10 μM). Data in panels B and C are mean efflux ratios (B-to-A/A-to-B) ± SD (3 experimental replicates). (D) Accumulation of raltegravir (1 μM) in Caco-2 cells at an extracellular pH of 5 to 8, with and without the ABCB1 inhibitor tariquidar (300 nM). Data are means ± SD (3 experimental replicates).
Table 1.
Effect of pH on raltegravir (50 μM) permeativity using a Caco-2 monolayer
| pH of apical compartmenta | Papp (10−6 cm/s)b |
Efflux ratioc | |
|---|---|---|---|
| A to B | B to A | ||
| 5 | 27.3 ± 1.2 | 11.9 ± 1.5 | 0.4 |
| 6 | 17.6 ± 1.7 | 13.1 ± 2.3 | 0.7 |
| 6.5 | 9.2 ± 1.1 | 14.5 ± 1.5 | 1.6 |
| 7 | 8.4 ± 1.2 | 14.5 ± 2.1 | 1.7 |
| 7.4 | 6.6 ± 0.8 | 16.4 ± 2.9 | 2.5 |
| 8 | 3.5 ± 0.5 | 13.8 ± 0.7 | 3.9 |
| 8.5 | 2.9 ± 0.3 | 14.1 ± 1.3 | 4.9 |
The pH of the basolateral compartment was maintained at 7.4.
Data are means ± SD for 3 experimental replicates.
Compared to the efflux ratio at pH 5, all other ratios had a P value of <0.05.
Impact of divalent and monovalent metals on raltegravir cell monolayer permeativity.
All Papp and efflux ratio calculations were made using samples taken after 30 min of incubation, and sink conditions were maintained at this time point. A positive correlation between concentration of magnesium chloride in the apical compartment and raltegravir efflux ratio is shown in Fig. 2B. When the apical compartment pH was 7.4, the raltegravir efflux ratio was significantly increased in comparisons of control wells with no additional metal salt (efflux ratio = 1.3) to wells containing 1 mM (efflux ratio = 1.6; P < 0.05), 10 mM (efflux ratio = 3.4; P < 0.05), or 25 mM (efflux ratio = 3.7; P < 0.05) magnesium chloride. The lopinavir efflux ratio was unaltered in comparisons of control wells with no additional metal salt (efflux ratio = 5.1) to wells containing 25 mM magnesium chloride (efflux ratio = 5.4; P = 0.83), illustrating that the effect of magnesium chloride on raltegravir cellular permeativity was not a result of general changes in monolayer integrity.
When the apical compartment pH was 5, the raltegravir efflux ratio was unchanged when control wells with no additional metal salt (efflux ratio = 0.8) were compared to wells containing 1 mM magnesium chloride (efflux ratio = 0.7, P = 0.28), but a significant increase in efflux ratio was observed with the addition of 10 mM (efflux ratio = 1.1; P < 0.05) or 25 mM (efflux ratio = 1.9; P < 0.05) magnesium chloride. Also, the effects of magnesium in a different salt form (magnesium sulfate), an additional divalent metal salt (calcium chloride), and a monovalent metal salt (potassium chloride) were investigated. The raltegravir efflux ratio was significantly increased in comparisons of control wells with no additional metal salt (efflux ratio = 0.8) to wells containing 25 mM magnesium sulfate (efflux ratio = 1.6; P < 0.05) or 25 mM calcium chloride (efflux ratio = 1.2; P < 0.05). The efflux ratio was unaltered when 25 mM potassium chloride was added to the apical compartment (efflux ratio = 0.8; P = 0.83). This supports the hypothesis that divalent metals, but not monovalent metals, have the potential to affect raltegravir cell membrane permeativity.
Impact of omeprazole on raltegravir cell monolayer permeativity.
Figure 2C depicts the efflux ratio of 1 μM raltegravir through Caco-2 cell monolayers in the presence and absence of omeprazole as an inhibitor (20 μM) or as an inducer (72 h preincubation with 10 μM omeprazole). All Papp and efflux ratio calculations were made using samples taken after 60 min of incubation, and sink conditions were maintained. In inhibition studies, there was no significant difference in raltegravir efflux ratio between control cells and cells coincubated with 20 μM omeprazole (efflux ratio of 2.7 versus 2.6; P = 0.83). Similarly, in induction studies, there was no significant difference in raltegravir efflux ratio between control cells and cells preincubated for 72 h with 10 μM omeprazole (efflux ratio of 2.7 versus 2.6; P = 0.51).
Impact of pH and ABCB1 inhibition on raltegravir cellular accumulation.
Figure 2D depicts the accumulation of raltegravir in Caco-2 cells that occurred when various pH buffers were used and tariquidar was used to inhibit ABCB1. Raltegravir accumulation in Caco-2 cells increased 3.4-fold when the incubation buffer pH was decreased from 8 to 5 (P < 0.05). This pH-related increase in raltegravir accumulation was also observed in cells treated with the ABCB1 inhibitor tariquidar (3.3-fold decrease; P < 0.05). Inhibiting ABCB1 led to increased raltegravir accumulation at pH 5 (1.4-fold; P < 0.05), 6 (1.5-fold; P < 0.05), and 7 (1.4-fold; P < 0.05) but not at pH 8 (P = 0.08). These results suggest that pH impacts cellular permeativity of raltegravir to a greater extent than ABCB1 transport and that ABCB1 transport of raltegravir is independent of pH.
DISCUSSION
In this study, the influence of pH on raltegravir solubility, lipophilicity, and cellular permeativity was investigated. Raltegravir exhibited solubility of less than 10 mM at pH 6.6 and below. It is likely that the drug obtains a negative charge at higher pH by deprotonation of the hydroxyl group at the 5 position of the 6-oxo-1,6-dihydropyrimidine ring. This negative charge would dramatically increase the solubility of the drug in aqueous buffer. Additionally, raltegravir log(P) was shown to decrease as pH is increased above 6, presumably due to the introduction of a charge to the drug. The solubility and lipophilicity data are supported by our UV spectroscopy study, where raltegravir pKa was calculated at 6.7. This pKa is located within the pH range that is encountered by the drug during absorption in the GI tract. This offers us a convincing explanation of the intra- and interpatient variability of raltegravir PK in healthy and HIV-1-infected subjects (3).
In experiments assessing raltegravir cellular permeativity, raltegravir was less able to cross a Caco-2 cell monolayer as the incubation buffer pH was increased from 5 to 8.5. This is most likely due to the introduced charge at the active site, preventing the drug from passing through the phospholipid bilayer of the cell membrane. Similar results were seen in accumulation studies, where less raltegravir was present in the cells when the pH was increased from 5 to 8. ABCB1 inhibition had less impact on raltegravir intracellular concentrations than changes in extracellular pH. Permeability and accumulation were assessed using raltegravir concentrations from 1 μM to 50 μM, and our solubility studies show that the drug is fully soluble at all pHs at these concentrations. Therefore, the introduction of a charge to raltegravir by increasing pH could have opposing consequences depending on the concentration of the drug. At high drug concentrations, the introduced charge is predicted to increase dissolution and therefore absorption. Conversely, at lower concentrations, solubility is unlikely to be a problem, and therefore the introduced charge may reduce the rate of absorption.
In previous clinical studies, coadministration of omeprazole and famotidine resulted in an increase in raltegravir Cmax (24). This may result from the predicted increased solubility of raltegravir in the stomach and the duodenum, where drug concentrations would be highest. The C12 of raltegravir was only slightly higher or unchanged when the drug taken with omeprazole or famotidine (24). It is tempting to speculate that over time, raltegravir dissolution normalizes in all subjects and the benefit of increased gastric pH is overcome. Inhibition and induction studies with omeprazole indicated that there were no direct effects on raltegravir cellular permeativity, indicating that the effect of omeprazole most likely results from its pH-altering properties.
Ingestion of a meal has been shown to result in an increase in gastric pH (5), which would be predicted to increase raltegravir solubility. However, only high-fat meals have been shown to increase raltegravir exposure, with low-fat meals resulting in a reduction (1). It is possible that a high-fat meal may dilute stomach acid to a greater extent or that the fat in the meal could increase the solubility of raltegravir.
Raltegravir cellular permeativity decreased in the presence of the divalent cations magnesium and calcium in the incubation medium. This decrease was not found when the monovalent cation potassium was used. Raltegravir may bind to the divalent metals and form a metal-drug complex which is unable to cross the cell membrane. It is important to note that binding of raltegravir to a divalent metal (magnesium) is a prerequisite for inhibition of HIV integrase (17). Antacids containing magnesium caused no significant change in raltegravir Cmax or AUC but did reduce C12, resulting in 75% of patients having a C12 less than the IC95 (15). The impact of antacids may be explained by a combination of the effects of pH and metal binding. We hypothesize that Cmax remains unchanged while raltegravir solubility improves with increased pH but that absorption is inhibited by magnesium binding. At later time points, when raltegravir solubility is no longer an issue, the elevated presence of magnesium in the gut may reduce the amount of raltegravir being absorbed, thus reducing raltegravir C12. If this is true, coadministration of products containing polyvalent metal cations that do not alter gastric pH (e.g., multivitamin tablets) would be expected to reduce raltegravir exposure. This is certainly now worthy of empirical determination.
These data may also have implications for the newer integrase strand transfer inhibitors elvitegravir and dolutegravir. Elvitegravir and dolutegravir exposure has been shown to be reduced when these drugs are taken with antacids containing magnesium and aluminum, with the reduction being less marked when the drug is taken 2 h before the antacid (19, 21). In healthy subjects, elvitegravir and dolutegravir absorption has also been shown to be influenced by food according to fat content (26, 9), and it is recommended that elvitegravir be taken with food (20). Interestingly, dolutegravir exposure has also been shown to be moderately reduced when the drug is taken with a multivitamin containing magnesium, calcium, iron, zinc, and copper (19).
In conclusion, the physicochemical properties of raltegravir are heavily influenced by environmental pH. Both pH and polyvalent metals have the potential to alter the pharmacokinetics of raltegravir, and these data help provide a rationale for the variability in raltegravir exposure seen in patients. The evaluation of how divalent metal-containing products, such as multivitamins that do not affect gastric pH, alter raltegravir pharmacokinetics in patients is now justified. A comprehensive knowledge of the mechanisms that underpin variability in disposition will help optimize future therapy with raltegravir.
ACKNOWLEDGMENTS
This study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. This work was funded by Merck & Co., Inc. (Whitehouse Station, NJ).
The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Brainard DM, et al. 2011. Effect of low-, moderate-, and high-fat meals on raltegravir pharmacokinetics. J. Clin. Pharmacol. 51:422–427 [DOI] [PubMed] [Google Scholar]
- 2. Brainard DM, Wenning LA, Stone JA, Wagner JA, Iwamoto M. 2011. Clinical pharmacology profile of raltegravir, an HIV-1 integrase strand transfer inhibitor. J. Clin. Pharmacol. 51:1376–1402 [DOI] [PubMed] [Google Scholar]
- 3. Cattaneo D, et al. 2011. Inter- and intra-patient variability of raltegravir pharmacokinetics in HIV-1-infected subjects. J. Antimicrob. Chemother. 67:460–464 [DOI] [PubMed] [Google Scholar]
- 4. Cattaneo D, et al. 2010. Exposure-related effects of atazanavir on the pharmacokinetics of raltegravir in HIV-1-infected patients. Ther. Drug Monit. 32:782–786 [DOI] [PubMed] [Google Scholar]
- 5. Charman WN, Porter CJ, Mithani S, Dressman JB. 1997. Physiochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J. Pharm. Sci. 86:269–282 [DOI] [PubMed] [Google Scholar]
- 6. Elsby R, Surry DD, Smith VN, Gray AJ. 2008. Validation and application of Caco-2 assays for the in vitro evaluation of development candidate drugs as substrates or inhibitors of P-glycoprotein to support regulatory submissions. Xenobiotica 38:1140–1164 [DOI] [PubMed] [Google Scholar]
- 7. Eron J, et al. 2011. QDMRK, a phase III study of the safety and efficacy of once daily vs twice daily RAL in combination therapy for treatment-naïve HIV-infected patients. CROI, Boston, MA [Google Scholar]
- 8. Espeseth AS, et al. 2000. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc. Natl. Acad. Sci. U. S. A. 97:11244–11249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. German P, et al. 2009. Effect of food on pharmacokinetics of elvitegravir, emtricitabine, tenofovir and the pharmacoenhancer GS-9350 as a fixed dose combination tablet, abstr. A1-1300. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. San Francisco, CA [Google Scholar]
- 10. Goodwin JT, Conradi RA, Ho NF, Burton PS. 2001. Physicochemical determinants of passive membrane permeability: role of solute hydrogen-bonding potential and volume. J. Med. Chem. 44:3721–3729 [DOI] [PubMed] [Google Scholar]
- 11. Hubatsch I, Ragnarsson EG, Artursson P. 2007. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2:2111–2119 [DOI] [PubMed] [Google Scholar]
- 12. Iwamoto M, et al. 2008. Lack of a pharmacokinetic effect of raltegravir on midazolam: in vitro/in vivo correlation. J. Clin. Pharmacol. 48:209–214 [DOI] [PubMed] [Google Scholar]
- 13. Iwamoto M, et al. 2009. Effects of omeprazole on plasma levels of raltegravir. Clin. Infect. Dis. 48:489–492 [DOI] [PubMed] [Google Scholar]
- 14. Kassahun K, et al. 2007. Metabolism and disposition in humans of raltegravir (MK-0518), an anti-AIDS drug targeting the human immunodeficiency virus 1 integrase enzyme. Drug Metab. Dispos 35:1657–1663 [DOI] [PubMed] [Google Scholar]
- 15. Kiser JJ, et al. 2010. Effect of antacids on the pharmacokinetics of raltegravir in human immunodeficiency virus-seronegative volunteers. Antimicrob. Agents Chemother. 54:4999–5003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li XQ, Andersson TB, Ahlstrom M, Weidolf L. 2004. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 32:821–827 [DOI] [PubMed] [Google Scholar]
- 17. Loizidou EZ, Kousiappa I, Zeinalipour-Yazdi CD, Van de Vijver DA, Kostrikis LG. 2009. Implications of HIV-1 M group polymorphisms on integrase inhibitor efficacy and resistance: genetic and structural in silico analyses. Biochemistry 48:4–6 [DOI] [PubMed] [Google Scholar]
- 18. Moss DM, et al. 2011. Raltegravir is a substrate for SLC22A6: a putative mechanism for the interaction between raltegravir and tenofovir. Antimicrob. Agents Chemother. 55:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel P, et al. 2011. Pharmacokinetics of the HIV integrase inhibitor S/GSK1349572 co-administered with acid-reducing agents and multivitamins in healthy volunteers. J. Antimicrob. Chemother. 66:1567–1572 [DOI] [PubMed] [Google Scholar]
- 20. Ramanathan S, Mathias AA, German P, Kearney BP. 2011. Clinical pharmacokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clin. Pharmacokinet. 50:229–244 [DOI] [PubMed] [Google Scholar]
- 21. Ramanathan S, Shen G, Hinkle J, Enejosa J, Kearney BP. 2007. Pharmacokinetic evaluation of drug interactions with ritonavir-boosted HIV integrase inhibitor GS-9137 (elvitegravir) and acid reducing agents, abstr. 69. 8th International Workshop on Clinical Pharmacology of HIV Therapy, Budapest, Hungary [Google Scholar]
- 22. Ramkumar K, Neamati N. 2010. Raltegravir: the evidence of its therapeutic value in HIV-1 infection. Core Evid. 4:131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reil H, Bukovsky AA, Gelderblom HR, Gottlinger HG. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhame F, et al. 2009. Effects of famotidine and omeprazole on raltegravir pharmacokinetics in HIV-infected individuals, abstr. PE4.1/1. 12th European AIDS Conference, Cologne, Germany [Google Scholar]
- 25. Shelton MJ, Akbari B, Hewitt RG, Adams JM, Morse GD. 2000. Eradication of Helicobacter pylori is associated with increased exposure to delavirdine in hypochlorhydric HIV-positive patients. J. Acquir. Immune Defic. Syndr. 24:79–82 [DOI] [PubMed] [Google Scholar]
- 26. Song I, et al. 2012. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob. Agents Chemother. 56:1627–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wenning LA, et al. 2009. Effect of rifampin, a potent inducer of drug-metabolizing enzymes, on the pharmacokinetics of raltegravir. Antimicrob. Agents Chemother. 53:2852–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]