Abstract
Pharmacokinetic differences between piperaquine (PQ) base and PQ tetraphosphate were investigated in 34 Papua New Guinean children aged 5 to 10 years treated for uncomplicated malaria with artemisinin-PQ (ART-PQ) base or dihydroartemisinin-PQ (DHA-PQ) tetraphosphate. Twelve children received ART-PQ base (two daily doses of 3 mg of ART and 18 mg of PQ base as granules/kg of body weight) as recommended by the manufacturer, with regular clinical assessment and blood sampling over 56 days. PQ concentrations in plasma samples collected from 22 children of similar ages with malaria in a previously published pharmacokinetic study of DHA-PQ tetraphosphate (three daily doses of 2.5 mg of ART and 20 mg of PQ tetraphosphate as tablets/kg of body weight) were available for comparison. The disposition of ART was also assessed in the 12 children who received ART-PQ base. Plasma PQ was assayed by high-performance liquid chromatography with UV detection, and ART was assayed using liquid chromatography-mass spectrometry. Multicompartment pharmacokinetic models for PQ and ART were developed using a population-based approach. ART-PQ base was well tolerated, and initial fever abatement and parasite clearance were prompt. There were no differences between the two treatments in the values for the PQ area under the concentration-time curve from time zero to infinity (AUC0–∞), with medians of 49,451 (n = 12) and 44,556 (n = 22) μg · h/liter for ART-PQ base and DHA-PQ tetraphosphate, respectively. Recurrent parasitemia was associated with lower PQ exposure. Using a two-compartment ART model, the median AUC0–∞ was 1,652 μg · h/liter. There was evidence of autoinduction of ART metabolism (relative bioavailability for the second dose, 0.27). These and previously published data suggest that a 3-day ART-PQ base regimen should be further evaluated, in line with World Health Organization recommendations for all artemisinin combination therapies.
INTRODUCTION
The most recent World Health Organization (WHO) recommendations for the treatment of uncomplicated malaria include a 3-day course of dihydroartemisinin plus piperaquine (DHA-PQ) as a first-line artemisinin (ART) combination therapy (ACT) (48). Various formulations of DHA-PQ are marketed in tropical countries (Duo-cotecxin, Combimal, and P-Alaxin; http://www.actwatch.info/resources/drugs_home03_search.asp) or are in development (Eurartesim) (20), and all employ PQ tetraphosphate as the DHA partner drug. DHA is a semisynthetic derivative of ART, and its production adds to the manufacturing cost, but, unlike ART, it does not exhibit autoinduction of metabolism. In addition, although the tetraphosphate salt of PQ has greater water solubility and therefore may have better oral bioavailability, incorporation of the lipid-soluble PQ base should also simplify production.
Artequick (Artepharm Co. Ltd., Guangzhou, China) is an ACT that contains ART in place of DHA and PQ base rather than PQ tetraphosphate. This combination is formulated as tablets but also as granules for pediatric use. It is marketed in Cambodia and some sub-Saharan African countries. The current manufacturer's recommendation is for Artequick to be given as a 2-day regimen, which contrasts with the 3 days recommended for all ACTs by the WHO (48). Although the tolerability, safety, efficacy, and pharmacokinetics (PK) properties of DHA-PQ tetraphosphate have been widely investigated in children and adults (27, 28, 36, 44, 49), there are limited data relating to the efficacy and tolerability of ART-PQ base (37, 46) and no studies of the pharmacokinetics of this novel combination in malaria-infected patients. Concerns have been raised regarding possible underdosing in children for a number of antimalarial drugs (8, 33), including PQ (27, 36, 49). Although children have been included in studies of PQ pharmacokinetics (28, 44), only one pharmacokinetic study of ART has specifically enrolled pediatric patients (40).
We have evaluated the population pharmacokinetics of ART-PQ base (Artequick) in children from Papua New Guinea (PNG) with uncomplicated malaria and compared the data with those of a previously published study of DHA-PQ tetraphosphate (Duo-cotecxin; Beijing Holley-Cotec, Beijing, China) in the same category of patients (29). The primary aims of the present study were to investigate pharmacokinetic differences between PQ base and PQ tetraphosphate and to describe the population pharmacokinetics of ART in PNG children. Secondary aims were to provide preliminary data relating pharmacokinetic factors to recurrent parasitemia and to use both pharmacokinetic and efficacy data to suggest improved dose regimens for these combinations.
MATERIALS AND METHODS
Patients.
Assessment and recruitment of children for the present and published DHA-PQ tetraphosphate studies were as described previously (29). Briefly, all subjects were children aged 5 to 10 years presenting to Alexishafen Health Centre, Madang Province, on the north coast of PNG. The clinic serves an area where Plasmodium falciparum and P. vivax are hyperendemic and where P. ovale and P. malariae are also transmitted (13). Children with an axillary temperature > 37.5°C or a history of fever in the previous 24 h were screened with a Giemsa-stained thick blood film read on site by a trained microscopist. Those with a monoinfection of P. falciparum (>1,000 asexual parasites μl of whole blood) or of P. vivax, P. ovale, or P. malariae (>250 asexual parasites μl of whole blood) were eligible provided that the child's parents gave informed consent, there were no features of severe malaria (47), they had not taken any antimalarial drug in the previous 14 days, there was no evidence of another cause of fever, and there were no features of malnutrition or other chronic comorbidity. Although the locations, populations, and enrollment procedures used in the two studies were identical, the DHA-PQ group was enrolled between August 2005 and January 2006 whereas the ART-PQ base group was enrolled from March 2008 to May 2008. The study was approved by the PNG Institute of Medical Research Institutional Review Board and the Medical Research Advisory Committee of the PNG Department of Health.
Clinical methods.
In the present study of ART-PQ base, a standardized history was taken and a clinical examination was performed. A 3-ml venous blood sample was taken for baseline blood film microscopy, for hemoglobin and blood glucose, and for subsequent drug assays of separated plasma. Each child was treated with granules of ART-PQ base (Artequick) according to body weight (approximately 3 mg of ART and 18 mg of PQ base/kg of body weight/day). This dose was repeated at 24 h, as recommended by the manufacturer, with the exact time of each dose recorded. All doses were given under direct observation. The full contents of each sachet were mixed with at least 50 ml of cow's milk (equivalent to 2 g of fat), as fat has been reported to increase the bioavailability of PQ tetraphosphate (25, 41). The volume of milk used was based on previous experience with its palatability and association with nausea in PNG children as well as on the amount of fat found to maximize the absorption of lumefantrine, another highly lipophilic antimalarial drug, in healthy adults (5).
Further venous blood samples were taken from an indwelling intravenous catheter at 1, 2, 4, 12, 24, 28, 36, and 48 h and then by venesection on days 3, 5, 7, 14, 28, 42, and 56. All samples were centrifuged promptly and red cells and separated plasma stored frozen at −80°C until assayed. Detailed clinical assessment, including a symptom questionnaire and determination of blood film, hemoglobin, and blood glucose data, was repeated on days 1, 2, 3, and 7, with additional clinical assessment and determination of blood film data on days 14, 28, 42, and 56. All blood smears taken at baseline and during follow-up were examined independently by at least two skilled microscopists in a central laboratory. Each microscopist viewed 100 fields at ×1,000 magnification before a slide was classified as negative. Any slide discrepant for positivity or negativity or for species identification was referred to a third microscopist for adjudication.
The clinical procedures followed for the DHA-PQ group have been previously described (29) and were similar to those followed for the ART-PQ base group. Differences in the previous study included (i) administration of 3 days of DHA-PQ tetraphosphate tablets at a dose of 2.5 mg of ART and 20 mg of PQ tetraphosphate/kg of body weight daily (equivalent to 11.5 mg of PQ base/kg of body weight daily), (ii) drug administration with water, and (iii) blood sampling and clinical follow-up only until day 42.
Laboratory methods.
A PQ tetraphosphate reference standard was obtained from Yick-Vic Chemicals and Pharmaceuticals, Ltd. (Hong Kong, China). Chloroquine (CQ) diphosphate and authentic ART were from Sigma-Aldrich (St. Louis, MO), and artemether (ARM) was from AAPIN Chemicals Ltd. (Abingdon, United Kingdom). Solid-phase extraction (SPE) Bond Elut PH columns were purchased from Varian Inc. (Palo Alto, CA). High-performance-liquid-chromatography (HPLC)-grade methanol was obtained from Merck Pty. Ltd. (Kilsyth, Australia), and liquid-chromatography—mass-spectrometry (LC-MS)-grade ammonium formate was from Sigma-Aldrich (Gillingham, United Kingdom). All other solvents and chemicals were of analytical grade.
For the ART-PQ base group, PQ in plasma was analyzed by high-performance liquid chromatography as described for the original DHA-PQ group (29) with minor modifications. Briefly, plasma was spiked with CQ as an internal standard, alkalinized, and extracted into 8 ml of hexane-isoamyl alcohol (99:1). Baseline samples were assayed for CQ prior to quantification of PQ to ensure no interference with the internal standard. After centrifugation, the supernatant was back extracted into 100 μl of 0.1 M HCl, aspirated, and recentrifuged. Aliquots of 80 μl were injected into a Phenomenex C6-phenyl column (Phenomenex, Torrance, CA) with a mobile phase of 11% acetonitrile–0.1 M phosphate buffer (pH 2.5) pumped at 1 ml/min. Retention times were 2.5 and 7.3 min for PQ and CQ, respectively, and PQ and CQ were detected at 340 nm. The linear assay range was 2 to 1,000 μg/liter, and the intraday relative standard deviations (RSDs) were 10.8%, 8.2%, and 9.4% and the interday RSDs were 11.6%, 4.4%, and 6.7% at 5, 100, and 1,000 μg/liter, respectively. The limits of quantification and detection were 2 μg/liter and 1 μg/liter, respectively.
For ART, the extraction procedure used a 1-ml C18 SPE column as previously described (9), with the following modifications. Briefly, the SPE column was preconditioned with 1 ml of methanol followed by 1 ml of 1 M acetic acid. Plasma samples (0.5 ml) were spiked with an internal standard (ARM; 1,000 μg/liter), loaded onto the preconditioned SPE column, and drawn through using a medium vacuum. The column was then washed with 1 M acetic acid (1 ml used in each of two successive washes) followed by 20% (vol/vol) methanol in 1 M acetic acid (1 ml). The column was dried under low-vacuum conditions for 30 min, and retained drugs were eluted with 2 ml of t-butyl chloride:ethyl acetate (80%:20% [vol/vol]). The eluate was evaporated in a vacuum evaporator at 35°C and then reconstituted in 50 μl of the mobile phase, and 5-μl aliquots were injected into the LC-MS system.
The LC-MS system used was a single quadrupole mass spectrometer (model 2020; Shimadzu, Kyoto, Japan) consisting of a binary pump (model 20AD), vacuum degasser, thermostated autosampler (model SIL 20ACHT), thermostated column compartment (model CTO 20A), photodiode detector (model SPD M 20A), and mass analyzer (model MS 2020) with both electrospray ionization (ESI) and atmospheric pressure ionization (APCI) systems. Analyses were performed in isocratic mode with a mobile phase of 20 mM ammonium formate (pH 4.8):methanol (20:80) pumped at a flow rate of 0.2 ml/min. Chromatographic separation was undertaken at 30°C on a Synergy fusion-RP C18 column (150-mm length by 2.0-mm inner diameter) coupled with a 5-μm-pore-size C18 guard column (Phenomenex, Lane Cove, Australia) (4-mm length by 3-mm inner diameter). Retention times were 4.2 min and 7.5 min for ART and ARM, respectively. Optimized mass spectra were acquired with an interface voltage of 4.5 kV, a detector voltage of 1 kV, a heat block temperature of 400°C, and a desolvation gas temperature of 250°C. Nitrogen was used as a nebulizer gas at a flow rate of 1.5 liter/min and a dry-gas flow rate of 10 liter/min.
Quantification was performed by selected ion monitoring (SIM), using the DUIS mode, in which ACPI and ESI are used simultaneously. All standard curves were linear, with r2 ≥ 0.999. Chromatographic data (peak area ratio of ART/ARM) were processed using the LAB Solution software package (version 5; Shimadzu, Japan). Responses from analysis of samples containing three different ART concentrations (5, 200, and 2,000 μg/liter) and one ARM concentration (1,000 μg/liter) spiked into five separate plasma samples were used to determine matrix effects (ion suppression/enhancement), absolute recovery, and process efficiency (32), which were between 90% and 98%, 82% and 93%, and 86% and 91%, respectively. The assay intraday RSDs were 9.3, 7.2, and 3.7% and interday RSDs were 9.5, 7.1, and 6.5% at 5, 200, and 2,000 μg/liter, respectively. The limits of quantification and detection for ART were 2.5 and 1 μg/liter, respectively.
Pharmacokinetic modeling.
Loge plasma concentration-time data sets for PQ and ART were analyzed by nonlinear mixed effects modeling using NONMEM (version 6.2.0; ICON Development Solutions, Ellicott City, MD) with an Intel Visual FORTRAN 10.0 compiler. The PQ plasma concentration-versus-time data from a previously published study of DHA-PQ performed by our group, which were originally analyzed using a patient rather than a population approach (29), were pooled with the PQ concentration data from the present study. The first-order conditional estimation (FOCE) with interaction estimation method was used. The minimum values of the objective function (OFV) and conditional weighted residuals (CWRES) plots were used to choose suitable models during the model-building process. Allometric scaling was employed a priori, with volume terms multiplied by (WT/70)1.0 and clearance terms by (WT/70)0.75 (3), where WT represents total body weight. Residual variability (RV) data were estimated as additive errors for the log-transformed data. Secondary pharmacokinetic parameters, including the area under the concentration-time curve from time zero to infinity (AUC0–∞) and elimination half-life (t1/2), were obtained for the participants from post hoc Bayesian predictions using NONMEM and the final model parameters. Base models were parameterized using ka (absorption rate constant), VC/F (central volume of distribution), CL/F (clearance), and VP/F and Q/F (peripheral volumes of distribution[s] and their respective intercompartmental clearance[s]).
For the PQ data set, two- and three-compartment models (ADVAN 4 and 12) with first-order absorption with and without lag time were tested. Since inspection of the time-concentration curves indicated that there was significant variability in the absorption phase, a transit compartment model was also tested (39). In this model, the dose passes through a series of transit compartments before entering the absorption compartment in order to model the delay often associated with drug absorption. A single rate constant (ktr) represents entry and exit for all transit compartments. Using a previously described implementation of the transit compartment model in NONMEM (39), the number of transit compartments (NN) and the mean transit time [MTT = (1 + NN)/ktr] were estimated as continuous variables. For the ART data set, 1- and 2-compartment models (ADVAN 2 and 4) with first-order absorption with and without lag time were evaluated. Once the structure of the models was established, interindividual variability (IIV), interoccasion variability (IOV), and correlations between IIV terms were estimated, where supported by the data.
As two different formulations of PQ with different water/lipid solubilities were used, potential differences in their relative levels of bioavailability were assessed. The difference in relative bioavailability levels between first and subsequent doses of PQ and ART was also investigated. For PQ, this was achieved by estimating the differences between the relative bioavailability levels of the first dose of PQ phosphate (fixed to 1) and the two doses of PQ base as well as the two subsequent doses of PQ phosphate. Similarly, for ART, the relative bioavailability of the first dose was fixed to 1 and potential differences between this and subsequent doses were assessed. The inclusion of an extra parameter to account for differences in relative bioavailability was considered only if accompanied by a significant (>6.63; P < 0.01) fall in the OFV and an improvement in the CWRES plot. Differences in absorption parameters (ka, NN, and MTT) between the two groups were also assessed within NONMEM. As described below, the effect size (percent) of the difference was estimated. To maintain the extra parameter estimating this difference, a significant (>6.63; P < 0.01) fall in the OFV was required. Differences between clearance and volume terms for the two formulations were not assessed, as the idea of differences between a salt and base formulation of the same drug is biologically implausible.
Finally, relationships between model parameters and the covariates age, sex, log (baseline parasitemia), and fever were identified through inspection of scatter plots and box plots of post-hoc values for individuals obtained from IIV distributions versus covariate and were subsequently evaluated within NONMEM. The effect size (percent) of categorical data (sex, fever) was assessed, while both linear and power relationships were evaluated for continuous covariates (age, log [baseline parasitemia]). For effect size, the individual parameter value = population parameter value × (1 + effect parameter × covariate value [0 or 1]). For linear relationships, the individual parameter value = population parameter value × [1 + effect parameter × (covariate value for individual/average value of covariate)]. For power relationships, the individual parameter value = population parameter value × [(covariate value for individual/average value of covariate)effect parameter]. A stepwise forward inclusion and backward elimination method was used, with a significance of P < 0.05 required for inclusion of a covariate relationship and P < 0.01 to retain a covariate relationship.
As CQ was used as the internal standard in the PQ assay, the potential impact of residual CQ in the plasma of the children on pharmacokinetic parameters was assessed through simulation. A previous study in a similar group of children resident in the same study area demonstrated that approximately 50% had a measurable plasma CQ concentration when hospitalized (12). Using plasma CQ concentrations from a previous pharmacokinetic study of Madang children (29), we simulated conditions such that (i) half of the children had, at random, received a treatment course of CQ finishing 14 days prior to the study (just before the exclusion period for such treatment) and (ii) only children from one of the treatment groups received CQ treatment 14 days prior to the study. The latter simulation represents the worst-case scenario in terms of the effect of residual CQ on the comparative pharmacokinetic properties of the two PQ formulations through exogenous augmentation of the internal standard.
Model evaluation.
Initially, plots of observed versus individual and population predicted values and of time versus CWRES were assessed. A bootstrap analysis using Perl-speaks-NONMEM with 1,000 samples was performed (for NQ, this was stratified according to dose regimen), and the parameters derived from this analysis were summarized as the median and 2.5th and 97.5th percentiles (with 95% empirical confidence intervals [CI]) to facilitate evaluation of final model parameter estimates. In addition, prediction-corrected visual predictive checks (pcVPCs) (11) and numerical predictive checks (NPCs) were performed with 1,000 data sets simulated from the final models, and these were stratified according to treatment group for PQ. The observed 10th, 50th, and 90th percentiles were plotted with their respective simulated 95% CIs to assess the predictive performance of the model. NPCs were assessed by comparing the actual with the expected numbers of data points within the 20%, 40%, 60%, 80%, 90%, and 95% prediction intervals (PI). These were also stratified according to treatment group for the PQ model.
Statistical analysis.
Comparisons between the baseline characteristics and secondary pharmacokinetic parameters of the subjects in the DHA-PQ and ART-PQ base studies were assessed using the Mann-Whitney U test for continuous variables and the Fisher exact test for categorical variables. A two-tailed level of significance of 0.05 was considered significant for all comparisons.
RESULTS
Clinical characteristics and course.
The baseline characteristics for all the children in the study are summarized in Table 1. Of those who received ART-PQ base, 11 had a monoinfection with P. falciparum and 1 had a monoinfection with P. vivax. One child was lost to follow-up after day 14. ART-PQ base treatment was well tolerated; reported symptoms were mild and short-lived (<2 days) and consistent with clinical features of uncomplicated malaria. Initial fever clearance occurred in <24 h in all cases, and parasite clearance occurred in <48 h in all but one child, in whom it occurred within 72 h. The child with P. vivax at enrollment cleared parasitemia promptly and remained slide negative for the 56 days of follow-up. Of the 11 children with P. falciparum, 1 developed slide-positive P. falciparum on day 28, another on day 42, and 2 more by day 56. As PCR was not performed, it was not possible to determine if these represented recrudescence or reinfection. Only one child with P. falciparum at entry became slide positive for P. vivax, on day 56. The mean hemoglobin concentration, where data were available, increased as a result of treatment regardless of malaria status during follow-up, with mean (95% CI) increases from baseline of 1.9 (0.40 to 3.3) (n = 9), 1.1 (0.15 to 2.5) (n = 11), and 1.5 (0.20 to 2.5) (n = 10) g/dl on days 28, 42, and 56, respectively (P = 0.027, P = 0.19, and P = 0.041). No cases of hypoglycemia were recorded.
Table 1.
Baseline characteristics of study participants
| Parameter | Duo-cotecxin values (29) (historical) (n = 22)a | Artequick values (present study) (n = 12)a | P value |
|---|---|---|---|
| Age (yr) | 6.9 ± 1.4 | 7.1 ± 1.5 | 0.790b |
| Sex (% male) | 17 (86) | 8 (66) | 0.687c |
| Weight (kg) | 19.1 ± 3.8 | 18.3 ± 3.1 | 0.986b |
| Axillary temp (°C) | 37.2 ± 1.2 | 36.3 ± 0.7 | 0.034b |
| No. (%) with P. falciparum parasitemia | 19 (86) | 11 (92) | 1.00c |
| Parasite density (per μl of whole blood) | 13,360 [6,900–51,650] | 26,270 [3,480–35,30] | 0.736b |
| No. (%) with P. vivax parasitemia | 2 (9.1) | 1 (8) | 1.00c |
| No. (%) with P. malariae parasitemia | 1 (4.5) | 0 (0) | 1.00c |
| Hemoglobin (g/dl) | 8.6 ± 1.8 | 9.3 ± 2.1 | 0.168b |
| Total PQ base dose (mg/kg) | 35.3 ± 4.4 | 38.3 ± 5.8 | 0.136b |
| Total DHA dose (mg/kg) | 7.7 ± 1.0 | ||
| Total ART dose (mg/kg) | 6.4 ± 1.0 |
Data represent number (%), mean ± SD, or median [interquartile range {IQR}], as indicated.
Mann-Whitney U test.
Fisher's exact test.
Pharmacokinetic modeling.
There were 298 and 174 individual plasma PQ concentrations available from the DHA-PQ (n = 22) and ART-PQ base (n = 12) studies, respectively. No drug concentrations were below the limit of quantification during the 56-day follow-up period. A 3-comparment model fitted the data better than a 2-compartment model, with a significant decrease in the OFV (ΔOFV = −109.232, P < 0.001). Although the addition of a lag time improved the model significantly (ΔOFV = −31.059, P < 0.001), the absorption phase was poorly described, with first-order absorption determined with or without lag time. Therefore, a transit compartment model was tested where the number of transit compartments (NN) and the mean transit time (MTT) through the transit compartments were estimated as continuous variables. The transit compartment model was significantly better than a model with lag time, resulting in a 37.173-point reduction in the OFV (P < 0.001). Further testing of the combined data sets with models in which the absorption processes of the two formulations of PQ differed (for example, use of a lag-time model for PQ base and a transit compartment model for PQ tetraphosphate) were also tested and offered no advantage over the use of a single-transit-compartment model. A three-compartment model remained superior to a two-compartment model with the use of a transit compartment absorption determination (ΔOFV = −57.937, P < 0.001).
The structural model parameters were ka, NN, MTT, VC/FPQ, VP1/FPQ, VP2/FPQ, CL/FPQ, Q1/FPQ, and Q2/FPQ. There was poor precision for the estimate of ka (RSE % > 100) as well as a high (>0.95) correlation between ka and MTT. Therefore, with the data available in this study, these two parameters could not be estimated simultaneously and ka was set to the same value as ktr, i.e., equal to (1 + NN)/MTT. Interindividual variability was estimable for MTT, CL/FPQ, VC/FPQ, andVP1/FPQ. Correlation between IIV terms was estimated for CL/FPQ and VC/FPQ and for VC/FPQ andVP1/FPQ. The IOV for FPQ was also estimable and was accompanied by significant falls in OFV (ΔOFV = −69.12, P < 0.001) and RV (35% to 29%). There was no significant difference between the relative bioavailabilities of the two formulations or between the subsequent doses of PQ base or tetraphosphate and the first dose. Although inspection of the concentration-time curves appeared to indicate a difference between the two formulations in their absorption phases, when differences in NN and MTT were evaluated, they did not improve the model. Likewise, none of the tested covariates improved the model.
The impact of residual CQ proved to be minimal as assessed using the simulations, with population pharmacokinetic parameter estimates differing by <9%. When all participants in the same formulation group were presumed to have taken CQ 14 days prior to the start of the study, there was still no significant difference between the population pharmacokinetic parameter estimates for the two PQ formulations.
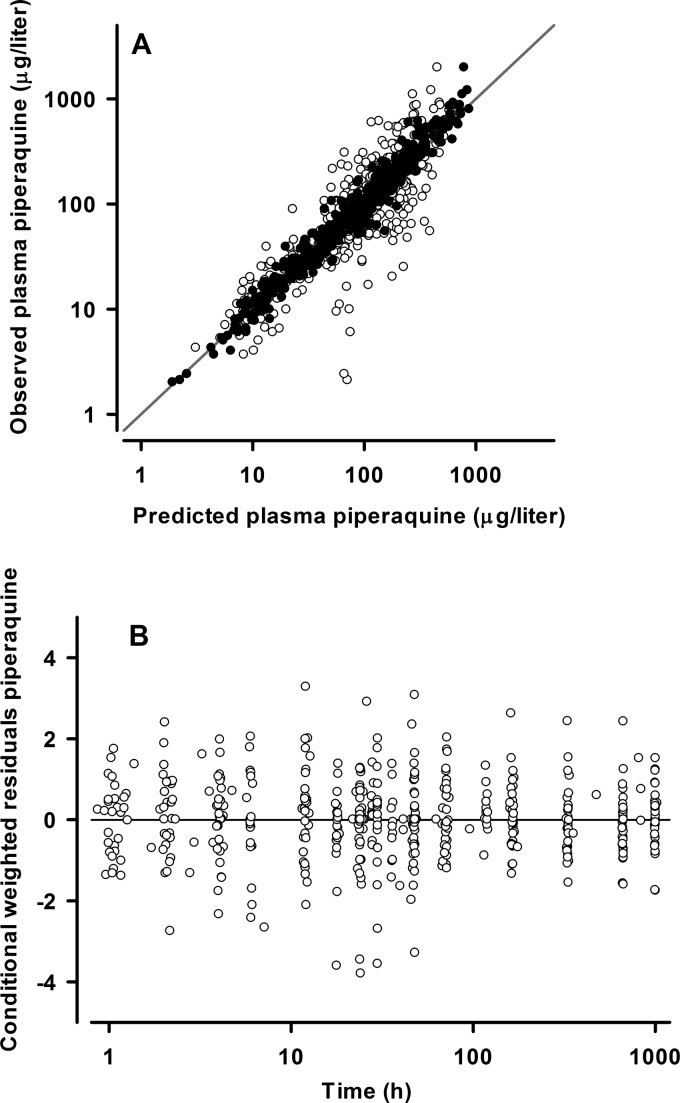
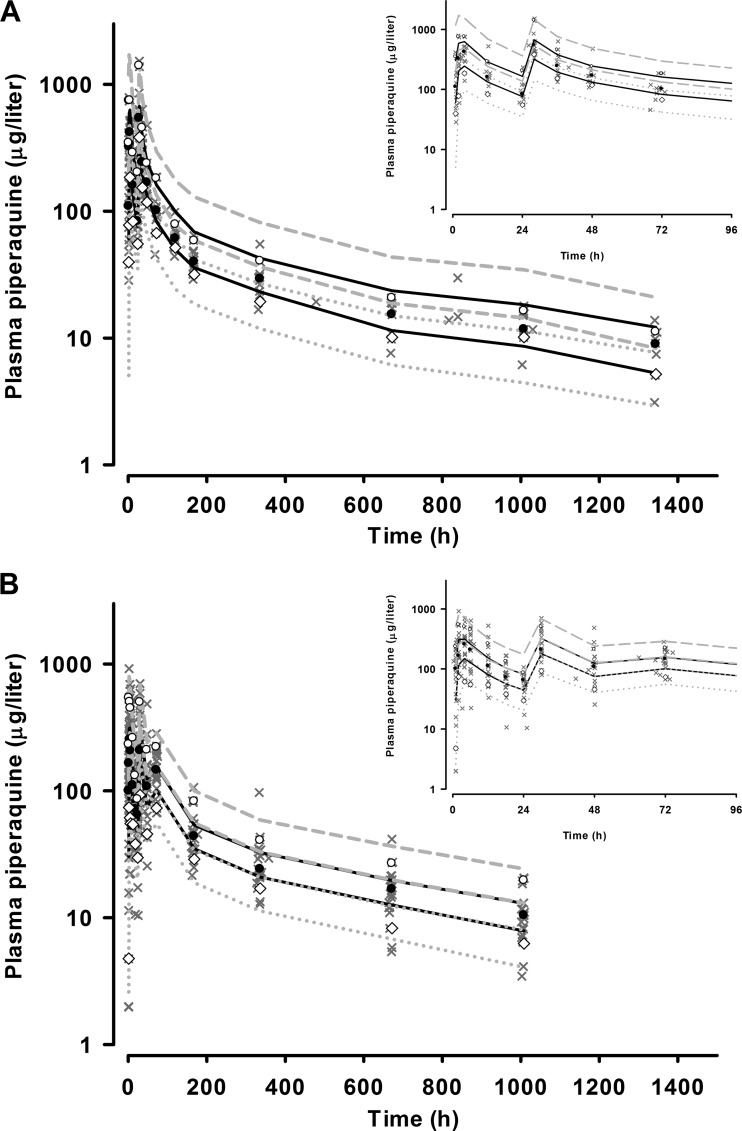
The final model parameter estimates and the bootstrap results for both PQ formulations are summarized in Table 2. Bias was <10% for all fixed and random model parameters. With the exception of IIV in CL/FPQ, all parameters were reasonably well estimated, with relative standard errors of <33%. The correlation between CL/FPQ and VC/FPQ displayed a wide 95% CI (−0.186 to 0.710). Figures 1 and 2 show goodness-of-fit plots and pcVPCs, respectively. The pcVPCs showed wide 95% confidence intervals for the 10th, 50th, and 90th percentiles due to relatively small numbers of children. The actual 10th, 50th, and 90th percentiles fell into their respective 95% CI ranges for all time points for both groups. The stratified NPCs demonstrated good predictive performance, with the expected number of points above and below the 20%, 40%, 60%, 80%, 90%, and 95% PIs. The half-life, total AUC0–∞, and dose-adjusted AUC0–∞ values are shown in Table 3. There were no significant differences between the two PQ compounds in any of these secondary parameters. The first distribution, second distribution, and terminal elimination t1/2 values for all participants had median values of 4.5, 36.0, and 512 h, respectively. The median PQ AUC0–∞ values for the Artequick and Duo-cotecxin formulations were 49,451 μg · h/liter and 44,556 μg · h/liter, respectively.
Table 2.
Final population pharmacokinetic estimates and bootstrap results for piperaquinea
| Parameter | Mean (RSE %) | Bootstrap median [95% CI] |
|---|---|---|
| Structural and covariate model parameters | ||
| MTT (h) | 1.27 (11) | 1.25 [1.12–1.58] |
| NN | 4.20 (19) | 3.70 [2.77–5.36] |
| CL/FPQ (liter/h/70 kg) | 40.1 (7) | 40.7 [36.6–45.1] |
| VC/FPQ (liter/70 kg) | 2,580 (13) | 2,550 [1,996–3,142] |
| Q1/FPQ (liter/h/70 kg) | 113 (21) | 119.0 [84.3–166.0] |
| VP1/FPQ (liter/70 kg) | 2,760 (24) | 3,440 [2,750–5,510] |
| Q2/FPQ (liter/h/70 kg) | 52.4 (15) | 52.9 [43.8–67.1] |
| VP2/FPQ (liter/70 kg) | 21,600 (8) | 22,300 [19,300–25,320] |
| Random model parameters | ||
| IOV in FPQ (%) | 46 (14) | 42 [36–54] |
| IIV in CL/FPQ (%) | 16 (53) | 16 [5–29] |
| IIV in VC/FPQ (%) | 53 (33) | 45 [31–71] |
| IIV in VP1/FPQ (%) | 68 (32) | 64 [16–93] |
| IIV in MTT (%) | 43 (13) | 42 [34–52] |
| Correlation coefficient (CL/FPQ, VC/FPQ) | 0.33 | 0.272 [–0.186–0.710] |
| Correlation coefficient (VC/FPQ, VP1/FPQ) | 0.85 | 0.874 [0.381–1.00] |
| Residual variability (%) | 29 (5) | 29 [27–32] |
Parameters are NN (number of transit compartments), MTT (mean transit time), CL/FPQ (clearance), VC/FPQ (central volume of distribution), VP1/FPQ and VP2/FPQ (peripheral volumes of distribution), Q1/FPQ and Q2/FPQ (intercompartmental clearance between VP1/FPQ and VC/FPQ and between VP2/FPQ and VC/FPQ respectively), and F1,Artequick (bioavailability of the first dose of Artequick relative to the first dose of Duo-cotecxin). RSE (relative standard error) values were calculated from bootstrap results. OFV in final model, −329.926; bootstrap OFV (median [95% CI]), −316.869 [−416.930 to 285.019].
Fig 1.
(A) Population (○) and individual (●) predicted versus observed plasma piperaquine concentrations (in micrograms per liter on a log10 scale) for the final model. The line of identity is also shown. (B) Conditional weighted residuals versus time (log scale) for piperaquine final model.
Fig 2.
Visual predictive check showing observed 50th (●), 10th (◇), and 90th (○) percentiles with simulated 95% CIs for the 50th (solid black line), 10th (gray dotted lines), and 90th (dashed gray lines) percentiles for plasma piperaquine concentrations (micrograms per liter on a log10 scale) versus time (h) for Artequick (A) and Duo-cotecxin (B) from the final model. The observed data are superimposed as gray crosses. The inset shows data for the first 96 h.
Table 3.
Secondary pharmacokinetic parameters for piperaquine derived from post hoc Bayesian estimates for study participants and day 7 plasma piperaquine levels
| Parametera | Median [IQR] |
P valueb | |
|---|---|---|---|
| PQ (Duo-cotecxin) n = 22 | PQ (Artequick) n = 12 | ||
| t½α (h) | 4.44 [3.43–5.30] | 4.52 [3.76–6.41] | 0.48 |
| t½β (h) | 36.1 [33.0–45.2] | 35.3 [28.1–58.7] | 0.82 |
| t½γ (h) | 513 [503–574] | 512 [497–566] | 0.82 |
| Day 7 plasma piperaquine level (μg/liter) | 39.3 [34.9–45.9] | 42.0 [34.6–55.6] | 0.56 |
| AUC0–∞ (μg · h/liter) | 49,451 [40,507–52,438] | 44,556 [33,215–51,873] | 0.36 |
| AUC0–∞ (μg · h/liter)/total PQ dose (mg/kg) | 1.27 [1.06–1.50] | 1.37 [1.09–1.65] | 0.40 |
t½α, t½β, and t½γ represent the first distribution half-live, second distribution half-live, and terminal elimination half-live, respectively.
Mann-Whitney U test.
Of the 96 ART drug concentrations (ART-PQ base group, n = 12) that were available for analysis, six (6.25%) were below the limit of quantification but above the limit of detection. As these represented a small proportion of the data, they were included at their measured values. All 12 children has measurable levels of ART to 48 h. Initial modeling of the ART data set demonstrated that a two-compartment model was significantly better than a one-compartment model (ΔOFV = −73.417, P < 0.001) and that the absorption phase was best represented by first-order absorption without a lag time. Therefore, the structural model parameters were ka, VC/FART, VP/FART,CL/FART, and Q/FART. The IIV of VC/FART was estimable, as was the IOV on FART. The data supported the estimation of a relative bioavailability term for the second dose of ART (F2,ART), with its addition resulting in a significant fall in the OFV (ΔOFV = −24.029, P < 0.001). The bioavailability of the second dose was 0.270 relative to the first. No significant covariate relationships were identified.
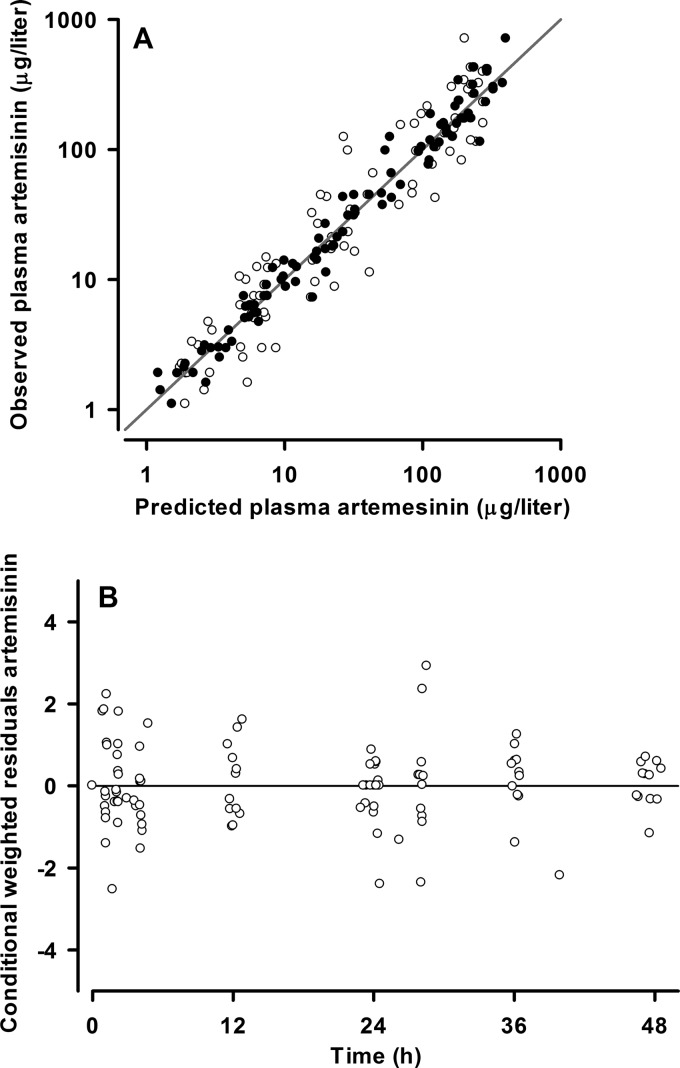
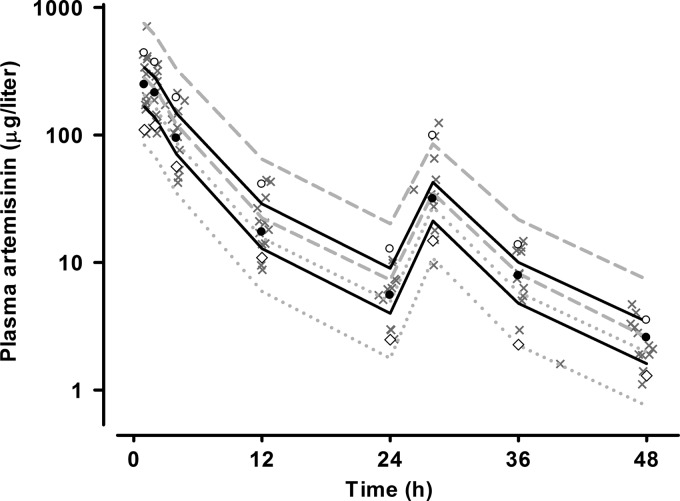
The final model parameter estimates and the bootstrap results for ART are summarized in Table 4. Bias was <10% for all fixed and random parameters. ka was not well estimated, with a relative standard error of 55% and a 4-fold range in the nonparametric 95% CI. Figures 3 and 4 show goodness-of-fit plots and pcVPCs, respectively. The pcVPC showed that all observed 10th, 50th, and 90th percentile values were within their simulated 95% CIs. Due to the small numbers used in the analysis, these CIs were wide and overlapping. The NPC demonstrated good predictive performance, with the expected number of points above and below the 20%, 40%, 60%, 80%, 90%, and 95% PIs. The t1/2 and the AUC0–∞ for each dose as well as the total AUC0–∞ for the study participants are shown in Table 5. The median distribution and terminal elimination t1/2 values were 1.55 and 7.43 h, while the median total AUC0–∞ value was 1,652 μg · h/liter.
Table 4.
Final population pharmacokinetic estimates and bootstrap results for artemisinin (n = 12)a
| Parameter | Mean (RSE %) | Bootstrap median [95% CI] |
|---|---|---|
| Structural model parameters | ||
| ka (per h) | 1.67 (55) | 1.62 [1.01–4.40] |
| CL/FART (liter/h/70 kg) | 124 (12) | 125 [99–157] |
| VC/FART (liter/70 kg) | 590 (30) | 533 [318–874] |
| Q/FART (liter/h/70 kg) | 43.7 (38) | 46.4 [19.5–79.4] |
| VP/FART (liter/70 kg) | 435 (26) | 456 [259–696] |
| F2,ART − relative bioavailability of 2nd dose | 0.270 (17) | 0.275 [0.192–0.368] |
| Random model parameters | ||
| IOV in FART (%) | 43 (27) | 39 [15–58] |
| IIV in CL/FART (%) | 12 (29) | 12 [4–18] |
| Residual variability (%) | 33 (11) | 32 [26–38] |
Parameters are ka (absorption rate constant), CL/FART (clearance), VC/FART (central volume of distribution), VP/FART (peripheral volume of distribution), Q/FART (intercompartmental clearance between VP/FART and VC/FART), and F2,ART (relative bioavailability of 2nd dose of ART). RSE (relative standard error) values were calculated from bootstrap results. OFV in final model, −63.562; bootstrap OFV (median [95% CI], −73.838 [−110.720 to 43.043].
Fig 3.
(A) Population (○) and individual (●) predicted versus observed plasma artemisinin concentrations (micrograms per liter on a log10 scale) for the final model. The line of identity is also shown. (B) Conditional weighted residuals versus time for artemisinin final model.
Fig 4.
Visual predictive check showing observed 50th (●), 10th (♢), and 90th (○) percentiles with simulated 95% CIs for the 50th (solid black line), 10th (gray dotted lines), and 90th (dashed gray lines) percentiles for plasma artemisinin concentrations (micrograms per liter on a log10 scale) versus time (h) from the final model. The observed data are superimposed as gray crosses.
Table 5.
Secondary pharmacokinetic parameters for artemisinin derived from post hoc Bayesian estimates for study participants
| Parametera | ART (Artequick) values (median [IQR]) (n = 12) |
|---|---|
| t½α (h) | 1.55 [1.49–1.60] |
| t½β (h) | 7.43 [7.22–7.68] |
| AUC (μg · h/liter) − first dose | 1,347 [1,065–1,594] |
| AUC (μg · h/liter) − second dose | 312 [253–438] |
| AUC0–∞ (μg · h/liter) − total | 1,652 [1,333–2,177] |
t½α and t½β represent the distribution half-life and terminal elimination half-life, respectively. AUCs for each dose were calculated using the standard pharmacokinetics formula to determine the relative contribution of each dose to the total AUC0–∞ value.
Relationship between drug exposure and treatment outcome.
In the eight children whose samples became slide positive for P. falciparum by day 42 (two in the ART-PQ base group and six in the DHA-PQ group), the AUC0–∞ value for PQ was significantly lower than that seen with those who remained free of P. falciparum infection (median, 39,297 versus 49,776 μg · h/liter; P = 0.0060). Clearance and terminal elimination t1/2 values were not significantly different; however, these children received a lower total dose of PQ (median, 31.4 versus 35.7 mg/kg of PQ base; P = 0.11). When adjusted for dose, the differences in the AUC0–∞ values were no longer significant between those children with and without slide positivity results for P. falciparum by day 42 (median, 1.16 versus 1.42 μg · h/liter per mg/kg of PQ base; P = 0.14). There was a significant positive correlation between AUC0–∞ values and day 7 drug levels that did not reach significance (r = 0.70; 95%CI, 0.48 to 0.84; P < 0.001). Unlike AUC0–∞ values, day 7 levels were not significantly lower in those children whose samples showed P. falciparum slide positivity (n = 8) compared to those whose samples did not (n = 26) (median, 44.1 versus 48.0 μg/liter; P = 0.22). Similar results were evident when the two children from the DHA-PQ group whose samples developed slide positivity for P. vivax by day 42 were included in the analysis (data not shown). For the child who took >48 h to clear initial parasitemia, the AUC0–∞ values for ART and PQ were within the ranges of those of the other patients.
DISCUSSION
The development of ACTs has seen a variety of different combinations, formulations, and dose regimens introduced into clinical use without a detailed assessment of tolerability, safety, pharmacokinetics, and efficacy. One recently marketed ACT, Artequick, appears to be relatively inexpensive to produce but uses component drugs that have not been investigated extensively, especially in a pediatric setting. The present pharmacokinetic and preliminary efficacy study in PNG children adds to the available data (30, 37), suggesting that there would be benefits in extending the Artequick manufacturer's recommended 2-day regimen to 3 days, as this would increase PQ exposure and thus limit late recurrence of parasitemia. However, the selection of a relatively low dose of ART (3 mg/kg of body weight versus the 10 to 20 mg/kg dose conventionally recommended), a drug that induces its own metabolism, may have implications for efficacy, especially in patients with limited immunity to malaria or in geographical areas where artemisinin resistance has started to develop (18).
Children in the DHA-PQ tetraphosphate group were given a mean of 35.3 mg/kg PQ base over 3 days (29) compared with 38.3 mg/kg PQ base over 2 days in the present children treated with ART-PQ base. Overall, the exposures to PQ were similar for the two formulations, and no differences in the post hoc pharmacokinetic parameters were identified. Although this suggests that the tablet and granule formulations have similar bioavailabilities and that the small amount (2 g) of fat we administered with each dose was unlikely to influence exposure to PQ, it is not possible to differentiate the influences of food and formulation with the current study design. Two of three studies involving healthy adults found that fat-containing foods increased exposure to PQ tetraphosphate (31, 34, 41), but the volunteers in those studies consumed relatively large quantities of fat (17 to 54 g). Consistent with the present results, 6.4 g of fat did not increase the exposure to PQ tetraphosphate in adults with malaria (4). However, PQ base is less water soluble than PQ tetraphosphate, and exogenous lipids are known to increase the solubility of lipophilic drugs and thus affect the extent of absorption (35). PQ base may behave similarly to lumefantrine, another highly lipophilic drug, and may require a smaller amount of fat to maximize absorption (5). Granule formulations have been reported to increase bioavailability relative to tablets (15), which is consistent with the increased surface area available for dissolution compared to tablets, but our data suggest that this is not a major effect in the case of Artequick. Future studies evaluating the effect of food and drug formulation on the disposition of PQ base in malaria could help refine dose regimens for therapies employing drugs such as Artequick.
A model with three compartments and a transit sequence prior to absorption best represented the PQ concentration-time data set. Most previous studies have used a two-compartment model (28, 31, 44). One study in healthy adults found that, although a three-compartment model represented the postadministration profile better, there were insufficient data to support its use over a two-compartment model (38). The mean elimination t1/2, a parameter influenced by the duration of sampling (45), was 512 h, a value within the previously reported range of 224 to 667 h (1, 14, 25, 28, 31, 34, 38, 44). Since there was substantial variability in the absorption phase of the plasma PQ concentration profile, a transit compartment model was tested and proved better than simpler absorption models that used lag time, as has been found in studies of other drugs (39).
It has recently been suggested that children should be given a higher dose of PQ than adults due to lower day 7 plasma concentrations (36) and reduced efficacy (27, 36, 49). This is supported by comparative pharmacokinetic studies in children and adults that found that children had a higher clearance (28) or a lower PQ exposure at critical times during the illness (44). These concerns have also been raised for other antimalarial drugs (8, 33) and reflect pharmacokinetic effects due to the effects of body size, maturation, and organ function (3). Although only children aged between 5 and 10 years were included in the present study, we found that recurrence of parasitemia was associated with a lower PQ AUC resulting from a lower milligram/kilogram dose, consistent with other studies of DHA-PQ tetraphosphate (16). As PCR was not performed, these cases may represent either recrudescence (treatment failure) or reinfection (failure of posttreatment prophylaxis).
The dose-adjusted PQ exposures in our children were similar to that found in Caucasian and Vietnamese healthy adults (14, 38, 41) and Thai adults with malaria (4) but were from three to six times lower than those found in studies of Vietnamese and Chinese healthy adults, respectively (25, 31), suggesting that there are PQ pharmacokinetic differences between populations. Currently recommended PQ tetraphosphate doses are 18 mg/kg/day (10 mg/kg/day PQ base) for 3 days (48). A higher average daily dose of PQ base in the Artequick group (19 mg/kg/day) was well tolerated when given for 2 days, and the same dose given on the third day might both satisfy WHO recommendations for duration of ACT and address the issue of the need for higher milligram/kilogram doses in children.
The use of CQ as an internal standard for PQ is potentially problematic for samples taken from an area of malaria endemicity where CQ is widely available and used empirically for treatment of fever. Utilizing CQ usage and pharmacokinetic data from other studies in children from the Madang area (12, 29), we investigated whether the 14-day exclusion for prior antimalarial treatment was sufficient to limit such potential confounding. Even in the worst-case scenario, there was only a small effect on the estimated PK parameters, and that effect did not produce falsely significant differences between the results obtained for the two formulations. Although this is reassuring, it would be best if future similar studies employed an alternative internal standard.
A number of published studies have evaluated the pharmacokinetics of ART in healthy adults (6, 7, 10, 19, 23, 43) and adults with malaria (2, 9, 21, 22, 24, 26, 42), but only one has included children with malaria (40). In the latter Vietnamese study, 23 children aged 2 to 12 years were given 5 days of ART dosed according to body weight (approximately 10 mg) and 31 adults received 500 mg of ART daily for 5 days. Sparse sampling was used to characterize ART population pharmacokinetics in plasma by the use of NONMEM after the first and final doses, with two samples collected from each patient on day 1 and a single sample collected on day 5 from some patients. A one-compartment model was used, with clearance and volume terms for children and adults estimated separately. The median weights and ages of the children were lower in the present study (18.3 versus 20 kg and 7.1 versus 9 years, respectively). Although our value for ka was comparable to that in the Vietnamese study (2.0/h versus 1.7/h), a two-compartment model provided a better fit in the present study, with distribution and elimination t1/2s of 1.9 h and 8.3 h, respectively, compared to a t1/2 of 1.8 h in the previous study (40). This difference may reflect the longer sampling duration in the present study (24 h versus 8 h postdose), which enabled the identification of a second exponential phase in the elimination of ART.
The elimination t1/2 of ART has been reported to be between 1.4 and 4.8 h in noncompartmental (2, 6, 7, 9, 21, 22, 26, 42, 43) and compartmental (10, 19, 23, 24, 40) analyses. The present analysis supports a biexponential disposition for ART, while most previous compartmental analyses have reported a monoexponential disposition. A shorter sampling duration may be responsible for this difference, as sampling was confined to <10 h after the last dose in all but one of the studies reported to date. In addition, assay sensitivity may also contribute by limiting quantification of ART to those samples taken <12 h after dose administration (10, 19). One study of healthy adults given a single dose of ART (6) also reported a biexponential disposition and found longer distribution (2.61 h versus 1.55 h) and shorter elimination (4.34 h versus 7.43 h) t1/2s compared to those determined in the present study. Although this study sampled blood to 24 h postdose, ART could be quantified in samples only up to 8 h.
The present median AUC0–∞ of the first dose of ART (1,347 μg · h/liter) was within the range reported for healthy adults (1,190 to 2,690 μg · h/liter) (6, 7, 10, 19, 43) but well below that of adults with malaria (2,601 to 2,780 μg · h/liter) (2, 9, 26) who received 500 mg of ART. Our children received a lower dose of ART (3.2 mg/kg/day), and, when adjusted for the relative dose administered, the AUC0–∞ for the first dose was above those seen in adults (2, 9, 26). The autoinduction of ART metabolism has been well characterized, with a primary effect on the bioavailability of subsequent doses rather than on systemic clearance (23). It is likely, therefore, that this represents an increase in the activity of gut wall rather than liver metabolism.
We found a difference in the PK of ART for the second dose that was explained by a lower relative bioavailability of 0.27 compared to the first dose. In comparisons of the AUCs of different doses in previous studies, the relative bioavailability after 4 to 7 days was between 0.13 and 0.29 (7, 9, 22, 26, 40, 43). One study of African adults with malaria who received 500 mg of ART daily for 3 days and a single dose of mefloquine (42) measured ART in saliva and found that relative bioavailability was lower (0.45) only on the third day when mefloquine was given after the last dose of ART. However, when mefloquine was given on the first day, at the same time as the first dose of ART, the relative bioavailabilities of both the second and third doses of ART were lower, at 0.23 and 0.25, respectively.
Our data are in agreement with a rapid mean autoinduction time of 1.9 h as estimated using a semiphysiological model for ART (23), indicating that all doses after the first had a lower relative bioavailability. If a third daily dose of ART-PQ base were to be given, its relative bioavailability would also be low. The rapid initial parasite clearance in Artequick-treated children seen in the present study despite relatively low and short-lived plasma ART concentrations may reflect the level of malarial immunity in this geographical area of intense transmission (17). It is likely that relatively low ART doses, even if given over 3 days rather than 2, would not be as effective where transmission and consequent immunity are less or where artemisinin resistance has started to develop (18).
Compared to 3 days of DHA-PQ tetraphosphate administration, the efficacy of 2 days of administration of Artequick in adults was equivalent in one study (46) and inferior in another (37). A 3-day Artequick regimen (3.2 and 16.0 mg/kg/day of ART and PQ base, respectively) has been found to be both well tolerated and more effective than a 2-day regimen (30). Our preliminary data suggest that the efficacy of 2 days of Artequick administration appeared similar to that of 3 days of Duo-cotecxin administration in PNG children. However, the weight of evidence from previous studies (30, 37), the low proportion of ART in Artequick and its autoinduction at a time when the specter of artemisinin resistance has emerged (18), and the issue of potential PQ underdosing in children all support further evaluation of a theoretically more efficacious 3-day Artequick regimen, as recommended by the WHO for all ACTs (48).
ACKNOWLEDGMENTS
We are most grateful to Valsi Kurian and the staff of Alexishafen Health Centre for their kind cooperation during the study. We also thank Jovitha Lammey, Christine Kalopo, and Bernard (“Ben”) Maamu for clinical and/or logistic assistance. Harin Karunajeewa is acknowledged for his pivotal role in coordinating the original DHA/PQ tetraphosphate study. We thank Artepharm Co. Ltd. for kind provision of Artequick.
The National Health and Medical Research Council (NHMRC) of Australia funded the study (grant 634343). T.M.E.D. is supported by an NHMRC Practitioner Fellowship.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 2 April 2012
REFERENCES
- 1. Ahmed T, et al. 2008. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J. Clin. Pharmacol. 48:166–175 [DOI] [PubMed] [Google Scholar]
- 2. Alin MH, Ashton M, Kihamia CM, Mtey GJ, Bjorkman A. 1996. Clinical efficacy and pharmacokinetics of artemisinin monotherapy and in combination with mefloquine in patients with falciparum malaria. Br. J. Clin. Pharmacol. 41:587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson BJ, Holford NH. 2009. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 24:25–36 [DOI] [PubMed] [Google Scholar]
- 4. Annerberg A, et al. 2011. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob. Agents Chemother. 55:3971–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ashley EA, et al. 2007. How much fat is necessary to optimize lumefantrine oral bioavailability? Trop. Med. Int. Health 12:195–200 [DOI] [PubMed] [Google Scholar]
- 6. Ashton M, et al. 1998. Artemisinin pharmacokinetics in healthy adults after 250, 500 and 1000 mg single oral doses. Biopharm. Drug Dispos. 19:245–250 [DOI] [PubMed] [Google Scholar]
- 7. Ashton M, et al. 1998. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab. Dispos. 26:25–27 [PubMed] [Google Scholar]
- 8. Barnes KI, et al. 2006. Sulfadoxine-pyrimethamine pharmacokinetics in malaria: pediatric dosing implications. Clin. Pharmacol. Ther. 80:582–596 [DOI] [PubMed] [Google Scholar]
- 9. Batty KT, et al. 1996. Selective high-performance liquid chromatographic determination of artesunate and alpha- and beta-dihydroartemisinin in patients with falciparum malaria. J. Chromatogr. B Biomed. Appl. 677:345–350 [DOI] [PubMed] [Google Scholar]
- 10. Benakis A, Paris M, Loutan L, Plessas CT, Plessas ST. 1997. Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am. J. Trop. Med. Hyg. 56:17–23 [DOI] [PubMed] [Google Scholar]
- 11. Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 13:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bönsch C, et al. 2010. Chloroquine and its derivatives exacerbate B19V-associated anemia by promoting viral replication. PLoS Negl. Trop. Dis. 4:e669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cattani JA, et al. 1986. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am. J. Trop. Med. Hyg. 35:3–15 [DOI] [PubMed] [Google Scholar]
- 14. Chinh NT, et al. 2009. Pharmacokinetics and bioequivalence evaluation of two fixed-dose tablet formulations of dihydroartemisinin and piperaquine in Vietnamese subjects. Antimicrob. Agents Chemother. 53:828–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crauwels HM, et al. 2010. Relative bioavailability of a concept paediatric formulation of TMC278, an investigational NNRTI. Abstr. 18th Int. AIDS Soc. Conf., abstr THPE0158 [Google Scholar]
- 16. Denis MB, et al. 2002. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin. Infect. Dis. 35:1469–1476 [DOI] [PubMed] [Google Scholar]
- 17. Djimdé AA, et al. 2003. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 69:558–563 [PubMed] [Google Scholar]
- 18. Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duc DD, et al. 1994. The pharmacokinetics of a single dose of artemisinin in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 51:785–790 [DOI] [PubMed] [Google Scholar]
- 20. European Medicines Agency 2011. Eurartesim. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/001199/WC500108010.pdf Accessed September 1, 2011
- 21. Gordi T, Hai TN, Hoai NM, Thyberg M, Ashton M. 2000. Use of saliva and capillary blood samples as substitutes for venous blood sampling in pharmacokinetic investigations of artemisinin. Eur. J. Clin. Pharmacol. 56:561–566 [DOI] [PubMed] [Google Scholar]
- 22. Gordi T, Huong DX, Hai TN, Nieu NT, Ashton M. 2002. Artemisinin pharmacokinetics and efficacy in uncomplicated-malaria patients treated with two different dosage regimens. Antimicrob. Agents Chemother. 46:1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordi T, et al. 2005. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br. J. Clin. Pharmacol. 59:189–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordi T, Xie R, Jusko WJ. 2005. Semi-mechanistic pharmacokinetic/pharmacodynamic modelling of the antimalarial effect of artemisinin. Br. J. Clin. Pharmacol. 60:594–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hai TN, Hietala SF, Van Huong N, Ashton M. 2008. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop. 107:145–149 [DOI] [PubMed] [Google Scholar]
- 26. Hassan Alin M, Ashton M, Kihamia CM, Mtey GJ, Bjorkman A. 1996. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans. R. Soc. Trop. Med. Hyg. 90:61–65 [DOI] [PubMed] [Google Scholar]
- 27. Hasugian AR, et al. 2007. Dihydroartemisinin-piperaquine versus artesunate-amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug-resistant Plasmodium falciparum and Plasmodium vivax malaria. Clin. Infect. Dis. 44:1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hung TY, et al. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br. J. Clin. Pharmacol. 57:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karunajeewa HA, et al. 2008. Pharmacokinetics and efficacy of piperaquine and chloroquine in Melanesian children with uncomplicated malaria. Antimicrob. Agents Chemother. 52:237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krudsood S, et al. 2007. Dose ranging studies of new artemisinin-piperaquine fixed combinations compared to standard regimens of artemisisnin combination therapies for acute uncomplicated falciparum malaria. Southeast Asian J. Trop. Med. Public Health 38:971–978 [PMC free article] [PubMed] [Google Scholar]
- 31. Liu C, et al. 2007. Pharmacokinetics of piperaquine after single and multiple oral administrations in healthy volunteers. Yakugaku Zasshi 127:1709–1714 [DOI] [PubMed] [Google Scholar]
- 32. Matuszewski BK, Constanzer ML, Chavez-Eng CM. 2003. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 75:3019–3030 [DOI] [PubMed] [Google Scholar]
- 33. Mwesigwa J, et al. 2010. Pharmacokinetics of artemether-lumefantrine and artesunate-amodiaquine in children in Kampala, Uganda. Antimicrob. Agents Chemother. 54:52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen TC, et al. 2008. Pharmacokinetics of the antimalarial drug piperaquine in healthy Vietnamese subjects. Am. J. Trop. Med. Hyg. 79:620–623 [PubMed] [Google Scholar]
- 35. Porter CJ, Trevaskis NL, Charman WN. 2007. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 6:231–248 [DOI] [PubMed] [Google Scholar]
- 36. Price RN, et al. 2007. Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria. Antimicrob. Agents Chemother. 51:4090–4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pyar KP, Myint WW, Kyaw MP, Zin T, Than M. 2009. Efficacy and safety of artemisinin-piperaquine (Artequick) compared to dihydroartemisinin-piperaquine (Artekin) in uncomplicated falciparum malaria in adults. Myanmar Health Sci. Res. J. 21:78–82 [Google Scholar]
- 38. Röshammar D, Hai TN, Friberg Hietala S, Van Huong N, Ashton M. 2006. Pharmacokinetics of piperaquine after repeated oral administration of the antimalarial combination CV8 in 12 healthy male subjects. Eur. J. Clin. Pharmacol. 62:335–341 [DOI] [PubMed] [Google Scholar]
- 39. Savic RM, Jonker DM, Kerbusch T, Karlsson MO. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 34:711–726 [DOI] [PubMed] [Google Scholar]
- 40. Sidhu JS, et al. 1998. Artemisinin population pharmacokinetics in children and adults with uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 45:347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sim IK, Davis TM, Ilett KF. 2005. Effects of a high-fat meal on the relative oral bioavailability of piperaquine. Antimicrob. Agents Chemother. 49:2407–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Svensson US, Alin H, Karlsson MO, Bergqvist Y, Ashton M. 2002. Population pharmacokinetic and pharmacodynamic modelling of artemisinin and mefloquine enantiomers in patients with falciparum malaria. Eur. J. Clin. Pharmacol. 58:339–351 [DOI] [PubMed] [Google Scholar]
- 43. Svensson US, et al. 1998. Artemisinin induces omeprazole metabolism in human beings. Clin. Pharmacol. Ther. 64:160–167 [DOI] [PubMed] [Google Scholar]
- 44. Tarning J, et al. 2008. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob. Agents Chemother. 52:1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tarning J, et al. 2005. Pitfalls in estimating piperaquine elimination. Antimicrob. Agents Chemother. 49:5127–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trung TN, Tan B, Van Phuc D, Song JP. 2009. A randomized, controlled trial of artemisinin-piperaquine vs dihydroartemisinin-piperaquine phosphate in treatment of falciparum malaria. Chin. J. Integr. Med. 15:189–192 [DOI] [PubMed] [Google Scholar]
- 47. World Health Organization Communicable Diseases Cluster 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1–S90 [PubMed] [Google Scholar]
- 48. World Health Organization 2010. Guidelines for the treatment of malaria— 2nd ed World Health Organization, Geneva, Switzerland [Google Scholar]
- 49. Zwang J, et al. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. [DOI] [PMC free article] [PubMed] [Google Scholar]