Abstract
An innovative liposomal formulation of meglumine antimoniate (LMA) was recently reported to promote both long-term parasite suppression and reduction of infectivity to sand flies in dogs with visceral leishmaniasis. However, 5 months after treatment, parasites were still found in the bone marrow of all treated dogs. In order to improve treatment with LMA, the present study aimed to evaluate its efficacy in combination with allopurinol. Mongrel dogs naturally infected with Leishmania infantum were treated with six doses of LMA (6.5 mg Sb/kg of body weight/dose) given at 4-day intervals, plus allopurinol (20 mg/kg/24 h per os) for 140 days. Comparison was made with groups treated with LMA, allopurinol, empty liposomes plus allopurinol, empty liposomes, and saline. Dogs remained without treatment from day 140 to 200 after the start of treatment. The drug combination promoted both clinical improvement of dogs and significant reduction in the parasitic load in bone marrow and spleen on days 140 and 200 compared to these parameters in the pretreatment period. This is in contrast with the other protocols, which did not result in significant reduction of the bone marrow parasite load on day 200. Strikingly, the combined treatment, in contrast to the other regimens, induced negative quantitative PCR (qPCR) results in the liver of 100% of the dogs. Both xenodiagnosis and skin parasite determination by qPCR indicated that the drug combination was effective in blocking the transmission of skin parasites to sand flies. Based on all of the parasitological tests performed on day 200, 50% of the animals that received the combined treatment were considered cured.
INTRODUCTION
Visceral leishmaniasis (VL) is a systemic parasitic disease which leads to high rates of morbidity and mortality in humans worldwide. Even with scientific advances related to diagnosis, treatment, and prevention over the past 10 years, VL still is a neglected disease leading to ∼60,000 human deaths/year. The clinical manifestations of VL are attributed to obligatory intracellular protozoa of the Leishmania donovani complex and, depending on the etiological agent, the disease presents two distinct forms: anthroponotic VL that is endemic in India and Central Africa, caused by L. donovani, and zoonotic VL that occurs in countries of the Mediterranean basin, Central Asia, and Americas, caused by Leishmania infantum. Domestic dogs are the most important urban reservoirs of L. infantum, which is transmitted to humans and dogs through bites of infected female sand flies of the genera Lutzomyia and Phlebotomus (Diptera: Psychodidae; Phlebotominae) in the New World and Old World, respectively (7, 33). The disease in dogs is characterized by a marked pleomorphism, and the clinical signs vary according to the immune response of the animals toward the infection. In general, the main clinical signs of canine visceral leishmaniasis (CVL) are various degrees of dermopathy, lymphadenopathy, onychogryposis, weight loss, abnormalities of the musculoskeletal system, eye lesions, hematopoietic disorders, renal disease, and lesions originating from immune complex deposition in tissues (e.g., vasculitis and arthritis) (30).
The treatment of dogs affected with VL has been practiced in Europe since the middle of the 20th century (1). Since then, the pentavalent antimonials, including meglumine antimoniate (MA) and sodium stibogluconate, have been the main class of drugs used to treat VL in both humans and dogs (1, 17). However, treatment with antimonial drugs does not promote parasitological cure in infected dogs, leading to frequent relapses (23) and necessitating continuous administration of the drugs, which are both poorly tolerated and expensive.
The purine analog allopurinol is an alternative orally active drug (6) which presents low toxicity, is effective in reverting the clinical signs of CVL, and prevents recurrence of the disease (1, 8). However, allopurinol also is unable to promote parasitological cure in dogs with VL (23).
Currently, the combination of MA and allopurinol constitutes the first-line pharmaceutical protocol for CVL. The basis for the use of this combination is the synergism between these drugs against Leishmania parasites, as previously demonstrated in vitro (22) and in vivo (1, 15, 24, 27). However, although most dogs recover clinically after therapy, complete elimination of the parasite is usually not achieved, and infected dogs may eventually relapse. Because of its limited parasiticidal efficacy and the potential risk of long-term treatment for selection of Leishmania strains resistant to antimonial drug, WHO strongly supports the search for more effective therapeutic protocols (21, 23, 33).
In the 1970s, a major advance occurred when it was found that liposome-encapsulated antimonial drugs were hundreds of times more effective than the free drugs against experimental VL based on parasite suppression in the liver (4). Since then, much effort has been devoted to the search for efficacious liposomal formulations in CVL (16). In this context, an innovative liposomal formulation of MA (LMA) was recently developed that has significant pharmaceutical and pharmacological advantages over conventional formulations (18, 29). Treatment of dogs naturally infected by L. infantum with four intravenous (i.v.) doses of LMA at 6.5 mg Sb/kg of body weight at 4-day intervals (28) promoted both long-term parasite suppression and reduction of infectivity to sand flies. However, parasites were still found in the bone marrow of all treated dogs.
With the aim of further improving the treatment efficacy of LMA in dogs, a new protocol was designed which combines LMA with allopurinol and takes advantage of the synergistic antileishmanial actions of allopurinol and antimonial drugs. In the present work, the efficacy of the combination of LMA with allopurinol as a mean of achieving parasitological cure in dogs naturally infected with L. infantum has been established for the first time, through clinical and parasitological parameters as well as assessment of the infectivity of dogs to sand flies.
MATERIALS AND METHODS
Materials and drugs.
Cholesterol and dicetylphosphate were purchased from Sigma Co. (St. Louis, MO in the supplemental material). Distearoylphosphatidylcholine was obtained from Lipoid (Ludwigshafen, Germany). N-Methyl-d-glucamine and antimony pentachloride (SbCl5, 99%) were obtained from Aldrich Chemical Co. (Milwaukee, WI). Allopurinol was purchased from a commercial laboratory for pharmaceutical products (Vetfarma, Brazil) as a formulation of oral capsules at individual dosages of 20 mg/kg of body weight. Meglumine antimoniate (MA) was synthesized, as previously described (14), from equimolar amounts of N-methyl-d-glucamine and pentavalent antimony oxyhydrate freshly prepared from the hydrolysis of antimony pentachloride in water. The resulting product contained 28% Sb by weight. As previously established (18), synthetic MA may be replaced by commercial MA (Glucantime; Sanofi-Aventis Farmacêutica Ltda., São Paulo, Brazil) in the preparation of the liposomal formulation.
Animals.
The present study was approved by the Ethical Committee for Animal Experimentation of the Universidade Federal de Minas Gerais (protocol number 211/2007), and all procedures were carried out according to the international guidelines (Principles of Laboratory Animal Care [26]).
Fifty-two mongrel dogs (32 males and 20 females) weighing 12.3 ± 5.2 kg (mean ± standard deviation), of unknown age, and naturally infected with Leishmania infantum were obtained by donation from the Center for Zoonosis Control of Ribeirão das Neves City (Minas Gerais State, Brazil), an area where canine visceral leishmaniasis is endemic. These dogs were previously identified by serological tests and captured as part of the activities of the municipality's Visceral Leishmaniasis Control Program.
The serological diagnosis was confirmed at the Serology Laboratory of the Institute of Biological Sciences (ICB), Federal University of Minas Gerais (UFMG) using indirect immunofluorescence assay (IFAT) and enzyme-linked immunosorbent assay (ELISA) (5). All animals were found to be positive by IFAT (≥1:40 dilution) and ELISA (optical density, ≥0.100; 1:400 dilution). In addition, specific PCR of bone marrow aspirate was used to confirm the infection of the animals by L. infantum (13). The animals were kept in the experimental kennel of the ICB-UFMG with drinking water and balanced commercial food (Nero Refeição; Total Alimentos, Brazil) ad libitum during the entire experimental period. Prior to treatment, dogs were treated against intestinal helminths (Helfine cães; Agener União, Brazil) and ectoparasite infestations (Frontiline Top Spot; Merial, Brazil) and immunized against rabies (Defensor; Pfizer Saúde Animal, Brazil) and other infectious diseases (Vanguard HTLP 5/CV-L; Pfizer Saúde Animal, Brazil).
Preparation and characterization of liposomes.
Liposome-encapsulated meglumine antimoniate (LMA) was prepared as previously described (29). Briefly, small unilamellar vesicles (SUVs) were obtained by mixing distearoylphosphatidylcholine, cholesterol, and dicetylphosphate (molar ratio of 5:4:1) following ultrasonication in deionized water at a final lipid concentration of 55 g/liter. Then, the SUV suspension was filtered with a sterile 0.22-μm membrane and mixed with sucrose (sugar/lipid mass ratio of 3:1; final sugar concentration of 0.3 M), and the resulting mixture was immediately frozen in liquid nitrogen and subsequently dried (freeze dryer, 4.5 liters; Labconco, United Kingdom). At this point, the freeze-dried liposomal formulation may be stored at −20°C for at least 6 months without any change of final vesicle size and drug encapsulation efficiency characteristics. The day before administration, the lyophilized powder was rehydrated with a solution of MA in water (antimony concentration of 0.65 M, corresponding to 40% of the original SUV volume) and the resulting suspension was vortexed and incubated for 30 min at 55°C. Then, the same volume of phosphate-buffered saline (PBS; 0.15 M NaCl, 0.01 M phosphate, pH 7.2) was added to the mixture, followed by vortexing and incubation for 30 min at 55°C. The resulting liposome suspension was diluted in PBS and centrifuged (25,000 × g for 30 min at 4°C) in order to separate LMA from nonencapsulated drug. After centrifugation, the liposome pellet was washed twice with isotonic saline and resuspended in sterile saline solution, and the final antimony concentration was adjusted to 10 g/liter. The liposome suspension was stored at 4°C until administration. The drug encapsulation efficiency was 37% ± 5%, as determined by atomic absorption spectroscopy (AA600; PerkinElmer, Inc., United States). The mean hydrodynamic diameter of the vesicles was 350 ± 58 nm, with a polydispersity index of 0.30 ± 0.07, as determined by photon correlation spectroscopy (Malvern Zetasizer Nano ZS90; Malvern Instruments, United Kingdom). In parallel, lyophilized SUVs were rehydrated with a solution of N-methyl-d-glucamine (0.65 M, pH 7.2) instead of MA, using the same method as described above. The final suspension, called empty liposomes (LEMP), presented similar values of mean hydrodynamic vesicle diameter and polydispersity index.
Treatment protocols.
Dogs were randomly distributed into six groups as follows. In the LMA+Allop group, eight animals were treated with six doses of LMA (6.5 mg Sb/kg of body weight/dose) given at 4-day intervals and allopurinol (20 mg/kg of body weight/24 h per os) for 140 days starting from the first dose of LMA. In the LMA group, eight animals were treated with six doses of LMA (6.5 mg Sb/kg of body weight/dose) given at 4-day intervals. In the Allop group, eight animals were treated with allopurinol (20 mg/kg of body weight/24 h per os) for 140 days and six doses of isotonic saline given at the same volume and time intervals as LMA in the LMA+Allop group. In the LEMP+Allop group, eight animals were treated with six doses of empty liposomes given at the same volume and time intervals as LMA in the LMA+Allop group and allopurinol (20 mg/kg of body weight/24 h per os) for 140 days. In the LEMP group, eight animals were treated with six doses of empty liposomes given at the same volume and time intervals as LMA in the LMA group. In the saline group, 12 animals received six doses of isotonic saline given at the same volume and time intervals as LMA in the LMA+Allop group.
Following day 140 after the beginning of treatment, all animals of the six experimental groups were kept in the kennel for 60 days without any intervention, representing a total experimental period of 200 days. Animals were clinically monitored during the experimental period and were submitted to clinical and parasitological evaluations just before and on days 140 and 200 after the beginning of treatment. At day 200, the animals were euthanized using an overdose of sodium thiopental (i.v.) under general anesthesia.
During the experimental period, seven animals died: two dogs in the LMA+Allop group, two in the LMA group, and one in the LEMP group, due to wounds caused by fights, and two dogs (one in the saline group and one in the LEMP group), most probably due to the natural evolution of the disease.
Clinical evaluation.
Just before treatment and on days 140 and 200 after the start of treatment, the dogs were inspected for the presence of clinical signs of CVL, and serum and plasma (using EDTA as an anticoagulant) were collected for quantitative evaluation of anti-Leishmania antibodies (IFAT), hemogram, and levels of serum urea, creatinine, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total proteins, globulins, albumins, and albumin/globulin (A/G) ratio.
Based on the results of the physical examination, levels of anti-Leishmania antibodies determined by IFAT (5), and laboratory findings in hemogram and serum biochemistry, the animals were classified according to a previously proposed staging system (30), with a few modifications, as shown in Table 1. Each animal received a score ranging from 0 to 4, where score 0 corresponds to absence of clinical signs of CVL, negative serology (IFAT ≤ 1:40), and no abnormalities in hemogram and serum biochemistry, and score values from 1 to 4 correspond to the mild, moderate, severe, and extremely severe disease clinical stage, respectively.
Table 1.
Staging system used for classification of dogs with visceral leishmaniasis
| Clinical stage | IFAT titer | Clinical signs | Clinicopathological abnormalities | Score |
|---|---|---|---|---|
| Stage I: mild disease | Negative or ≤1:160 | Peripheral lymphadenopathy and/or one slight dermatological alteration, such as papular dermatitis, exfoliative dermatitis, seborrheic dermatitis, or onychogryposis | No clinicopathological abnormalities, creatinine <1.4 mg/dl, urea <25 mg/dl, and A/G ratio ≥0.6 | 1 |
| Stage II: moderate disease | ≥1:80 | Peripheral lymphadenopathy associated with two or more signs, as follows: cutaneous alterations (papular dermatitis, exfoliative dermatitis, seborrheic dermatitis, onychogryposis, or ulcerative dermatitis), anorexia, mucopurulent conjunctivitis, keratoconjunctivitis, fever, and epistaxis | Mild nonregenerative anemia and/or hypergammaglobulinemia, hypoalbuminemia, creatinine <1.4 mg/dl, urea >25 mg/dl, and A/G ratio ≤0.6 | 2 |
| Stage III: severe disease | ≥1:160 | Clinical signs of stage II and lesions associated with immune complex, such as vasculitis, arthritis, and uveitis | Mild nonregenerative anemia and hypergammaglobulinemia, hypoalbuminemia, creatinine 1.4-2 mg/dl, urea >25 mg/dl, and A/G ratio ≤0.6 | 3 |
| Stage IV: very severe disease | ≥1:640 | Clinical signs of stage III and signs of chronic kidney disease, such as nephrotic syndrome and uremic crisis | Nonregenerative anemia and hypergammaglobulinemia, hypoalbuminemia, creatinine >2 mg/dl, urea >25 mg/dl, and A/G ratio ≤0.6 | 4 |
Parasitological evaluation.
Quantitative real-time PCR (qPCR) was used to determine the parasite loads in bone marrow and spleen just before treatment and on days 140 and 200 after the start of treatment. Dogs were submitted to general anesthesia using the combination of 2 mg/kg of body weight of xylazine chloridrate (Calmium; União Química Farmacêutica S/A, Brazil) and 11 mg/kg of body weight of ketamine chloridrate (Ketamina Agener; União Química Farmacêutica S/A, Brazil) by intramuscular injection. Then, 1.0 ml of sternal bone marrow aspirate was collected, followed by 1.0 ml of spleen aspirate after previous localization of the organ with portable ultrasound equipment (SonoSite SonoHeart Elite Superior 180 Plus; SonoSite Inc., United States). At euthanasia (day 200 after starting treatment), samples of liver and skin of the internal face of the right ear were collected to perform qPCR, in addition to the bone marrow and spleen aspirates. Skin samples were also obtained just before treatment to be used as controls. All samples were stored at −80°C until required for further processing.
Total DNA extraction of the samples was carried out with the DNeasy blood and tissue kit (Qiagen, Inc., United States), used according to the manufacturer's instructions. In order to quantify parasite burdens, primers (forward, 5′ TGT CGC TTG CAG ACC AGA TG 3′, and reverse, 5′ GCA TCG CAG GTG TGA GCA C 3′) that amplified a 90-bp fragment of a single-copy-number gene of DNA polymerase of L. infantum (GenBank accession number AF009147) were used (9). PCR was carried out with a final reaction mixture volume of 25 μl containing 200 nM forward and reverse primers, 1× SYBR Green PCR master mix (Applied Biosystems, United States), and 5 μl of template DNA. The PCR conditions were as follows: an initial denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. Standard curves were prepared for each run using 10-fold serial dilutions in DNase-/RNase-free water ranging from 1 to 106 molecules/μl of pGEM-T Easy vector system plasmids (Promega, United States) containing the 90-bp fragment of the DNA polymerase gene of L. infantum (3, 9). The same procedure was carried out for the housekeeping β-actin gene using primers that amplified a 307-bp fragment (forward, 5′ CTT CTA CAA CGA GCT GCG CG 3′, and reverse, 5′ TCA TGA GGT AGT CGG TCA GG 3′) in order to verify the integrity of the samples and to normalize the initial concentrations of DNA. The number of copies of L. infantum in the samples was adjusted using the β-actin correction factor obtained for each sample. In all assays, the efficiency of the amplification was close to 100% and the standard curves presented correlation coefficients between plasmid concentrations and threshold cycle (CT) values ranging from 0.97 to 0.99. In our assays, standard curves allowed the detection of a limit of 0.8 parasites/ml of bone marrow and 0.25, 0.84, and 1.1 parasites/mg of liver, spleen, and skin, respectively. The reaction mixtures were processed and analyzed in the ABI Prism 7500 sequence detection system (Applied Biosystems, United States).
Bone marrow aspirates (1.0 ml) were collected on day 200 and immediately seeded into a biphasic culture medium, NNN (Novy, McNeal, and Nicole) medium, enriched with minimum essential medium (MEM α; Gibco, United States). Cultures were maintained at 23 ± 1°C and examined each 10 days for 30 days in order to identify the presence of promastigote forms.
Xenodiagnosis.
Xenodiagnosis was performed as previously described (12), with some modifications, in order to verify the ability of treated dogs to infect the sand flies. Briefly, just before treatment and on days 140 and 200 after the start of treatment, animals were submitted to general anesthesia as described above and the internal surface of the right ear was shaved. Then, 40 to 50 4-day-old females of Lutzomyia longipalpis from the colony of the Laboratory of Physiology of Haematophagous Insects (Department of Parasitology, ICB/UFMG) were placed in a round plastic box called a FleboContainer (11), and the sand flies were allowed to feed directly on the right ear for 30 min in a dark room. After the blood meal, the sand flies were fed daily with a 50% fructose solution in distilled water and kept at 28°C in the insectary for 5 days. On the fifth day, the females of L. longipalpis were dissected in a drop of PBS solution and midguts were examined under an optical microscope at 400× magnification to verify the presence of promastigote forms and to determine the infection ratio. The estimated number of promastigotes in each midgut was determined as follows: −, absence of promastigotes; +, presence of 1 to 50 promastigotes; ++, 51 to 200 promastigotes; and +++, >201 promastigotes (32).
Cure criteria.
The criteria established to consider an animal as cured of CVL on day 200 after the start of treatment, independent of the treatment protocol received, were (i) absence of parasites in bone marrow aspirate culture, (ii) negative results for parasitological evaluations by qPCR of bone marrow, spleen, liver, and ear skin, and (iii) negative results in xenodiagnosis.
Statistical analysis.
Statistical analyses were performed with the aid of GraphPad Prism version 5.00 for Windows (GraphPad Software, United States). According to the Kolmogorov-Smirnov test, experimental data were not normally distributed. All data were not normally distributed. Then, the Kruskal-Wallis test followed by Dunn's multiple comparison test or the Mann-Whitney test was used to compare clinical stage and tissue parasitic loads. The Friedman test was used to compare the clinical stages and the tissue parasite loads in each group just before and on days 140 and 200 after the start of treatment. Comparison of qPCR results for skin samples before and after treatment was performed using Wilcoxon matched pairs. Fisher's exact test was used to compare the sand fly infection efficiencies and the proportions of cured dogs between different groups. A significance level of 95% was applied in all statistical tests.
RESULTS
Treatment of mongrel dogs naturally infected with Leishmania infantum was performed with six doses of LMA (6.5 mg Sb/kg/dose) given at 4-day intervals plus allopurinol (20 mg/kg/24 h per os) (LMA+Allop) for 140 days starting from the first dose of LMA. The efficacy of the combined treatment was evaluated on the basis of clinical and parasitological parameters determined just before treatment and on days 140 and 200 after the start of treatment. Comparison was performed with experimental groups treated with LMA, allopurinol (Allop), or empty liposomes plus allopurinol (LEMP+Allop). Control groups received either saline or empty liposomes (LEMP).
Clinical parameters.
Before treatment, all animals presented clinical signs of CVL, as established by the inclusion criteria. Dermatological alterations, such as exfoliative dermatitis, seborrheic dermatitis, localized alopecia, ulcerative dermatitis over joint prominences, and onychogryposis, were the main clinical signs observed (90.4% of the animals). The other most frequent clinical signs were lymphadenopathy and mucopurulent conjunctivitis/keratoconjunctivitis, observed in 75.0% and 32.7% of the animals, respectively. Normocytic normochromic anemia (31.0%), thrombocytopenia (23.0%), leukocytosis (24.5%), serum urea (84.6%), and globulins (32.6%) above and A/G ratio (69.2%) below the reference values were the main abnormalities found in hemograms and serum biochemistry of the animals. All animals were also positive according to IFAT, and 95% of these dogs presented high levels of anti-Leishmania antibody titers (≥1:640).
Taking into account clinical, laboratory, and serological data and a recently proposed clinical classification of CVL (30), each animal received a specific score, the highest score corresponding to the most severe pattern of the disease. The distribution of animals was as follows: 7.7% of dogs were in stage I, 51.9% in stage II, and 40.4% in stage III.
Treatment of infected dogs with LMA in combination with allopurinol (LMA+Allop) promoted a marked reduction in anti-Leishmania antibody titers. Quantitative serology of this group, as determined by IFAT endpoint, showed a 20.3-fold reduction in antibody titer, ranging from 1:8,133 before treatment to 1:400 on day 200. For comparison, during the same period, the average titer reductions in the other groups were as follows: LMA, 1.9-fold (from 1:3,867 to 1:2,000); Allop, 1.3-fold (from 1:3,940 to 1:3,040); LEMP+Allop, 2.5-fold (from 1:2,800 to 1:1,140); LEMP, 1.4-fold (from 1:5,013 to 1:3,627); and saline, 2.5-fold (from 1:6,749 to 1:2,755). Interestingly, only in the LMA+Allop group was the median IFAT titer significantly lower on days 140 and 200 than prior to treatment (P < 0.05; Friedman).
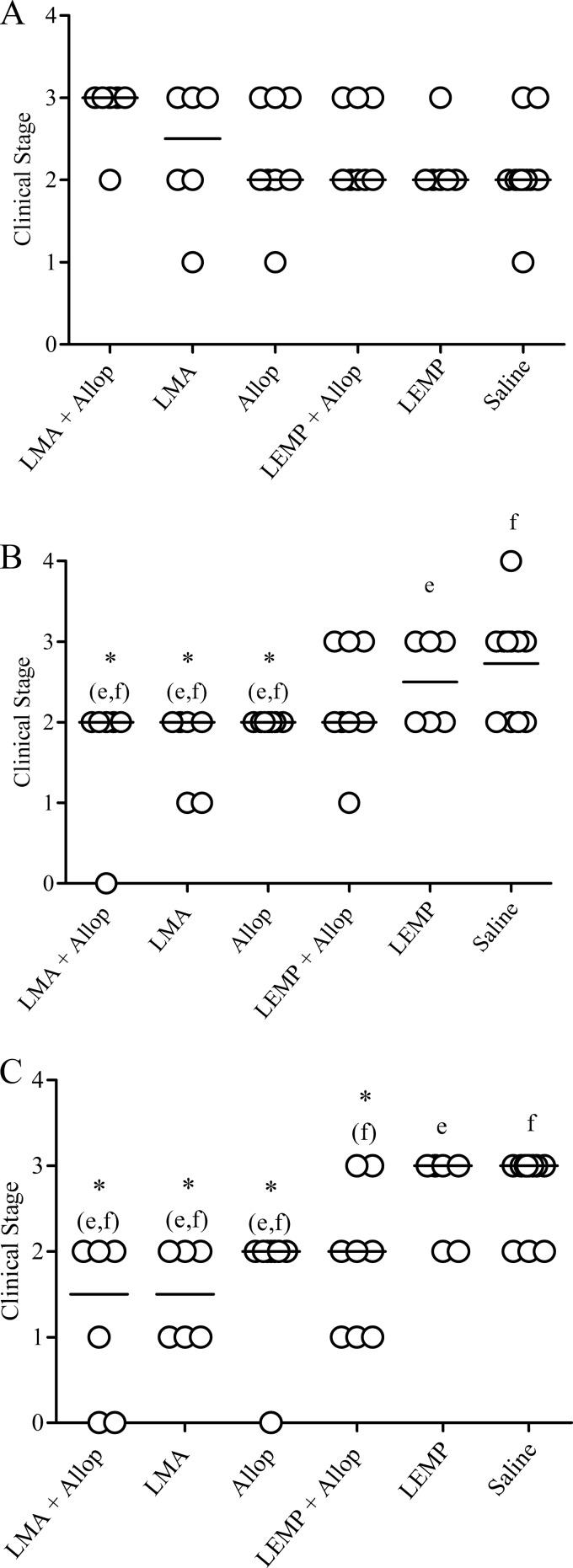
Figure 1 compares the more general and predictive clinical stage parameters between the different experimental groups, taking into account the results of quantitative serology, physical examination, and clinicopathological findings. The data indicate that the LMA+Allop, LMA, and Allop groups presented significantly lower clinical scores (severity of stages) on days 140 and 200 than control groups (LEMP and saline) (P < 0.05; Kruskal-Wallis).
Fig 1.
Clinical staging of dogs naturally infected with Leishmania (L.) infantum before and after treatment with liposomal meglumine antimoniate (LMA), allopurinol (Allop), empty liposomes (LEMP), or the LMA+Allop or LEMP+Allop combination. (A) Staging prior to treatment. (B and C) Staging on days 140 and 200, respectively, after the start of treatment. LMA (6.5 mg Sb/kg/dose), empty liposomes (same dose of lipid), and saline (same volume) were given intravenously as six doses at 4-day intervals. Allopurinol was given at 20 mg/kg/24 h per os for 140 days. Clinical scores are defined in detail in Table 1. Score 0, absence of clinical signs and clinicopathological alterations suggestive of CVL and negative serology; score 1, mild clinical stage; score 2, moderate stage; score 3, severe stage; score 4, extremely severe disease stage. Data are shown as dot plots, and lines correspond to the median of each group (n = 6 to 11). *(e,f) and *(f), P < 0.05 according to Kruskal-Wallis test followed by Dunn's multiple comparison test, for comparison with LEMP (e) and/or saline (f).
Among the different treatment protocols, the LMA-allopurinol combination was the one that promoted the most pronounced improvement of the clinical status of infected animals. The reduction in the median clinical score of the LMA+Allop group was significant on days 140 and 200 compared to the median score before treatment (P < 0.05; Friedman). Importantly, all the animals of this group (100%) presented a lower clinical score at both times than prior to treatment. At day 200, two dogs were completely asymptomatic, with no alterations in physical examination, normal hemogram and serum biochemistry, and negative serology (score = 0). Another dog was classified as stage I, showing no clinicopathological alterations but medium levels of anti-Leishmania antibody titers (IFAT, 1:320). The other three animals of this group were classified in clinical stage II. They presented medium levels of anti-Leishmania antibody titers (IFAT, 1:320 to 1:640) and, associated with this alteration, one dog had a low platelet level, another showed seborrheic dermatitis and lymphadenopathy, and the third presented a low platelet level, hypoalbuminemia, and a low A/G ratio (data not shown).
The LMA group presented a significant reduction in the median clinical score on day 200 compared to the median score in the pretreatment period (P < 0.05; Friedman). At day 200, 83.3% of these dogs presented a better clinical score than prior to treatment, and the animals were classified as stage I (50%) or stage II (50%).
The Allop and LEMP+Allop groups presented an improvement in general clinicopathological abnormalities, but no statistical difference was found when comparing the median clinical scores between the three time points.
In contrast, most dogs from the control groups presented with worse clinical stages on days 140 and 200 compared to the stage at the pretreatment period. In these animals, both a progressive increase of the number and severity of the lesions noted in the physical examination and worsening of hemogram and serum biochemistry parameters were observed. Saline-treated animals presented a significantly greater median clinical score on day 200 than before treatment (P < 0.05; Friedman).
Parasitological evaluations in bone marrow and spleen.
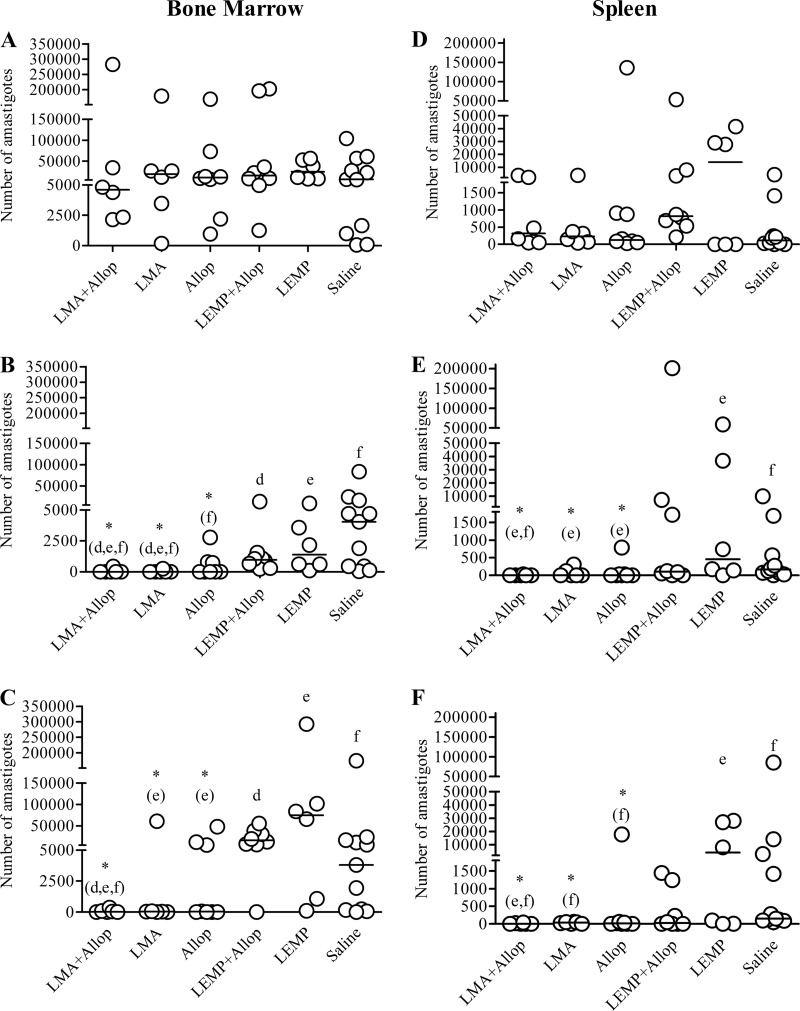
Parasitological evaluation of dogs by qPCR indicated that the LMA-allopurinol combined treatment was the most effective protocol for reducing the parasite burden in bone marrow and spleen. As illustrated in Fig. 2, this combination promoted a significant reduction in bone marrow parasite loads on days 140 and 200 compared to the results of the LEMP+Allop, LEMP, and saline protocols (P < 0.05; Kruskal-Wallis). Similarly, the LMA+Allop group presented with significantly lower parasite loads in the spleen on days 140 and 200 than the LEMP and saline groups (P < 0.05; Kruskal-Wallis) (Fig. 2).
Fig 2.
Parasite burdens in the bone marrow (A, B, and C) and spleen (D, E, and F) of dogs naturally infected with Leishmania (L.) infantum before and after treatment with liposomal meglumine antimoniate (LMA), allopurinol (Allop), empty liposomes (LEMP), or the LMA+Allop or LEMP+Allop combination. Parasite burdens prior to treatment (A and D), at day 140 (B and E), and at day 200 (C and F) are shown. LMA (6.5 mg Sb/kg/dose), empty liposomes (same dose of lipid), and saline (same volume) were given intravenously as six doses at 4-day intervals. Allopurinol was given at 20 mg/kg/24 h per os for 140 days, starting from the first dose of LMA. Parasite burdens were determined by qPCR as described in Materials and Methods. Data are shown as dot plots, and lines correspond to the median of each group (n = 6 to 11). *(d,e,f), *(e,f), *(e), and *(f), P < 0.05 according to Kruskal-Wallis test followed by Dunn's multiple comparison test, for comparison with LEMP+Allop (d), LEMP (e), and/or saline (f).
The effectiveness of the LMA-allopurinol combination for reducing the parasite burden in the bone marrow and spleen was also evident when the parasite loads were compared before and on days 140 and 200 after the start of treatment. The parasite burdens in both tissues were significantly reduced on days 140 and 200 compared to the burdens in the pretreatment period (P < 0.05; Friedman). On day 140, five animals (83.3%) were negative by qPCR in both the bone marrow and spleen. At the end of the experimental period (day 200), three of these animals remained negative in both the spleen and bone marrow. Another dog was still negative only in the spleen. The median numbers of parasites in bone marrow and spleen aspirates, as determined by qPCR on day 140, were about 770 and 245 times lower, respectively, than before treatment. This substantial reduction in the parasite load was still observed at the end of the experimental period (parasite burdens were about 660 and 104 times lower, respectively).
The LMA and Allop groups showed results similar to those for the LMA+Allop group with respect to the reduction of parasite loads in spleen and bone marrow on day 140 (Fig. 2). However, on day 200, treatment with LMA or allopurinol (with or without empty liposomes) did not show a significant reduction of the bone marrow parasite burden compared to that in the pretreatment period, confirming the inability of these drugs alone to maintain low parasite levels in this tissue after interruption of treatment.
Parasitological evaluations in the liver.
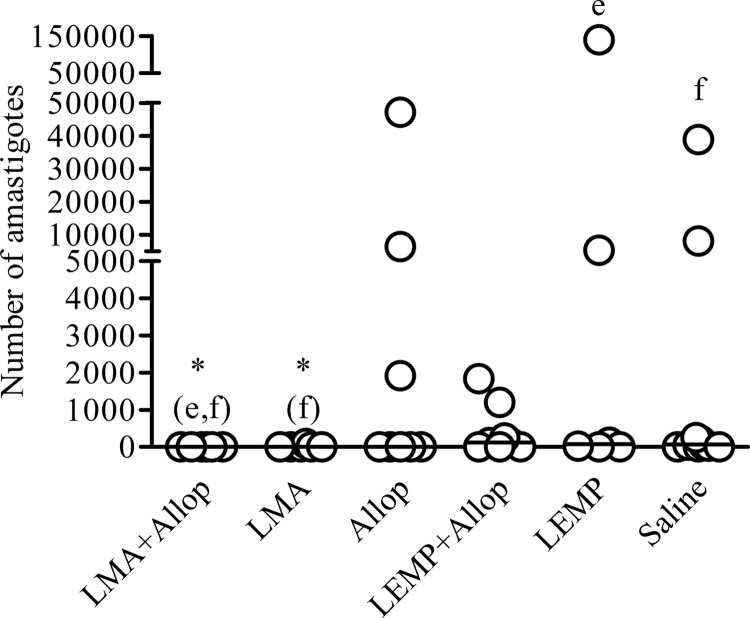
The parasite loads in the liver of treated dogs on day 200 after the start of treatment were determined by qPCR. Significant parasite suppression in the liver of dogs in comparison to the parasite suppression in control groups was achieved in the groups that received LMA (with or without allopurinol) but not in those receiving allopurinol without LMA. All animals (100%) treated with the LMA-allopurinol combination presented negative qPCR results. As shown in Fig. 3, the parasite burdens in the livers of the LMA+Allop group were significantly lower than the parasite burdens in the LEMP and saline groups (P < 0.05; Kruskal-Wallis). In the LMA group, five animals (87.3%) were found to be negative and the median number of parasites in this organ was significantly lower than that in the saline group (P < 0.05; Kruskal-Wallis).
Fig 3.
Parasite burdens in the liver of dogs naturally infected with Leishmania (L.) infantum after treatment with liposomal meglumine antimoniate (LMA), allopurinol (Allop), empty liposomes (LEMP), or the LMA+Allop or LEMP+Allop combination. LMA (6.5 mg Sb/kg/dose), empty liposomes (same dose of lipid), and saline (same volume) were given intravenously as six doses at 4-day intervals. Allopurinol was given at 20 mg/kg/24 h per os for 140 days, starting from the first dose of LMA. Parasite burdens were determined by qPCR as described in Materials and Methods. Data are shown as dot plots, and lines correspond to the median of each group (n = 6 to 11). *(e,f) and *(f), P < 0.05 according to Kruskal-Wallis test followed by Dunn's multiple comparison test, for comparison with LEMP (e) and/or saline (f).
Parasitological evaluations in the skin.
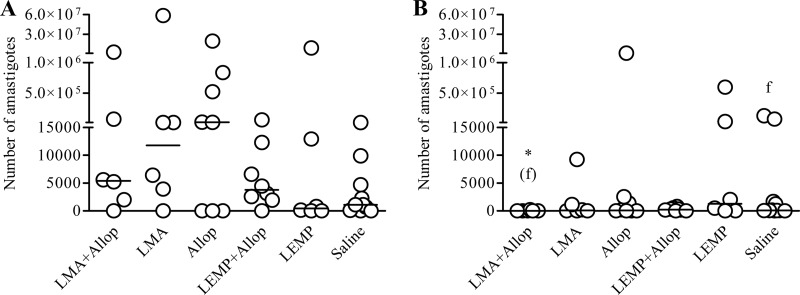
Parasitological evaluation was carried out by qPCR in the ear skin of dogs before treatment and on day 200 after the start of treatment. No statistical difference was observed between the six groups in the median number of parasites in the ear skin on day 200 (P > 0.05; Kruskal-Wallis). Nevertheless, as illustrated in Fig. 4, the LMA+Allop group was the only group to show a significantly lower parasite load than the saline group (P < 0.05; Mann-Whitney). Furthermore, the parasite loads in the skin of animals from the LMA+Allop, LMA, and LEMP+Allop groups were significantly lower on day 200 than before treatment (P < 0.05; Wilcoxon matched pairs). Prior to treatment, one animal (16.7%) from the LMA+Allop group was negative according to skin qPCR. At day 200, the proportion of skin-negative dogs from this group was 83.3%, corresponding to the highest proportion of negative dogs among all experimental groups. In the dog whose qPCR remained positive, the median number of parasites decreased about 314-fold (from 69,776 amastigotes prior to treatment to 222 parasites on day 200).
Fig 4.
Parasite burdens in the ear skin of dogs naturally infected with Leishmania (L.) infantum before and after treatment with liposomal meglumine antimoniate (LMA), allopurinol (Allop), empty liposomes (LEMP), or the LMA+Allop or LEMP+Allop combination. Parasite burdens prior to treatment (A) and at day 200 (B) are shown. LMA (6.5 mg Sb/kg/dose), empty liposomes (same dose of lipid), and saline (same volume) were given intravenously as six doses at 4-day intervals. Allopurinol was given at 20 mg/kg/24 h per os for 140 days, starting from the first dose of LMA. Data are shown as dot plots, and lines correspond to the median of each group (n = 6 to 11). *(f), P < 0.05 for comparison with saline group according to the Mann-Whitney test, for comparison with saline (f).
Xenodiagnosis.
The effect of the treatment of dogs on their infectivity to sand flies was investigated through xenodiagnosis in the different experimental groups just before and on days 140 and 200 after the start of treatment, using females of Lutzomyia longipalpis. As shown in Table 2, the LMA+Allop and Allop groups exhibited the best results in the xenodiagnosis evaluations.
Table 2.
Frequency of positive dogs in xenodiagnosis and proportion and intensity of infection of Lutzomyia longipalpis fed on dogs naturally infected with Leishmania (L.) infantum before and after treatment
| Treatment groupa | Frequency of positive dogs in xenodiagnosis (%)b |
Proportion of infected sand flies (%)c |
Intensity of infection (%)d |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prior to treatment | Day 140 | Day 200 | Prior to treatment | Day 140 | Day 200 | Prior to treatment |
Day 140 |
Day 200 |
|||||||
| + | ++ | +++ | + | ++ | +++ | + | ++ | +++ | |||||||
| LMA+Allop | 50 | 0 | 0 | 19.1 | 0e | 0e | 42.5 | 40 | 17.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| LMA | 50 | 16.7 | 33.3 | 11.9 | 1.4e | 1.9e | 28 | 12 | 60 | 33.3 | 66.7 | 0 | 75 | 25 | 0 |
| Allop | 50 | 0 | 0 | 35.0 | 0e | 0e | 11.2 | 11.2 | 77.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| LEMP+Allop | 62.5 | 0 | 12.5 | 34.3 | 0e | 2.5e | 22.9 | 24 | 53.1 | 0 | 0 | 0 | 28.6 | 57.1 | 14.3 |
| LEMP | 33.3 | 33.3 | 50 | 16.7 | 26.2f | 30.0f | 28.6 | 22.9 | 48.5 | 23.7 | 14.5 | 61.8 | 22.2 | 20.6 | 57.2 |
| Saline | 36.4 | 45.5 | 63.6 | 3.9 | 11.4f | 16.4f | 46.7 | 33.3 | 20 | 45.8 | 25 | 29.2 | 41.6 | 27.7 | 30.7 |
Liposomal meglumine antimoniate (LMA; 6.5 mg Sb/kg/dose), empty liposomes (LEMP; same dose of lipid), and saline (same volume) were given intravenously as six doses at 4-day intervals; allopurinol (Allop) was given at 20 mg/kg/24 h per os for 140 days, starting from the first dose of LMA.
Proportions of dogs in each group whose promastigotes were identified in the midgut of Lutzomyia longipalpis females 5 days after their blood meal on the internal surface of the right ear of these animals.
Proportions of infected Lutzomyia longipalpis females in relation to total numbers of insects dissected 5 days after their blood meal on each experimental group of dogs.
Distribution of midgut infections according to the estimated numbers of promastigotes, categorized as follows: +, 1 to 50 promastigotes; ++, 51 to 200 promastigotes; and +++, >201 promastigotes.
P < 0.05 according to Fisher's exact test, showing a significantly lower proportion of infected sand flies than in the pretreatment period.
P < 0.05 according to Fisher's exact test, showing a significantly greater proportion of infected sand flies than in the pretreatment period.
In the LMA+Allop group, three animals (50%) infected sand flies just before treatment, with 38% infection efficiency (given by the ratio of infected to fed sand flies in positive dogs), whereas xenodiagnosis was negative in all animals (100%) on days 140 and 200. As shown in Table 2, significant reductions in the proportions of infected sand flies were observed on both day 140 and 200 compared to the proportions in the pretreatment period (P < 0.05; Fisher's exact test).
Among the other groups, the Allop but not the LMA and LEMP+Allop groups showed 100% noninfective dogs on day 200. In the LMA, Allop, and LEMP+Allop groups, significant reductions in the proportions of infected sand flies on days 140 and 200 were also observed compared to the proportions in the pretreatment period (P < 0.05; Fisher's exact test) (Table 2). This is in contrast with the LEMP and saline groups, in which the proportions of infected sand flies were significantly increased on days 140 and 200 compared to the proportions in the pretreatment period.
Parasitological cure.
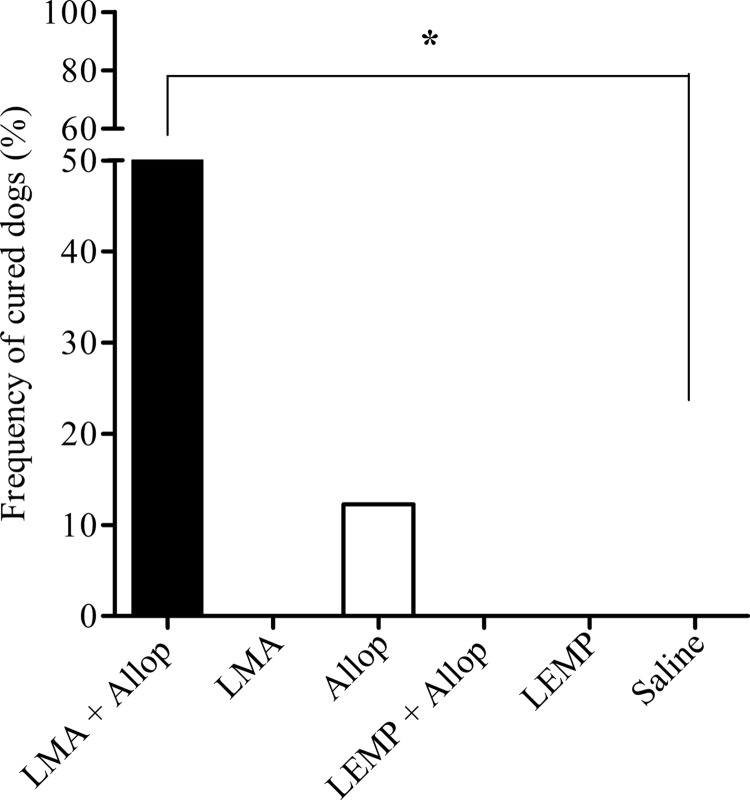
Each animal was evaluated on day 200 using different parasitological tests, including culture from bone marrow aspirates, qPCR of bone marrow, spleen, liver, and skin samples, and xenodiagnosis. Animals that showed negative results in all the parasitological tests were considered cured of CVL. According to these cure criteria, the LMA-allopurinol combination promoted parasitological cure in 50% of treated animals. An additional immunohistochemistry test was performed, as described previously (28), in those tissues tested by qPCR. Strikingly, negative results were obtained in all samples collected from the six animals of this group (data not shown). Among the other five experimental groups, parasitological cure was achieved in only one animal that was treated with allopurinol.
As illustrated in Fig. 5, the proportion of cured dogs achieved after treatment with the LMA-allopurinol combination was significantly greater than that in the saline group (P < 0.05; Fisher's exact test).
Fig 5.
Proportions of dogs cured of Leishmania (L.) infantum infection after treatment with liposomal meglumine antimoniate (LMA), allopurinol (Allop), empty liposomes (LEMP), or the LMA+Allop or LEMP+Allop combination. LMA (6.5 mg Sb/kg/dose), empty liposomes (same dose of lipid), and saline (same volume) were given intravenously as six doses at 4-day intervals. Allopurinol was given at 20 mg/kg/24 h per os for 140 days, starting from the first dose of LMA. Dogs were considered cured when they showed negative results in all the parasitological tests performed on day 200, including culture from bone marrow aspirates, qPCR of bone marrow, spleen, liver, and skin, and xenodiagnosis. *, P < 0.05 according to Fisher's exact test.
DISCUSSION
With the aim of further improving the treatment efficacy of CVL with an innovative liposomal formulation of MA, a new therapeutic regimen was designed which combines this formulation with allopurinol, a well-tolerated and effective leishmaniostatic drug capable of reaching all infected sites. In comparison to our previous protocol (28), LMA was given at the same dosage (6.5 mg Sb/kg/dose) and time intervals, but animals received six doses instead of four. Thus, a more effective protocol was also expected from the more prolonged treatment with LMA. The protocol used for the administration of allopurinol (20 mg/kg/24 h) differed slightly from the conventional one in that a frequency of dosing of 12 h was used (15, 20). Animals also remained without any therapeutic intervention from day 140 to 200, to uncover possible relapse of CVL.
Analysis of the clinical, parasitological, and xenodiagnosis findings indicates at least an additive effect for LMA and allopurinol, since animals treated with this protocol presented much better clinical and parasitological profiles than those treated with each drug alone.
Among the six experimental groups, the LMA+Allop group was the one that exhibited the most pronounced improvement of clinical signs and clinicopathological parameters. However, the improvements were gradual and improvements were also observed in the groups treated with LMA or allopurinol alone. During the experimental period, most of the animals (72% ± 19%) treated with these protocols showed weight gain, complete regression or marked reduction in the number and degree of skin lesions, absence of lymphadenopathy and eye lesions, and alterations in the color of the mucous membranes (data not shown). Improvement in the clinical condition was observed until day 140 and was also accompanied by the normalization of most hemogram and serum biochemistry parameters, such as protein electrophoresis, A/G ratio, number of erythrocytes, hemoglobin concentration, hematocrit, and platelets (data not shown).
From day 140 to the end of the experimental period, a tendency toward improvement was observed only in the LMA+Allop group, suggesting that, besides reversion of the physical and clinicopathological abnormalities, the drug combination promoted a long-term action resulting in the prevention of disease relapse. In contrast, the LMA, Allop, and LEMP+Allop groups presented a slight worsening of their clinical status from day 140 to 200. This fact can be attributed to the inability of allopurinol to prevent relapse after interruption of its use (10, 27) and to the transitory positive effect of LMA on the same parameters, as previously described by our group (28).
The clinical improvement observed in the LMA+Allop group was accompanied by a significant reduction in the parasitic load, as determined by qPCR. The choice of qPCR to assess the parasite burdens in the tissues of the dogs was based on the high sensitivity and specificity of the technique for the absolute quantification of parasites (25). The LMA-allopurinol combination had the greatest impact on parasite burden, reducing by hundreds of times the numbers of parasites in bone marrow, spleen, and skin following treatment.
Importantly, treatment with the drug combination resulted in significant decreases in the parasite loads in the bone marrow and spleen on days 140 and 200 compared to the parasite loads in the pretreatment period. This is in contrast with the other groups, which did not show significant reductions in the bone marrow parasite loads on day 200. Furthermore, the drug combination reduced the parasite loads in bone marrow to a significantly greater degree than the LEMP-allopurinol protocol. These results, taken together, indicate at least additive effects of LMA and allopurinol.
The importance of the use of splenic aspirates in monitoring treatment was previously demonstrated in dogs infected with Ehrlichia canis treated with doxycycline (19). To the best of our knowledge, the present study is the first to evaluate the efficacy of treatment of CVL according to the parasite burden in the spleen by using qPCR prior to and during treatment. The use of an ultrasound device to guide the aspiration of the spleen allowed the safe collection of samples from this organ without complications such as hemorrhages or ruptures. Given the importance of the spleen in the context of CVL (31), evaluation of the parasite burden in this organ during treatment could help in monitoring relapses and response to therapeutics.
A major benefit of LMA-allopurinol combination is its ability to produce negative qPCR results in the liver of all treated dogs. This remarkable effect is most probably due to the extremely high accumulation of antimony in the liver promoted by the liposome formulation (29). In accordance with this interpretation, the LMA group showed only one animal (18.7%) with positive qPCR of liver tissue, whereas the Allop and LEMP+Allop groups presented 37.5 and 62.5% positive animals.
Because of their hematophagic behavior, sand flies need direct contact with the skin of vertebrate hosts. The skin of dogs is the site where transmission of parasites occurs, both from infected dogs to noninfected sand flies and from infected sand flies to noninfected dogs, which spreads the disease to other dogs and humans (33).
Therefore, one of the most important objectives of the treatment of CVL is the blockade of transmission to sand flies by eliminating parasites in the skin or, at least, reducing the number of parasites to such a level that transmission does not occur (24). Thus, some authors (24) have proposed reduction in the infectivity of dogs to sand flies through treatment as a key measure in control programs designed to eradicate active foci of CVL.
In our study, the capacity of L. longipalpis to be infected with L. infantum after feeding in infected dogs was investigated through xenodiagnosis. Although it is the most accurate experimental tool for assessing the epidemiologic impact of treatment of CVL, xenodiagnosis is not a simple methodology and basically is restricted to research institutions (11, 24). Since our data show a positive correlation between the xenodiagnosis result and the number of parasites in the ear skin (Spearman's correlation, 0.6518; P < 0.0001), in accordance with a recent study (5), skin qPCR may be used as an alternative protocol to assess the infectivity of dogs to the sand flies.
Both the xenodiagnosis and skin parasite load results showed that the LMA-allopurinol combination was the most effective protocol for inhibiting the transmission of skin parasites to L. longipalpis. Indeed, it promoted the blockade of transmission of parasites to sand flies on both days 140 and 200 and resulted in the highest percentage (83.3%) of dogs negative according to skin qPCR. This is in contrast with the results obtained with the LMA and LEMP+Allop groups, which both presented infective animals on day 200 and lower percentages of negative dogs (50 and 25%, respectively).
An important question to be answered is whether LMA-allopurinol combined treatment is capable of promoting parasitological cure of infected dogs. This is a crucial question for the control of visceral leishmaniasis, since no fully effective treatment has been reported so far (2, 30) and an effective treatment would block the transmission of the parasite to sand flies and humans and reduce the risk of emergence of resistance to antimony. Based on the absence of parasites in bone marrow, spleen, liver, and ear skin and negative xenodiagnosis on day 200, the LMA-allopurinol combination was found to promote parasitological cure in 50% of the dogs treated. In the other groups, cured animals were found only in the Allop group, but at a much lower rate (8%). These findings confirm our previous report that LMA alone cannot promote parasitological cure (28).
The criteria used here for parasitological cure are based on the absence of parasites in critical sites of infection and on the blockade of parasite transmission to the sand flies at a specific time. Importantly, this evaluation was performed in treated dogs after a 60-day period without treatment, to allow the occurrence of possible relapses. Thus, in the LMA+Allop group, two of five animals initially negative in bone marrow and spleen (on day 140) became positive on day 200. In this context, our claim for achievement of parasitological cure should be taken cautiously, since not all tissues were evaluated and one cannot completely exclude the possibility of other relapses after a longer period of time without treatment.
As a major advance, the present study displays for the first time a new, highly effective therapeutic alternative for CVL, based on the combination of nanotechnology and conventional therapy, with the prospect of achieving parasitological cure. In future studies, new protocols based on this combination should be designed to confirm the achievement of cure in treated dogs and to further enhance the cure rate of CVL. A higher cure rate may be expected from an increase in the number of doses of LMA, from the use of allopurinol for a more prolonged period of time, and from the improvement of the liposomal formulation for more effectively reaching less accessible sites of infection.
ACKNOWLEDGMENTS
We acknowledge the Brazilian agencies CNPq (grants 303046/2009-0, 473534/2010-0, and 473601/2009-5), FAPEMIG (grants REDE–221/08, REDE–40/11, APQ–01935-09, APQ–01355-09, PRONEX 2009, and PPM–00382-11), and CAPES for financial support. R.R.R. was the recipient of a postdoctoral fellowship from FAPEMIG.
We are also grateful to Oscar Bruna-Romero from ICB-UFMG for his helpful assistance with qPCR assays.
Footnotes
Published ahead of print 12 March 2012
REFERENCES
- 1. Alvar J, Canavate C, Molina R, Moreno J, Nieto J. 2004. Canine leishmaniasis. Adv. Parasitol. 57:1–88 [DOI] [PubMed] [Google Scholar]
- 2. Alvar J, et al. 1994. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann. Trop. Med. Parasitol. 88:371–378 [DOI] [PubMed] [Google Scholar]
- 3. Alves CF, et al. 2009. Expression of IFN-gamma, TNF-alpha, IL-10 and TGF-beta in lymph nodes associates with parasite load and clinical form of disease in dogs naturally infected with Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 128:349–358 [DOI] [PubMed] [Google Scholar]
- 4. Alving C. 1986. Liposomes as drug carriers in leishmaniasis and malaria. Parasitol. Today 2:101–107 [DOI] [PubMed] [Google Scholar]
- 5. Amorim IF, et al. 2011. Toll receptors type-2 and CR3 expression of canine monocytes and its correlation with immunohistochemistry and xenodiagnosis in visceral leishmaniasis. PLoS One 6:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balana-Fouce R, Reguera RM, Cubria JC, Ordonez D. 1998. The pharmacology of leishmaniasis. Gen. Pharmacol. 30:435–443 [DOI] [PubMed] [Google Scholar]
- 7. Baneth G, Koutinas AF, Solano-Gallego L, Bourdeau P, Ferrer L. 2008. Canine leishmaniasis—new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 24:324–330 [DOI] [PubMed] [Google Scholar]
- 8. Baneth G, Shaw SE. 2002. Chemotherapy of canine leishmaniosis. Vet. Parasitol. 106:315–324 [DOI] [PubMed] [Google Scholar]
- 9. Bretagne S, et al. 2001. Real-time PCR as a new tool for quantifying Leishmania infantum in liver in infected mice. Clin. Diagn. Lab. Immunol. 8:828–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cavaliero T, et al. 1999. Clinical, serologic, and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. J. Vet. Intern. Med. 13:330–334 [DOI] [PubMed] [Google Scholar]
- 11. da Costa-Val AP, et al. 2007. Canine visceral leishmaniasis: relationships between clinical status, humoral immune response, haematology and Lutzomyia (Lutzomyia) longipalpis infectivity. Vet. J. 174:636–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. da Silva SM, et al. 2010. First report of infection of Lutzomyia longipalpis by Leishmania (Leishmania) infantum from a naturally infected cat of Brazil. Vet. Parasitol. 174:150–154 [DOI] [PubMed] [Google Scholar]
- 13. da Silva SM, et al. 2009. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet. Parasitol. 166:159–162 [DOI] [PubMed] [Google Scholar]
- 14. Demicheli C, et al. 2003. Pentavalent organoantimonial derivatives: two simple and efficient synthetic methods for meglumine antimonate. Appl. Organomet. Chem. 17:226–231 [Google Scholar]
- 15. Denerolle P, Bourdoiseau G. 1999. Combination allopurinol and antimony treatment versus antimony alone and allopurinol alone in the treatment of canine leishmaniasis (96 cases). J. Vet. Intern. Med. 13:413–415 [DOI] [PubMed] [Google Scholar]
- 16. Frézard F, Demicheli C. 2010. New delivery strategies for the old pentavalent antimonial drugs. Expert Opin. Drug Deliv. 7:1343–1358 [DOI] [PubMed] [Google Scholar]
- 17. Frézard F, Demicheli C, Ribeiro RR. 2009. Pentavalent antimonials: new perspectives for old drugs. Molecules 14:2317–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frézard F, Michalick MS, Soares CF, Demicheli C. 2000. Novel methods for the encapsulation of meglumine antimoniate into liposomes. Braz. J. Med. Biol. Res. 33:841–846 [DOI] [PubMed] [Google Scholar]
- 19. Harrus S, et al. 2004. Comparison of simultaneous splenic sample PCR with blood sample PCR for diagnosis and treatment of experimental Ehrlichia canis infection. Antimicrob. Agents Chemother. 48:4488–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koutinas AF, et al. 2001. A randomised, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniosis. Vet. Parasitol. 98:247–261 [DOI] [PubMed] [Google Scholar]
- 21. Maia C, Campino L. 2008. Methods for diagnosis of canine leishmaniasis and immune response to infection. Vet. Parasitol. 158:274–287 [DOI] [PubMed] [Google Scholar]
- 22. Martinez S, Looker DL, Berens RL, Marr JJ. 1988. The synergistic action of pyrazolopyrimidines and pentavalent antimony against Leishmania donovani and L. braziliensis. Am. J. Trop. Med. Hyg. 39:250–255 [DOI] [PubMed] [Google Scholar]
- 23. Miró G, Cardoso L, Pennisi MG, Oliva G, Baneth G. 2008. Canine leishmaniosis—new concepts and insights on an expanding zoonosis: part two. Trends Parasitol. 24:371–377 [DOI] [PubMed] [Google Scholar]
- 24. Miró G, Galvez R, Fraile C, Descalzo MA, Molina R. 2011. Infectivity to Phlebotomus perniciosus of dogs naturally parasitized with Leishmania infantum after different treatments. Parasit. Vectors 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mortarino M, et al. 2004. Quantitative PCR in the diagnosis of Leishmania. Parassitologia 46:163–167 [PubMed] [Google Scholar]
- 26. National Institutes of Health 1985. Principles of laboratory animal care. NIH publication 85-23. National Institutes of Health, Bethesda, MD [Google Scholar]
- 27. Noli C, Auxilia ST. 2005. Treatment of canine Old World visceral leishmaniasis: a systematic review. Vet. Dermatol. 16:213–232 [DOI] [PubMed] [Google Scholar]
- 28. Ribeiro RR, et al. 2008. Reduced tissue parasitic load and infectivity to sand flies in dogs naturally infected by Leishmania (Leishmania) chagasi following treatment with a liposome formulation of meglumine antimoniate. Antimicrob. Agents Chemother. 52:2564–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schettini DA, et al. 2006. Improved targeting of antimony to the bone marrow of dogs using liposomes of reduced size. Int. J. Pharm. 315:140–147 [DOI] [PubMed] [Google Scholar]
- 30. Solano-Gallego L, et al. 2009. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 165:1–18 [DOI] [PubMed] [Google Scholar]
- 31. Strauss-Ayali D, Jaffe CL, Burshtain O, Gonen L, Baneth G. 2004. Polymerase chain reaction using noninvasively obtained samples, for the detection of Leishmania infantum DNA in dogs. J. Infect. Dis. 189:1729–1733 [DOI] [PubMed] [Google Scholar]
- 32. Travi BL, Ferro C, Cadena H, Montoya-Lerma J, Adler GH. 2002. Canine visceral leishmaniasis: dog infectivity to sand flies from non-endemic areas. Res. Vet. Sci. 72:83–86 [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization 2010. Control of the leishmaniases, p 201 Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010 Technical report series 949 World Health Organization, Geneva, Switzerland [Google Scholar]