Abstract
The in vitro cardiac properties of dihydroartemisinin (DHA) plus piperaquine phosphate (PQP) were compared with those of other antimalarial compounds. Results with antimalarial drugs, chosen on the basis of their free therapeutic maximum concentration in plasma (Cmax), were expressed as the fold of that particular effect with respect to their Cmax. The following tests were used at 37°C: hERG (human ether-à-go-go-related gene) blockade and trafficking, rabbit heart ventricular preparations, and sodium and slow potassium ion current interference (INa and IKs, respectively). Chloroquine, halofantrine, mefloquine, and lumefantrine were tested in the hERG studies, but only chloroquine, dofetilide, lumefantrine, and the combination of artemether-lumefantrine were used in the rabbit heart ventricular preparations, hERG trafficking studies, and INa and IKs analyses. A proper reference was used in each test. In hERG studies, the high 50% inhibitory concentration (IC50) of halofantrine, which was lower than its Cmax, was confirmed. All the other compounds blocked hERG, with IC50s ranging from 3- to 30-fold their Cmaxs. In hERG trafficking studies, the facilitative effects of chloroquine at about 30-fold its Cmax were confirmed and DHA blocked it at a concentration about 300-fold its Cmax. In rabbit heart ventricular preparations, dofetilide, used as a positive control, revealed a high risk of torsades de pointes, whereas chloroquine showed a medium risk. Neither DHA-PQP nor artemether-lumefantrine displayed an in vitro signal for a significant proarrhythmic risk. Only chloroquine blocked the INa ion current and did so at about 30-fold its Cmax. No effect on IKs was detected. In conclusion, despite significant hERG blockade, DHA-PQP and artemether-lumefantrine do not appear to induce potential torsadogenic effects in vitro, affect hERG trafficking, or block sodium and slow potassium ion currents.
INTRODUCTION
Torsade de pointes (TdP) caused by drugs is a life-threatening form of polymorphic ventricular tachycardia which is associated clinically with a long QT interval prolongation. This kind of deleterious effect has been described in several classes of drugs, such as antihistamines, psychotropics, and antibiotics (40). However, QT prolongation is not a strong predictor of the risk of TdP, and several factors may contribute to an individual patient's risk of TdP. Various models have been developed to assess the potential in vitro cardiac toxicity of drugs and their potential to generate TdP (9). Many antimalarials are associated with prolongation of the corrected QT interval (QTc) at therapeutic concentrations (49). However, despite large-scale use, information on the rate of TdP with these drugs is limited, because these drugs are mostly used in developing countries, where pharmacovigilance data are lacking. Dihydroartemisinin (DHA) plus piperaquine phosphate (PQP) is a fixed-combination antimalarial treatment with excellent efficacy and good tolerance (3, 4, 14, 16, 29, 38, 46). Derivatives of artemisinin are generally considered to be safe in terms of cardiotoxic potential, with no clinically significant changes in QT observed in the treatment of malaria (49). QTc prolongation appears to be limited with DHA and PQP (49). However, PQP is structurally similar to chloroquine, for which significant electrophysiological effects on the heart have been described (43, 45), even if the clinical differences have not been well considered.
One common electrophysiological finding for those antimalarial drugs associated with QTc prolongation is blockade of the repolarizing potassium channel hERG (human ether-à-go-go-related gene) (24, 35, 45). To investigate the electrophysiological profile of DHA and PQP, the hERG channel-blocking profile of these compounds, alone and in combination, was characterized in stably expressing human embryonic kidney (HEK-293) cells. For comparison, the hERG channel-blocking effects of chloroquine, dofetilide, halofantrine, lumefantrine, and mefloquine were also evaluated. Previous hERG studies with antimalarials were performed in vitro at room temperature (24, 35, 45). Under our experimental conditions, the hERG assay was evaluated at 37°C, which provides a more conservative safety evaluation of hERG inhibition (27).
The potential torsadogenic risk of DHA, PQP, and their combination was evaluated in a rabbit heart wedge preparation (33), an established experimental model for the prediction of drug-induced proarrhythmia (26). In this model, the torsadogenic risk score ranges from −2 up to 14, with a higher score being worse (33). The results with the combination of DHA and PQP were compared to those with chloroquine, artemether combined with lumefantrine, and dofetilide. Dofetilide was used as a positive control because of the numerous reports of TdP associated with this medication (1). Additionally, three in vitro tests were performed with DHA, PQP, and their combination: hERG trafficking (50) and sodium and slow potassium ion current interference (INa and IKs, respectively) measurements (11). These tests give additional insights into the possible mechanism of cardiac toxicity.
The aim of the present work was to evaluate the effects of the combination of DHA and PQP and its components on in vitro tests used to predict cardiac proarrhythmic risk.
MATERIALS AND METHODS
hERG. (i) Transfection and cell culture.
Because rapid potassium ion current interference (IKr) is regionally expressed in human heart and difficult to record in native cells, the cloned equivalent of the human IKr (hERG) was used in this study. The pharmacology of this cloned channel expressed in a human cell line has been shown to be very similar to that determined in native cardiac tissues. The hERG channel is expressed in an HEK-293 cell line that lacks endogenous hERG channels. HEK-293 cells were stably transfected through the Lipofectamine method (42) with the hERG clone.
(ii) External and internal recording solution and components.
No expiration date for the individual components of the external recording solution was given by the manufacturer. An expiration date of 3 years was set for the individual components from the time of reception. Cells were maintained in the following medium in culture flasks with materials purchased at Cellgro (Mediatech): minimum essential medium with Earle's salts (86.9 ml), nonessential amino acids (1 ml), sodium pyruvate (1 ml), penicillin-streptomycin (1 ml), fetal bovine serum (10 ml), and Geneticin (100 μl). The external recording solutions were made up by NaCl (137 mM), KCl (4 mM), MgCl2 (1 mM), CaCl2 (1.8 mM), HEPES (10 mM), and dextrose (11 mM), adjusted to a pH of 7.4 with NaOH (Sigma). The external recording solution was not used for more than 2 weeks after preparation. The composition of internal recording solution was made up by KCl (130 mM), MgCl2 (1 mM), HEPES (5 mM), EGTA (5 mM), and NaCl (7 mM), adjusted to a pH of 7.2 using KOH. In a second set of experiments, Na-ATP (5 nM) was added to the internal recording solution (see below).
(iii) Experimental methods.
Experiments were performed at 37 ± 1°C. Currents were measured using the whole-cell variant of the patch clamp method. Glass pipettes were pulled from borosilicate glass by a horizontal puller (Sutter Instruments) and then fire polished to produce tip openings of 1 to 2 μm. Pipette tip resistance was approximately 1 to 2 MΩ when the pipette was filled with internal recording solution. Bath temperature was measured by a thermistor placed near the cell under study and was maintained by a thermoelectric device (model no. 806-7243-01; Cambion/Midland Ross, Cambridge, MA). An Axopatch 1-B amplifier (Axon Instruments, Foster City, CA) was used for whole-cell voltage clamping. Creation of voltage clamp pulses and data acquisition were controlled by a personal computer (PC) running pClamp software (Axon Instruments). From a holding potential of −75 mV, preparations were depolarized at +10 mV for 500 ms and repolarized to −40 mV for another 500 ms, before they were returned to the holding potential. After rupture of the cell membrane (entering whole-cell mode), current kinetics and amplitudes were allowed to stabilize as the cell was dialyzed with internal recording solution and stimulated at 0.1 Hz using the protocol described below. Current kinetics and amplitude typically stabilized in approximately 5 min. If the hERG current did not stabilize over this time period, the cell was discarded. Currents were considered stable if currents elicited by a series of voltage pulses given at 0.1 Hz were superimposable. Peak hERG current was measured as the maximum outward deflection of the tail current elicited upon return to −40 mV. In all experiments, drug was added in a cumulative manner.
Means ± standard errors of the means (SEMs) are given. Analyzed data are presented as the percent reduction of the current amplitude after a steady-state effect was reached in the presence of drug relative to the current amplitude before the test substance was superfused (control). Each cell served as its own control. Test substance effects were compared by a paired Student t test for significance (P < 0.05) using MicroCal Origin, version 6.0, software. Log-linear plots of the mean percent blockade ± SEM at the concentrations tested were created. A nonlinear curve-fitting routine was utilized to fit a three-parameter Hill equation to the results using MicroCal Origin, version 6.0, software. The equation is y = Vmax[xn/(kn + xn)], where y is the IC50, x is the concentrations, Vmax (which was equal to 100) is the maximum rate of metabolism, and k and n are unconstrained variables.
The experiment with DHA and PQP was repeated twice with different concentrations: six concentrations on five cells on the first set and three to five concentrations on four to five cells on the second set.
hERG trafficking studies.
The objective of the hERG trafficking studies was to obtain a rapid estimate of the in vitro effects of test articles on surface expression of the wild-type (WT) hERG (human ether-à-go-go-related gene) potassium channel. The cardiac potassium channel, hERG, is responsible for the rapid delayed rectifier current (IKr) in the human ventricle. This channel is selected because inhibition of IKr is the most common cause of cardiac action potential prolongation by noncardiac drugs (5, 48, 55). In addition to direct block, drug-induced trafficking inhibition of hERG has been linked to QT prolongation (18, 30). Increased action potential duration causes prolongation of the QT interval that has been associated with ventricular arrhythmia, or torsade de pointes.
(i) Cell culture procedures.
HEK-293 cells were transfected with hERG WT cDNA. Stable transfectants were selected by coexpression of the hERG cDNA and G418 resistance gene incorporated into the expression plasmid. Selection pressure was maintained by including G418 in the culture medium. Cells were cultured in Dulbecco's modified Eagle medium–nutrient mixture F-12 (DMEM/F-12) supplemented with 10% fetal bovine serum, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 500 μg/ml G418. Cells were maintained in tissue culture incubators at 37°C in a humidified 95% air, 5% CO2 atmosphere, with stocks maintained in cryogenic storage. Cells for HERG-Lite assays were plated in 96-well microplates.
(ii) Experimental methods.
Cells were incubated with a test article(s) overnight (minimum of 16 h) in a tissue culture incubator at 37°C in a humidified 95% air, 5% CO2 atmosphere. To assay hERG surface expression, cells were fixed with freshly prepared paraformaldehyde. Nonspecific antibody binding sites were blocked by incubation in 1% goat serum–phosphate-buffered saline. The cells were sequentially incubated with an antibody against an extracellular epitope incorporated into the hERG channel and a secondary antibody coupled to a light-generating enzyme. A DNA-binding fluorescent dye was added to the secondary antibody solution to monitor cell number. The fluorescent signals were captured in a microplate fluorescence reader, after which the chemiluminescent reagent detection mix was added to the cells and the luminescent signals were detected in a microplate luminometer.
Since some test articles may be toxic, a DNA-binding fluorescent dye was used in the HERG-Lite protocol to determine the cell number per well at the end of the experiment. A standard curve of fluorescence versus cell number was performed. Chemiluminescence values were corrected for cell loss up to 75%. If cell loss exceeded 75%, the test article was deemed too toxic at that concentration to obtain reliable data.
The mean surface expression (relative chemiluminescence units) of the test article wells was compared to the surface expression (mean ± standard deviation) of the control wells. A significant change in surface expression produced by the test article is indicated if the mean of the test article wells is 3 standard deviations or more away from the mean of the vehicle control. The surface expression changes were compared to those in the presence of the positive-control article.
Rabbit heart ventricular wedge preparations.
New Zealand White female rabbits weighing 2.2 to 3.0 kg were anticoagulated with heparin and anesthetized by endovenous injection of ketamine-xylazine (40 to 50 mg/0.5 to 1.0 mg per kg of body weight). The chest was opened via a left thoracotomy, and the heart was excised and placed in a cardioplegic solution consisting of cold (4°C) normal Tyrode's solution. Transmural wedges with dimensions of approximately 1.5 cm in width and 2 to 3 cm in length were dissected from the left ventricle as described previously (33). The tissue was cannulated via the small branch of the left anterior descending artery and perfused with cardioplegic solution. The preparation was then placed in a small tissue bath and arterially perfused with Tyrode's solution containing 4 mM K+ buffered with 95% O2 and 5% CO2 (temperature, 35.7 ± 0.1°C; perfusion pressure, 40 to 50 mm Hg). The ventricular wedge was allowed to equilibrate in the tissue bath until electrically stable for 1 h. The preparations were stimulated at basic cycle lengths (BCLs) of 1,000 and 2,000 ms using bipolar silver electrodes insulated except at the tip and applied to the endocardial surface.
(i) Electrophysiological recordings.
A transmural electrocardiographic (ECG) signal was recorded via an HP ECG amplifier (model 8811A) using extracellular silver/silver chloride electrodes placed in Tyrode's solution, bathing the preparation 1.0 to 1.5 cm from the epicardial and endocardial surfaces, along the same vector as the transmembrane recordings. The QT interval was defined as the time from the onset of the QRS to the point at which the final downslope of the T wave intersects with the isoelectric line. Transmembrane action potential from the endocardium (endo) was recorded at a BCL of 2,000 ms via a customer-made amplifier. Transmural dispersion of repolarization (TDR) was defined as the interval between the end (e) and the peak (p) of the T wave (Tp-e) (53, 54). All measured biological signals, including ECG and transmembrane action potentials, were sampled via a digital-to-analog converter (1401; Cambridge Electronic Design [CED], England) and stored on electronic media (compact disk). The raw signals of ECG and transmembrane action potentials were analyzed using Spike 2 software (CED, England).
(ii) Experimental methods.
Each compound or combination except DHA (2.4 μM)-PQP was tested at five concentrations in five wedge preparations; for DHA (2.4 μM)-PQP, six concentrations were tested in four preparations.
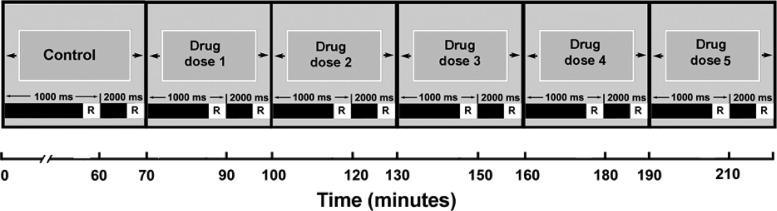
Each preparation was exposed to each compound or combination at each concentration for ≥30 min (Fig. 1). Two BCLs of 1,000 and 2,000 ms were used. Action potentials from endo, the QT and QRS intervals, and the interval Tp-e, an index of TDR, were measured at a BCL of 2,000 ms. Arrhythmic phenomena, including spontaneous early after depolarization (EAD), R-on-T ectopic beats, and TdP, were recorded if they occurred. The combination of these events originated the torsadogenic risk score (Table 1).
Fig 1.
Experimental protocol used to study the test article in the isolated arterially perfused rabbit ventricular preparations. The data recording (R) is made 30 to 60 s before the end of each stimulation period. Control, control perfusion.
Table 1.
Score system for estimate of risk of a compound for relative TdP risk using the isolated rabbit left ventricular wedge preparationa
| Score | Difference in QT interval (%) | Tp-e/QT ratio (%) | Phase 2 EAD |
|---|---|---|---|
| −1 | <−5 | <−5 | |
| 0 | −5–<10 | −5–<10 | |
| 1 | 10–<20 | 10–<20 | |
| 2 | 20–<30 | 20–<30 | ±EAD |
| 3 | >30 | >30 | |
| 4 | EAD without R on T | ||
| 6 | EAD with R on T | ||
| 8 | TdP |
Points for the QT interval, the Tp-e/QT ratio, and phase 2 EAD are provided (7). The maximal score is 14, and the minimum is −2. Basic cycle lengths (BCLs) are 2,000 ms. EAD, early after depolarization; ±EAD, equivocal EAD from endocardial action potential at a BCL of 2,000 ms when the QT interval is >30%; TdP, torsade de pointes; Tp-e, interval between the end (e) and the peak (p) of the T wave; QT, QT interval.
Data were analyzed using one-way analysis of variance (if data were parametric) or the Friedman test (if data were not parametric) for repeated measurements and then Holm-Sidak's or Dunn's method as post hoc statistical analysis.
INa and IKs currents. (i) Isolation of cardiac myocytes.
Human myocytes were obtained from specimens of human right atrial appendage obtained during surgery from hearts of patients undergoing cardiopulmonary bypass (12). Samples were quickly immersed in a cardioplegic solution consisting of 50 mM KH2PO4, 8 mM MgSO4, 10 mM NaHCO3, 5 mM adenosine, 25 mM taurine, 140 mM glucose, and 100 mM mannitol, titrated to a pH of 7.4, and bubbled with 100% O2 at 0 to 4°C. Specimens were minced into 0.5- to 1-mm cubes and transferred to a 50-ml conical tube containing an ultra-low-calcium wash solution containing 137 mM NaCl, 5 mM KH2PO4, 1 mM MgSO4, 10 mM taurine, 10 mM glucose, 5 mM HEPES, and 100 μmol/liter (μM) EGTA, pH 7.4 (22 to 24°C). The tissue was then gently agitated by continuous bubbling with 100% O2 for 5 min. The tissue was next incubated in 5 ml of solution containing 137 mM NaCl, 5 mM KH2PO4, 1 mM MgSO4, 10 mM taurine, 10 mM glucose, and 5 mM HEPES supplemented with 0.1% bovine albumin, 2.2 mg/ml collagenase type V, and 1.0 mg/ml protease type XXIV (Sigma Chemical), pH 7.4 (37°C), and bubbled continuously with 100% O2. The supernatant was removed after 20 min and discarded. The chunks were then incubated in a solution of the same ionic composition but supplemented with only collagenase and 100 μM CaCl2. Microscopic examination of the medium was performed every 5 to 10 min to determine the number and quality of the isolated cells. When the yield appeared to be maximal, the cell suspension was centrifuged for 2 min and the resulting pellet was resuspended in a modified Kraftbruhe solution containing 25 mM KCl, 10 mM KH2PO4, 25 mM taurine, 0.5 mM EGTA, 22 mM glucose, 55 mM glutamic acid, and 0.1% bovine albumin, pH 7.3 (22 to 24°C). In general, the isolation procedure produced an initial yield of approximately 40 to 60% rod-shaped, calcium-tolerant cells. Cells were used within 24 h after isolation.
(ii) Experimental methods for INa and IKs.
Experiments were performed at 36 ± 1°C. Currents were measured using the whole-cell variant of the patch clamp method. Glass pipettes were pulled from borosilicate glass by a horizontal puller (Sutter Instruments) and then fire polished to produce tip openings of 1 to 4 μm. Pipette tip resistance was approximately 1.0 to 2.0 MΩ when filled with internal solutions. Bath temperature was measured by a thermistor placed near the cell under study. An Axopatch 1-B amplifier (Axon Instruments, Foster City, CA) was used for whole-cell voltage clamping. Creation of voltage clamp pulses and data acquisition were controlled by a PC running pClamp software (version 9.2; Axon Instruments).
After rupture of the cell membrane (entering whole-cell mode), current kinetics and amplitudes were allowed to stabilize as the cell was dialyzed with internal solution and paced at 0.1 Hz (typically, 3 to 5 min). If the current did not stabilize over this time period, the cell was discarded. Currents were considered stable if currents elicited by a series of voltage pulses given at 0.1 Hz were superimposed.
INa was elicited by a pulse to −20 mV from a holding potential of −120 mV (pulse duration, 40 ms). Peak inward current was measured for INa. IKs was elicited by a 5-s voltage pulse to +10 mV from a holding potential of −40 mV. A pacing rate of 0.1 Hz was used for both ion currents. Drugs were added in a cumulative manner; i.e., 0.1 μM was added to a cell until steady state was reached, generally in 2 to 3 min, then 0.3 μM, and so on.
Drugs.
With the exception of PQP (Sigma-Tau Industrie Farmaceutiche Riunite, Rome, Italy), which was dissolved in water, all the other compounds, artemether (Tokyo Chemical Industry Co., Tokyo, Japan), chloroquine (Sigma-Aldrich, Milan, Italy), dofetilide (Sigma-Aldrich, Milan, Italy), dihydroartemisinin (DHA; Sigma-Tau Industrie Farmaceutiche Riunite, Rome, Italy), halofantrine (Sigma-Aldrich, Milan, Italy), lumefantrine (LGM Pharma Inc., Boca Raton, FL), and mefloquine (Sigma-Aldrich, Milan, Italy), were dissolved as stock solutions in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) at a maximum of 1% (vol/vol). Then, stock solutions were vortexed until the solution was clear and stored in a freezer, whose temperature ranged from −19 to −22°C. DMSO and geldanamycin were purchased from Sigma-Aldrich (St. Louis, MO). Drug concentrations were chosen to cover a wide range of subtherapeutic, therapeutic, and supratherapeutic concentrations using the estimated free fraction around the maximum concentration in plasma (Cmax) in humans as the therapeutic concentrations, as indicated in Table 2.
Table 2.
Drugs used for the various studies with references and total and free plasma concentrations in patients
| Reference(s) | Compound | Mol wt | % protein binding | Mean therapeutic Cmax |
|||
|---|---|---|---|---|---|---|---|
| ng/ml |
μMa |
||||||
| Total | Free | Total | Free | ||||
| Eurartesim SmPCb | Piperaquine 4H2PO4 | 927.50 | 99 | 448c | 4.5 | 0.836 | 0.008 |
| Dihydroartemisinin | 284.35 | 90 | 752 | 75 | 2.65 | 0.265 | |
| Coartem SmPC | Lumefantrine | 528.94 | 99 | 9,800 | 98 | 18.5 | 0.185 |
| Artemether | 298.37 | 95 | 84 | 3.9 | 0.281 | 0.013 | |
| 45, 49 | Chloroquine 2H2PO4 | 515.86 | 59 | 320 | 131 | 1 | 0.41 |
| 45, 49 | Halofantrine HCl | 536.88 | 83 | 1,799 | 305.8 | 3.35 | 0.57 |
| 45, 49 | Mefloquine HCl | 414.77 | 98 | 1,037 | 20.7 | 2.5 | 0.05 |
| 32 | Dofetilide | 441.56 | 70 | 2 | 0.6 | 1.36 | 0.41 |
Data are in nM for dofetilide.
SmPC, summary of product characteristics.
The datum was obtained from single-dose Cmax by applying an accumulation factor of 2.5.
RESULTS
hERG currents.
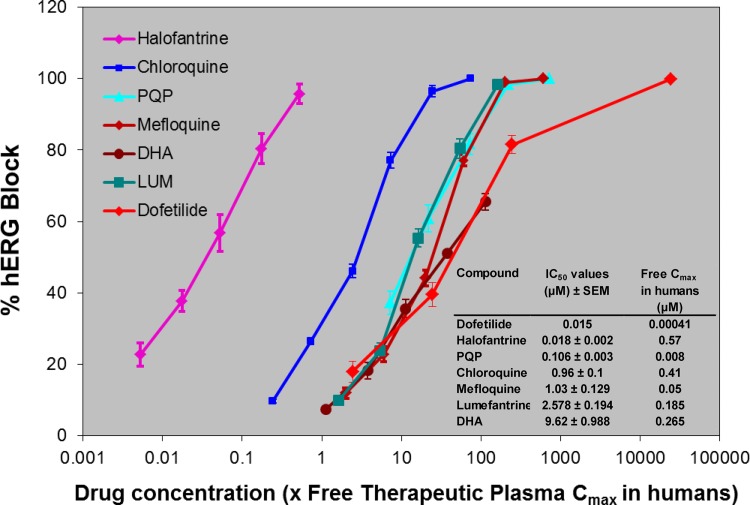
The various compounds decreased the hERG current in a concentration-related manner (Fig. 2). The combination of DHA at two concentrations (2.4 μM and 7.2 μM) in the presence of different concentrations of PQP produced a level of hERG block which was apparently less than that produced by PQP alone and somewhat less than that produced by DHA alone (Table 3). Historically, this channel in our lab is blocked by E-4031 (21, 22, 44), a selective and potent hERG blocker, with a 50% inhibitory concentration (IC50) of 18.1 nM (n = 6 to 11). In 3 cells, current rundown and the effects of the vehicle (DMSO) were assessed by monitoring current when cells were exposed to DMSO (1/300, vol/vol) over the time period of a typical experiment (6 to 8 min). In two cells, current was decreased by 2.7% and 3.1%, and in the other cell it was increased by 1.7%. This indicates that in the absence of test article there was no substantial reduction in hERG current amplitude.
Fig 2.
Effects of various antimalarial drugs on hERG current at 0.1 Hz. Five concentrations of drugs were used, and concentrations ranged from 0.003 to 0.3 nM for halofantrine, from 0.058 to 5.8 μM for PQP, and from 0.3 to 30 μM for lumefantrine (LUM) and DHA. Six concentrations were used for chloroquine and mefloquine, and concentrations ranged from 0.1 to 30 μM. Five different cells were used for each concentration. The actual IC50s are reported in the inset. Values represent mean ± SEM of five different cells.
Table 3.
IC50s on hERG current at 0.1 Hz by DHA and PQP and their combination in a second set of experiments
| Compound | IC50 (μM) ± SEMa |
|---|---|
| DHA | 7.7 ± 0.9 |
| PQP | 0.087 ± 0.003 |
| 2.4 μM DHA + PQP | 0.201 ± 0.001 |
| 7.2 μM DHA + PQP | 0.179 ± 0.008 |
Values represent the mean ± SEM of 4 to 5 cells.
hERG trafficking.
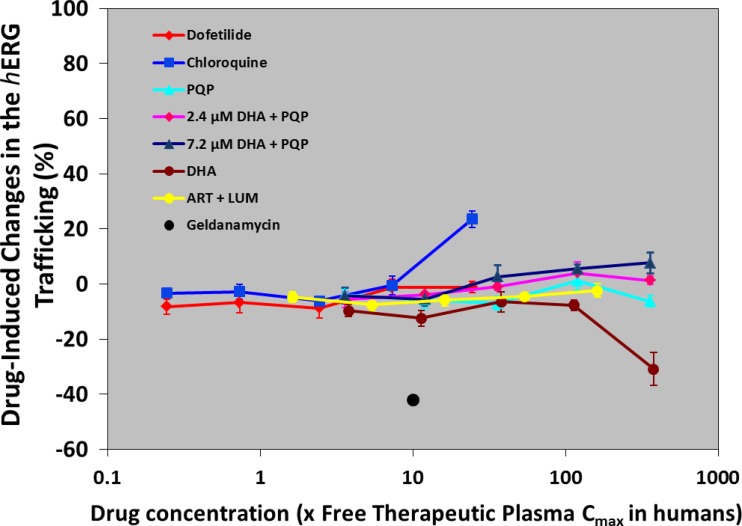
The only test article that produced significant inhibition of hERG trafficking was DHA at 100 μM (Fig. 3). However, cells in one vial with such a DHA concentration had a mortality rate higher than 75%, and therefore, the result from this vial was not considered. In contrast, chloroquine increased hERG trafficking, as already reported (50). The test system worked properly, as geldanamycin (1 μM), the positive-control trafficking inhibitor for WT hERG, produced the expected result with a 50% decrease in WT hERG surface expression (50).
Fig 3.
Effects of various antimalarial drugs on hERG trafficking. Five concentrations of drugs were used, and concentrations ranged from 0.003 to 3 μM for PQP (with or without 2.4 or 7.2 μM DHA), from 1 to 100 μM for DHA, from 0.1 to 10 μM for chloroquine, from 0.3 to 30 μM for lumefantrine (LUM; with 0.18 μM artemether [ART]), and from 0.1 to 10 nM for dofetilide. Geldanamycin was given at 1 μM (black spot). Values represent mean ± SEM of five different cells.
Rabbit ventricular wedge preparations.
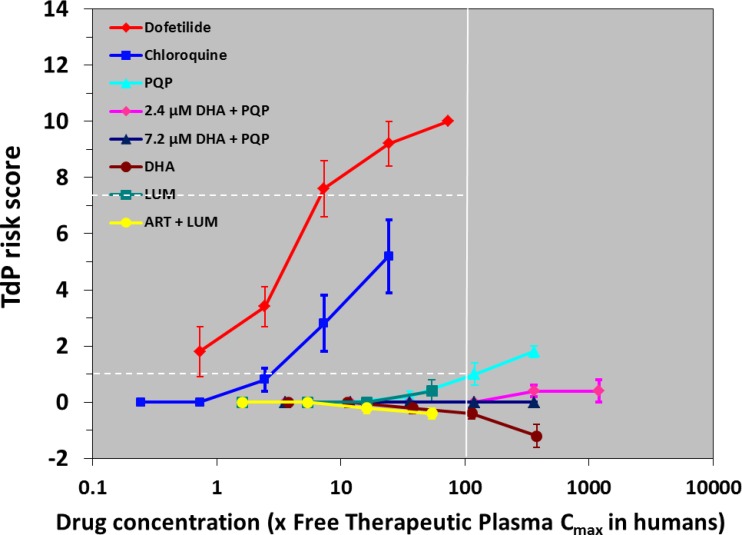
The system, i.e., the isolated arterially perfused rabbit left ventricular wedge preparation, was validated by using dofetilide, which produced concentration-dependent torsadogenic risk effects, starting from 0.3 nM (Fig. 4; Table 4). Moreover, prolonging effects on QT, Tp-e, and endocardial action potential duration (APD) measured at 90% repolarization (endo-APD90) were also observed at 3 nM (Table 4), and EADs were evident in four out of five preparations at 10 nM and in all five preparations at 30 nM.
Fig 4.
Effects of various antimalarial drugs on TdP risk score in comparison with those of dofetilide. Values represent mean ± SEM of five preparations. Concentrations of drugs ranged from 0.003/0.1 to 3/10 μM for PQP without or with 2.4 or 7.2 μM DHA, from 1 to 100 μM for DHA, from 0.1 to 10 μM for chloroquine, from 0.3 to 30 μM for lumefantrine (LUM; without or with 0.18 μM artemether [ART]), and from 0.3 to 30 nM for dofetilide. Horizontal dashed lines represent scores of 1 and 7.25, which are considered to be associated with moderate and marked risks of torsades de pointes, respectively, when they are reached at concentrations less than 100× the free therapeutic plasma Cmax (32).
Table 4.
Effects of various antimalarial drugs on rabbit heart ventricular wedge preparations in comparison with those of dofetilidea
| Drug and concn | QT (ms) at: |
Tp-e (ms) at 2,000 ms | Endo APD90 (ms) at 2,000 ms | QRS (ms) at 2,000 ms | TdP risk score | |
|---|---|---|---|---|---|---|
| 2,000 ms | 1,000 ms | |||||
| DHA | ||||||
| Control | 314.8 ± 12.7 | 275.2 ± 7.7 | 59.0 ± 4.0 | 257.7 ± 14.2 | 37.6 ± 2.2 | 0.0 ± 0.0 |
| 1 μM | 315.8 ± 14.5 | 273.4 ± 7.6 | 58.8 ± 4.1 | 257.0 ± 14.2 | 37.4 ± 2.2 | 0.0 ± 0.0 |
| 3 μM | 315.0 ± 14.8 | 276.8 ± 7.9 | 58.6 ± 4.1 | 260 ± 14.5 | 37.4 ± 2.2 | 0 0.0 ± 0.0 |
| 10 μM | 317.2 ± 12.3 | 271.2 ± 8.3 | 57.6 ± 3.8 | 260.2 ± 14.4 | 37.4 ± 2.4 | −0.2 ± 0.2 |
| 30 μM | 307.0 ± 8.7 | 268.8 ± 5.4 | 56.6 ± 3.3 | 257.9 ± 13.1 | 37.6 ± 2.5 | −0.4 ± 0.2 |
| 100 μM | 300.6 ± 7.6** | 266.8 ± 5.4** | 51.6 ± 2.1* | 251.5 ± 9.1 | 37.6 ± 2.2 | −1.2 ± 0.4** |
| PQP | ||||||
| Control | 299.2 ± 10.3 | 270.8 ± 6.8 | 57.2 ± 3.9 | 260.2 ± 13.5 | 39.4 ± 2.4 | 0.0 ± 0.0 |
| 0.03 μM | 304.6 ± 11.4 | 271.0 ± 8.3 | 57.8 ± 4.0 | 267.0 ± 13.5 | 39.8 ± 2.3 | 0.0 ± 0.0 |
| 0.1 μM | 313.2 ± 10.9* | 276.2 ± 6.8 | 60.2 ± 3.4 | 275 ± 12.4* | 39.8 ± 2.3 | 0.0 ± 0.0 |
| 0.3 μM | 313.6 ± 9.6* | 277.4 ± 7.1** | 62.8 ± 2.6* | 281.4 ± 16.0** | 40.0 ± 2.2 | 0.2 ± 0.2 |
| 1 μM | 326.6 ± 12.7* | 282.6 ± 6.8** | 68.6 ± 2.9* | 287.6 ± 13.3** | 39.8 ± 2.3 | 1.0 ± 0.4 |
| 3 μM | 335.2 ± 12.8** | 283.8 ± 6.9*** | 75.0 ± 3.6* | 290.7 ± 17.2*** | 39.4 ± 2.4 | 1.8 ± 0.3* |
| DHA (2.4 μM) + PQP | ||||||
| Control | 318 ± 15 | 279 ± 8 | 55.4 ± 4.3 | 272 ± 16 | 38.0 ± 1.8 | 0.0 ± 0.0 |
| 0.03 μM | 328 ± 13* | 286 ± 8 | 56.2 ± 4.2 | 277 ± 15 | 38.2 ± 1.5 | 0.0 ± 0.0 |
| 0.1 μM | 330 ± 12 * | 287 ± 7 | 57.2 ± 4.4 | 281 ± 16 | 38.0 ± 1.7 | 0.0 ± 0.0 |
| 0.3 μM | 336 ± 14** | 288 ± 8 | 58.8 ± 4.8 | 283 ± 16 | 38.2 ± 1.9 | 0.0 ± 0.0 |
| 1 μM | 338 ± 14** | 289 ± 7 | 60.0 ± 4.9 | 287 ± 15 | 38 ± 1.7 | 0.0 ± 0.0 |
| 3 μM | 347 ± 19*** | 295 ± 8* | 61.8 ± 3.6*** | 293 ± 17 | 38.2 ± 2.0 | 0.4 ± 0.2 |
| 10 μM | 334 ± 21*** | 286 ± 9 | 0.3 ± 5.6*** | 283 + 21 | 38.5 ± 2.2 | 0.4 ± 0.4 |
| DHA (7.2 μM) + PQP | ||||||
| Control | 290.6 ± 13.7 | 266.2 ± 9.3 | 54.8 ± 6.4 | 252.4 ± 15.3 | 36.6 ± 1.6 | 0.0 ± 0.0 |
| 0.03 μM | 293.6 ± 15.5 | 266.2 ± 9.2 | 55.6 ± 6.5 | 254.7 ± 15.3 | 37.0 ± 1.7 | 0.0 ± 0.0 |
| 0.1 μM | 293.8 ± 13.2 | 266.4 ± 8.9 | 55.4 ± 6.2 | 253.0 ± 13.8 | 37.0 ± 1.8 | 0.0 ± 0.0 |
| 0.3 μM | 294.8 ± 14.5 | 267.4 ± 8.2 | 57.0 ± 6.0 | 251.3 ± 14.8 | 37.0 ± 1.8 | 0.0 ± 0.0 |
| 1 μM | 304.6 ± 15.6** | 269.8 ± 8.3 | 58.2 ± 6.1 | 259.8 ± 14.4 | 36.8 ± 1.5 | 0.0 ± 0.0 |
| 3 μM | 306.2 ± 13.7*** | 276.4 ± 7.4* | 57.0 ± 7.0 | 262.4 ± 14.1 | 36.4 ± 1.3 | 0.0 ± 0.0 |
| Lumefantrine | ||||||
| Control | 280.4 ± 12.0 | 249.8 ± 11.0 | 50.2 ± 3.4 | 236.9 ± 19.0 | 36.0 ± 1.6 | 0.0 ± 0.0 |
| 0.3 μM | 292.0 ± 14.0 | 260.8 ± 11.2* | 51.2 ± 3.4 | 245.2 ± 21.5 | 36.0 ± 1.8 | 0.0 ± 0.0 |
| 1 μM | 295.0 ± 15.5 | 264.2 ± 10.6* | 51.6 ± 3.6 | 247.5 ± 21.2 | 36.0 ± 1.6 | 0.0 ± 0.0 |
| 3 μM | 297.2 ± 16.2* | 265.2 ± 10.8** | 52.0 ± 3.8 | 248.6 ± 22.2 | 35.8 ± 1.6 | 0.0 ± 0.0 |
| 10 μM | 311.8 ± 21.8* | 265.6 ± 11.3** | 54.2 ± 5.2 | 263.6 ± 25.4* | 36.2 ± 1.5 | 0.4 ± 0.4 |
| 30 μM | ND | ND | ND | ND | ND | ND |
| Artemether (0.18 μM) + lumefantrine | ||||||
| Control | 296.0 ± 10.3 | 277.6 ± 7.1 | 52.0 ± 4.0 | 258.3 ± 8.2 | 38.4 ± 3.1 | 0.0 ± 0.0 |
| 0.3 μM | 300.6 ± 10.7* | 283.0 ± 7.8* | 52.6 ± 4.1 | 259.8 ± 8.2 | 38.4 ± 3.1 | 0.0 ± 0.0 |
| 1 μM | 304.0 ± 11.0* | 282.6 ± 8.4 | 53.0 ± 4.6 | 265.0 ± 12.5 | 38.0 ± 3.2 | 0.0 ± 0.0 |
| 3 μM | 304.8 ± 10.5** | 282.2 ± 8.9 | 52.4 ± 4.5 | 261.2 ± 9.5 | 38.8 ± 3.3 | 0.2 ± 0.2 |
| 10 μM | 309.8 ± 9.9** | 282.4 ± 6.9 | 52.8 ± 5.1 | 266.7 ± 9.1 | 38.2 ± 3.0 | 0.4 ± 0.2 |
| 30 μM | ND | ND | ND | ND | ND | ND |
| Chloroquine | ||||||
| Control | 307.4 ± 7.9 | 280.2 ± 8.3 | 52.2 ± 3.4 | 278.7 ± 8.8 | 38.0 ± 2.8 | 0.0 ± 0.0 |
| 0.1 μM | 309.6 ± 7.6 | 281.2 ± 7.6 | 52.2 ± 4.1 | 281.0 ± 7.3 | 38.4 ± 2.8 | 0.0 ± 0.0 |
| 0.3 μM | 316.4 ± 11.0 | 286.4 ± 8.1 | 55.0 ± 4.0 | 290.9 ± 3.0 | 38.4 ± 2.9 | 0.0 ± 0.0 |
| 1 μM | 341.4 ± 10.5 | 299.6 ± 10.5 | 61.2 ± 3.8 | 314.5 ± 5.34 | 38.4 ± 2.9 | 0.8 ± 0.4 |
| 3 μM | 376.4 ± 18.7* | 318.8 ± 15.7* | 73.8 ± 7.1 * | 347.6 ± 16.2 | 41.6 ± 3.6 | 2.8 ± 1.0 |
| 10 μM | 477.0 ± 49.2* | 390.6 ± 20.9* | 106.6 + 19.2* | 445.1 ± 45.0* | 48.4 ± 4.2*** | 5.2 ± 1.3* |
| Dofetilide | ||||||
| Control | 289.2 ± 8.2 | 262.0 ± 2.8 | 51.2 ± 2.8 | 249.1 ± 13.2 | 41.6 ± 2.2 | 0.0 ± 0.0 |
| 0.3 nM | 330.8 ± 17.3 | 291.2 ± 13.2 | 65.2 ± 5.0 | 286.5 ± 26.9 | 41.6 ± 2.2 | 1.8 ± 0.9* |
| 1 nM | 353.8 ± 25.2 | 309.6 ± 17.7 | 76.6 ± 8.4 | 315.5 ± 34.9 | 41.8 ± 2.1 | 3.4 ± 0.7* |
| 3 nM | 432.0 ± 45.9** | 344.8 ± 24.6** | 103.6 ± 7.5** | 382.8 ± 51.6** | 41.8 ± 2.2 | 7.6 ± 1.0** |
| 10 nM | 547.8 ± 55.3** | 431.0 ± 16.3** | 170.4 ± 23.1** | 555.1 ± 59.3** | 41.8 ± 2.1 | 9.2 ± 0.8** |
| 30 nM | 647.4 ± 71.6*** | 464.8 ± 33.8*** | 170.4 ± 23.1*** | 574.0 ± 78.2*** | 41.6 ± 2.1 | 10.0 ± 0.0*** |
All values represent mean ± SEM of five preparations (except for 2.4 μM DHA plus 10 μM PQP, where only four preparations were used). Endo, endocardium;
, P < 0.05 versus control group;
, P < 0.02 versus control group;
, P < 0.01 versus control group; ND, no valid data available because the compound was not completely dissolved in the Tyroide's solution.
DHA had a mild negative effect on potential torsadogenic risk (Fig. 4; Table 4), which was statistically observed only at 100 μM (but see Discussion). PQP increased the QT interval, Tp-e, and endo-APD90 at 0.1 to 1 μM, but no arrhythmic events were observed (Table 4). PQP increased the TdP risk score only at 3 μM, i.e., more than 100-fold its Cmax. When DHA (at 2.4 and 7.2 μM) and PQP were coadministered, the TdP risk scores were not changed (Table 4), even if PQP was used at 10 μM (Table 4). Also in such a case, no EADs were observed. Lumefantrine did not induce TdP (Table 4); however, it significantly increased QT from 0.3 to 10 μM and APD90 at 10 μM (Fig. 4; Table 4). No EAD was observed. Likewise, the association of artemether and lumefantrine did not modify such effects (Table 4).
Chloroquine induced mild TdP risk scores at concentrations of ≥3 μM, at which QT and endocardial APD90 were significantly increased (Table 4). Chloroquine induced one EAD out of five preparations at 10 μM and at such a concentration increased the QRS interval.
INa current.
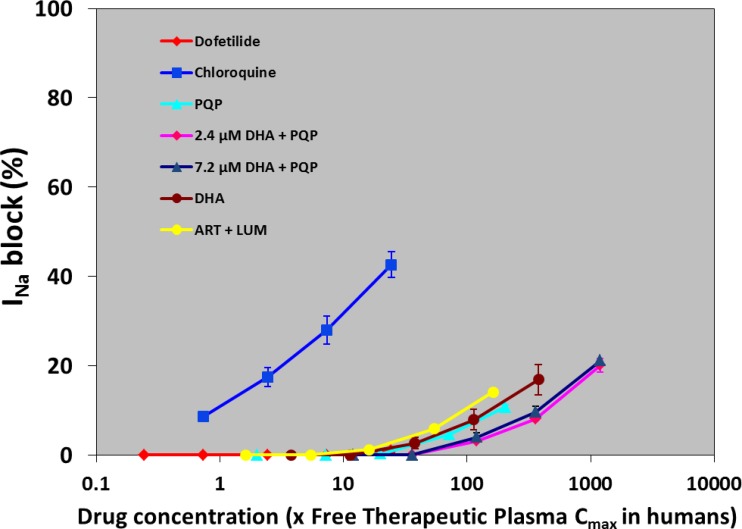
All of the compounds tested produced less than a 50% inhibition of the human cardiac sodium current (Fig. 5). The block of chloroquine (42.6%) is in the same range of the values (45%) already published for feline ventricular myocytes (43).
Fig 5.
Effects of various antimalarial drugs on INa ion current. Values represent mean ± SEM of five preparations. Five or six concentrations of drugs were used, and the concentrations ranged from 0.003/0.1 to 3/10 μM for PQP without or with 2.4 or 7.2 μM DHA, from 1 to 100 μM for DHA, from 0.1 to 10 μM for chloroquine, from 0.3 to 30 μM for lumefantrine (LUM; with 0.18 μM artemether [ART]), and from 0.1 to 10 nM for dofetilide.
IKs current.
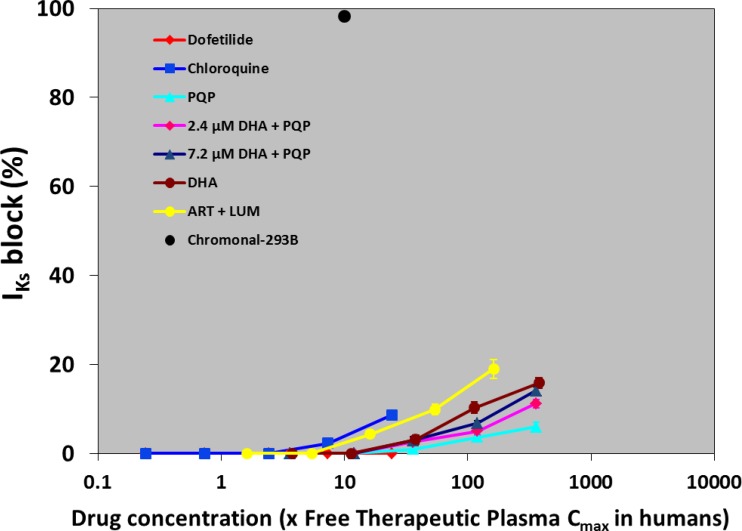
The IKs-blocking profiles of DHA, PQP, the DHA and PQP combination, chloroquine, and dofetilide (Fig. 6) produced less than 20% inhibition. In one cell, 50 μM chromonal 293B, a selective IKs blocker (15), was added to the bath solution. In this cell, IKs was blocked by 98.3%, confirming cell sensitivity.
Fig 6.
Effects of various antimalarial drugs on IKs ion current. Values represent mean ± SEM of five preparations. Five or six concentrations of drugs were used, and the concentrations ranged from 0.003/0.1 to 3/10 μM for PQP without or with 2.4 or 7.2 μM DHA, from 1 to 100 μM for DHA, from 0.1 to 10 μM for chloroquine, from 0.3 to 30 μM for lumefantrine (LUM; with 0.18 μM artemether [ART]), and from 0.1 to 10 nM for dofetilide.
DISCUSSION
The in vitro concentrations of nonclinical cardiac toxicity experiments should be consistent with the free therapeutic plasma concentrations (33, 41). For the present analyses, free therapeutic plasma concentrations were used, as reported in Table 2, to determine the central concentrations around which sub- and supratherapeutic concentrations were examined.
Consistent with the clinically observed QT prolongation, DHA combined with PQP and lumefantrine blocked IKr channel currents (hERG). The effects of PQP and DHA on hERG currents were quite stable, as they ranged, in the two different experiments, from 0.106 to 0.087 μM for PQP and 9.62 to 7.7 μM for DHA.
The IC50s on hERG are similar but somewhat more potent than those previously reported. For example, the IC50 for halofantrine has been reported to range from 21.6 to 40 nM (35, 45), while the present study yielded a value of 18 nM. Chloroquine, mefloquine, and lumefantrine have previously been reported to have IC50s of 2.5, 2.6 to 5.6, and 8.1 μM, respectively (25, 35, 45), while the present experiment yielded values of approximately 1.0, 1.0, and 2.6 μM, respectively. The increased potency in the present study is most likely explained by the use of physiologic temperatures (37°C) versus room temperature in previous studies. Increased potency at elevated temperatures has been reported for other drugs as well, such as erythromycin and sotalol (27, 51). The combination of DHA at two concentrations (2.4 and 7.2 μM) in the presence of PQP was also evaluated, and hERG blockade by DHA-PQP was less than that by PQP alone. However, retrospective analysis of the risk of TdP and the ability of a drug to inhibit this channel demonstrates that some compounds already on the market can block this channel without inducing TdP (6, 41). It is generally accepted that hERG blockade can predict clinical QT prolongation in many cases but not TdP (19, 34, 40). It should, however, be acknowledged that QT prolongation is found in humans at estimated free concentrations of PQP which are 10 times lower (0.008 μM) than those which block 50% of hERG activity in vitro (0.087 μM). A possible explanation for this discrepancy is that QT prolongation in vivo may occur at levels of hERG blockade in the 10% range (23).
DHA exerted blocking of hERG trafficking effects only at a concentration that induced cell deaths (>75%) in 1 out of 6 vials. This result is not surprising, as it has already been shown that DHA may already increase apoptosis at μM concentrations after several hours of incubation (31, 36, 56). It is worth mentioning that the execution of the hERG trafficking test requires 16 h of incubation with the test compounds. As already reported, chloroquine increased hERG trafficking (50). Thus, it seems that the effect of hERG blockade by the various antimalarial drugs involved increased protein hERG synthesis only by chloroquine.
The electrophysiological properties of the isolated rabbit ventricular wedge preparation are integrative functions of major depolarization and repolarization ion currents such as INa, IKr, and IKs (33). As also shown by the dedicated studies, neither DHA-PQP nor artemether-lumefantrine significantly affected Na or slow K currents. The lack of interaction with fast INa is also demonstrated by the absence of QRS prolongation, as reported, for example, for the Na channel blocker flecainide (28). The heart wedges are considered a sensitive and specific experimental model mimicking human pathological conditions characterized by a substantial reduction in repolarization reserve (24). These conditions (hypokalemia, bradycardia, etc.) are recognized risk factors for the potential of QT-prolonging agents to trigger TdP, a life-threatening arrhythmia. Similarly, the Tp-e interval describes transmural dispersion of repolarization that plays an important role in the development of TdP (53, 54). Moreover, the Tp-e/QT ratio provides important electrophysiological information about a drug (33): (i) when there is QT interval prolongation, a concurrent increase in the Tp-e/QT ratio signals strong IKr blockade because IKr blockade is accompanied by a preferential prolongation of endocardial action potential in the rabbit ventricular wedge preparation (33, 54); and (ii) when there is QT interval prolongation, an unchanged or decreased Tp-e/QT ratio signals IKs blockade or combined IKr and INa blockade if the QRS duration increases (33) or signals combined IKr and calcium current interference (ICa,L [where L is L-type calcium current]) blockade (47). Proarrhythmic events measurable in the rabbit ventricular wedge preparation include EAD as well as EAD-dependent phenomena (i.e., R-on-T extrasystole and TdP), ventricular tachycardia, and ventricular fibrillation. It is widely accepted that TdP is initiated by EAD-dependent R-on-T extrasystoles under conditions of QT prolongation (24, 54). EADs as well as EAD-dependent R-on-T extrasystoles and TdP often occur in the presence of potent IKr blockers in the rabbit ventricular wedge preparation (24, 33, 54). Therefore, the use of this parameter significantly reduces the probability of false positives by increasing the specificity of the preclinical study (24). On the other hand, strong use-dependent blockade of the fast sodium channel can result in monomorphic ventricular tachycardia and increase mortality in patients with coronary artery disease and left ventricular systolic dysfunction (8, 20).
However, DHA-PQP and artemether-lumefantrine, despite their hERG blockade, did not induce proarrhythmic events, as shown by any effect on the TdP risk score. In the case of lumefantrine, however, the difficulty in dissolving it at 30 μM was noticed when it was to have been infused into the wedge preparation. Thus, the effects with lumefantrine at 30 μM should be interpreted with caution, both in the hERG and hERG trafficking preparations and in the INa and IKs current studies. The TdP risk score was increased by chloroquine and was very clearly increased by dofetilide, with the latter inducing EADs in all five heart wedge preparations. These observations with dofetilide were never reported and confirm the predictivity of the test.
The aim of the present studies was to evaluate the potential arrhythmogenic effects of DHA-PQP. Despite the experimental limits (cytotoxicity at high DHA concentration, difficulty in dissolving 30 μM lumefantrine), our experiments suggest that both DHA-PQP and artemether-lumefantrine may have a low potential to induce TdP in the rabbit ventricular wedge model. Using this model, both combination compounds appear to be less active in generating a risk of TdP than chloroquine. Chloroquine has been reported to induce heart rate-corrected QT (QTc) prolongation between 14 and 30 ms (8, 10). A few reports (2, 13, 17, 39) describe an event consistent with TdP ventricular arrhythmia with chloroquine; however, there is no sign of sudden unexplained death associated with its use as an antimalarial. These results comparing chloroquine and dofetilide are consistent with data obtained in the model of isolated canine Purkinje fibers (8) to predict proarrhythmic risk, which also showed chloroquine to be likely to be associated with some arrhythmias.
Our results give a consistent message. DHA has little cardiac toxicity, as indicated in previous reports (49). PQP, which causes QT prolongation clinically but which has not been associated with TdP, demonstrated a blockage of the hERG channel which is not mediated by trafficking. In the rabbit ventricular wedge model ventricular preparations, PQP shows a low arrhythmogenic potential whether used alone or in combination with DHA.
In conclusion, it appears that DHA-PQP can be considered a valuable antimalarial therapeutic option from an in vitro cardiac safety point of view, considering that (i) the malaria parasite is resistant to the combination of artemether and lumefantrine, with a >10% failure rate reported in Cambodia and resistance also reported in Ghana and Burkina-Faso (52), (ii) DHA combined with PQP has never been reported to induce sudden death in malaria patients (57), and (iii) PQP is second only to chloroquine, in terms of human exposure, as treatment for malaria (37). Clinical pharmacovigilance data are needed to confirm this view.
ACKNOWLEDGMENTS
All contributors received financial support from Medicine for Malaria Venture to perform the present experiments.
Footnotes
Published ahead of print 5 March 2012
REFERENCES
- 1. Aktas MK, Shah AK, Akiyama T. 2007. Dofetilide-induced long QT and torsades de pointes. Ann. Noninvasive Electrocardiol. 12:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anonymous 2005. Medicines and QT prolongation. Aust. Adverse Drug React. Bull. 24:1–4 [Google Scholar]
- 3. Ashley EA, et al. 2004. Randomized, controlled dose-optimization studies of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria in Thailand. J. Infect. Dis. 190:1773–1782 [DOI] [PubMed] [Google Scholar]
- 4. Ashley EA, et al. 2005. A randomized controlled study of a simple once daily regimen of dihydroartemisinin-piperaquine for the treatment of uncomplicated multidrug-resistant falciparum malaria. Clin. Infect. Dis. 41:425–432 [DOI] [PubMed] [Google Scholar]
- 5. Belardinelli L, Shryock JC, Wu L, Song Y. 2005. Use of preclinical assays to predict risk of drug induced torsades de pointes. Heart Rhythm. 2:S16–S22 [DOI] [PubMed] [Google Scholar]
- 6. Bindschedler M, Lefevre G, Degen P, Sioufi A. 2002. Comparison of the cardiac effects of the antimalarials co-artemether and halofantrine in healthy participants. Am. J. Trop. Med. Hyg. 66:293–298 [DOI] [PubMed] [Google Scholar]
- 7. Bustos MDG, Gay F, Diquet B, Thomare P, Warot D. 1994. The pharmacokinetics and electrocardiographic effects of chloroquine in healthy subjects. Trop. Med. Parasitol. 45:83–86 [PubMed] [Google Scholar]
- 8. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators 1989. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N. Engl. J. Med. 321:406–412 [DOI] [PubMed] [Google Scholar]
- 9. Champeroux P, et al. 2005. Prediction of the risk of torsade de pointes using the model of isolated canine Purkinje fibres. Br. J. Pharmacol. 144:376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook JA, Randinitis EJ, Bramson CR, Wesche DL. 2006. Lack of a pharmacokinetic interaction between azithromycin and chloroquine. Am. J. Trop. Med. Hyg. 74:407–412 [PubMed] [Google Scholar]
- 11. Crumb W, et al. 2008. Cyamemazine metabolites: effect on human cardiac ion channels in vitro and on the QTc interval in guinea pigs. J. Pharm. Pharmacol. 60:1507–1513 [DOI] [PubMed] [Google Scholar]
- 12. Crumb WJ, Jr, Pigott JD, Clarkson CW. 1995. Comparison of Ito in young and adult human atria1 myocytes: evidence for developmental changes. Am. J. Physiol. 268(3 Pt 2):H1335–H1342 [DOI] [PubMed] [Google Scholar]
- 13. Demaziere J, et al. 1995. The hazards of chloroquine self prescription in West Africa. Clin. Toxicol. 33:369–370 [DOI] [PubMed] [Google Scholar]
- 14. Denis MB, et al. 2002. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin. Infect. Dis. 35:1467–1476 [DOI] [PubMed] [Google Scholar]
- 15. Ding WG, Toyoda F, Matsuura H. 2002. Blocking action of chromanol 293B on the slow component of delayed rectifier K+ current in guinea-pig sino-atrial node cells. Br. J. Pharmacol. 137:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital toll in efforts to eliminate malaria. Nat. Rev. Microbiol. 7:864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fauchier JP, Lanfranchi J, Ginies G, Raynaud R. 1974. Syncope through multifocal ventricular tachycardia during treatment with chloroquine. Study of the hisian electrogram and treatment by verapamil. Ann. Cardiol. Angeiol (Paris) 23:341–346 [PubMed] [Google Scholar]
- 18. Ficker E, et al. 2004. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol. Pharmacol. 66:33–44 [DOI] [PubMed] [Google Scholar]
- 19. Gintant G. 2011. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol. Ther. 129:109–119 [DOI] [PubMed] [Google Scholar]
- 20. Greene HL, et al. 1992. The Cardiac Arrhythmia Suppression Trial: first CAST … then CAST-II. J. Am. Coll. Cardiol. 19:894–898 [DOI] [PubMed] [Google Scholar]
- 21. Herzberg IM, Trudeau MC, Robertson GA. 1998. Transfer of rapid inactivation and sensitivity to the class III antiarrhythmic drug E-4031 from HERG to M-eag channels. J. Physiol. 511(Pt 1):3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imamura H, et al. 1998. Inhibition of delayed rectifier K+ current by dofetilide and E-4031 differentially affects electrical cardiac responses to vagus stimulation in anesthetized dogs. Jpn. J. Pharmacol. 76:31–37 [DOI] [PubMed] [Google Scholar]
- 23. Jonker DM, Kenna LA, Leishman D, Wallis R, Jonsson EN. 2005. A pharmacokinetic-pharmacodynamic model for the quantitative prediction of dofetilide QT prolongation from human ether-a-go-go-related gene current inhibition data. Clin. Pharmacol. Ther. 77:572–582 [DOI] [PubMed] [Google Scholar]
- 24. Joshi A, DiMino T, Vohra Y, Cui C, Yan GX. 2004. Preclinical strategies to assess QT liability and torsadogenic potential of new drugs: the role of experimental models. J. Electrocardiol. 37(Suppl):7–14 [DOI] [PubMed] [Google Scholar]
- 25. Kang J, Chen X-L, Wang L, Rampe D. 2001. Interactions of the antimalaric drug mefloquine with the human cardiac potassium channels nKvLQT1/minK and HERG. J. Pharmacol. Exp. Ther. 299:290–296 [PubMed] [Google Scholar]
- 26. Kannankeril P, Roden DM, Darbar D. 2010. Drug-induced long QT syndrome. Pharmacol. Rev. 62:760–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirsch GE, et al. 2004. Variability in the measurement of hERG potassium channel inhibition: effects of temperature and stimulus pattern. J. Pharmacol. Toxicol. Methods 50:93–101 [DOI] [PubMed] [Google Scholar]
- 28. Konzen G, Reichard B, Hauswirth O. 1990. Fast and slow blockade of sodium channel by flecainide in rabbit cardiac Purkinje fibres. Naunyn Schmiedebergs Arch. Pharmacol. 341:565–576 [DOI] [PubMed] [Google Scholar]
- 29. Kurunajeewa H, et al. 2004. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br. J. Clin. Pharmacol. 57:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuryshev YA, et al. 2005. Pentamidine-induced long QT syndrome and block of hERG trafficking. J. Pharmacol. Exp. Ther. 312:316–323 [DOI] [PubMed] [Google Scholar]
- 31. Lai H, Singh NP. 1995. Selective cancer cell cytotoxicity from exposure to dihydroartemisinin and halotransferrin. Cancer Lett. 91:41–46 [DOI] [PubMed] [Google Scholar]
- 32. Le Coz F, Funck-Brentanò C, Morell T, Ghadanfar MM, Jaillon P. 1995. Pharmacokinetics and pharmacodynamic modeling of the effects of oral and intravenous administrations of dofetilide on ventricular repolarization. Clin. Pharmacol. Ther. 57:533–542 [DOI] [PubMed] [Google Scholar]
- 33. Liu T, et al. 2006. Blinded validation of the isolated arterially perfused rabbit ventricular wedge in preclinical assessment of drug-induced proarrhythmias. Heart Rhythm 3:948–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu HR, et al. 2008. Predicting drug-induced changes in QT interval and arrhythmias: QT-shortening drugs point to gaps in the ICHS7B guidelines. Br. J. Pharmacol. 154:1427–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mbai M, Rajamani S, January CT. 2002. The anti-malarial drug halofantrine and its metabolite N-desbutylhalofantrine block HERG potassium channels. Cardiovasc. Res. 55:799–805 [DOI] [PubMed] [Google Scholar]
- 36. Morrissey C, et al. 2010. Effect of artemisinin derivatives on apoptosis and cell cycle in prostate cancer cells. Anticancer Drugs 21:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myint HY, et al. 2004. A systematic overview of published antimalarial drug trials. Trans. R. Soc. Trop. Med. Hyg. 98:73–81 [DOI] [PubMed] [Google Scholar]
- 38. Mytton OT, et al. 2007. Short report: electrocardiographic safety evaluation of dihydroartemisinin-piperaquine in the treatment of uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 77:447–450 [PubMed] [Google Scholar]
- 39. Mzayek F, et al. 2007. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin. Trials 2:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raehl CL, Patel AK, LeRoy M. 1985. Drug-induced torsade de pointes. Clin. Pharm. 4:675–690 [PubMed] [Google Scholar]
- 41. Redfern WS, et al. 2003. Relationship between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58:32–45 [DOI] [PubMed] [Google Scholar]
- 42. Saldeen J, et al. 1996. Efficient gene transfer to dispersed human pancreatic islet cells in vitro using adenovirus-polylysine/DNA complexes or polycationic liposomes. Diabetes 45:1197–1203 [DOI] [PubMed] [Google Scholar]
- 43. Sánchez-Chapula JA, Salinas-Stefanon E, Torres-Jacome J, Benavides-Haro DE, Navarro-Polanco RA. 2001. Blockade of currents by the antimalarial drug chloroquine in feline ventricular myocytes. J. Pharmacol. Exp. Ther. 297:437–445 [PubMed] [Google Scholar]
- 44. Shinmura K, Tani M, Hasegawa H, Ebihara Y, Nakamura Y. 1998. Effect of E4031, a class III antiarrhythmic drug, on ischemia- and reperfusion-induced arrhythmias in isolated rat hearts. Jpn. Heart J. 39:183–197 [DOI] [PubMed] [Google Scholar]
- 45. Traebert M, et al. 2004. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK-293 cells. Eur. J. Pharmacol. 484:41–48 [DOI] [PubMed] [Google Scholar]
- 46. Tran TH, et al. 2004. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomized clinical trial. Lancet 363:18–22 [DOI] [PubMed] [Google Scholar]
- 47. Wang D, Patel C, Cui C, Yan GX. 2008. Preclinical assessment of drug-induced proarrhythmias: role of the arterially perfused rabbit left ventricular wedge preparation. Pharmacol. Ther. 119:141–151 [DOI] [PubMed] [Google Scholar]
- 48. Weirich J, Antoni H. 1998. Rate-dependence of antiarrhythmic and proarrhythmic properties of class I and class III antiarrhythmic drugs. Basic Res. Cardiol. 93(Suppl 1):125–132 [DOI] [PubMed] [Google Scholar]
- 49. White NJ. 2007. Cardiotoxicity of antimalarial drugs. Lancet Infect. Dis. 7:549–558 [DOI] [PubMed] [Google Scholar]
- 50. Wible BA, et al. 2005. HERG-lite®: a novel comprehensive high-throughput screen for drug-induced hERG risk. J. Pharmacol. Toxicol. Methods 52:136–145 [DOI] [PubMed] [Google Scholar]
- 51. Witchel HJ, Milnes JT, Mitcheson JS, Hancox JC. 2002. Troubleshooting problems with in vitro screening of drugs for QT interval prolongation using HERG K+ channels expressed in mammalian cell lines and Xenopus oocytes. J. Pharmacol. Toxicol. Methods 48:65–80 [DOI] [PubMed] [Google Scholar]
- 52. World Health Organization 2010. Global report on antimalarial drug efficacy and drug resistance: 2000–2010. World Health Organization Press, Geneva, Switzerland [Google Scholar]
- 53. Yan GX, Antzelevitch C. 1998. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 98:1928–1936 [DOI] [PubMed] [Google Scholar]
- 54. Yan GX, et al. 2001. Phase 2 early after depolarization as a trigger of polymorphic ventricular tachycardia in acquired long-QT syndrome: direct evidence from intracellular recordings in the intact left ventricular wall. Circulation 103:2851–2856 [DOI] [PubMed] [Google Scholar]
- 55. Yap YG, Camm AJ. 1999. Lessons from antiarrhythmic trails involving class III antiarrhythmic drugs. Am. J. Cardiol. 84:83R–89R [DOI] [PubMed] [Google Scholar]
- 56. Zhou H-J, Zhang J-L, Li A, Wang Z, Lou X-E. 2010. Dihydroartemisinin improves the efficiency of chemotherapeutics in lung carcinomas in vivo and inhibits murine Lewis lung carcinoma cell line growth in vitro. Cancer Chemother. Pharmacol. 66:21–29 [DOI] [PubMed] [Google Scholar]
- 57. Zwang J, et al. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4:e6358. [DOI] [PMC free article] [PubMed] [Google Scholar]