Abstract
Ferroquine (SSR97193), a ferrocene-quinoline conjugate, is a promising novel antimalarial currently undergoing clinical evaluation. This study characterizes its pharmacokinetic properties. Young male African volunteers with asymptomatic Plasmodium falciparum infection were administered a single oral dose (n = 40) or a repeated oral dose (n = 26) given over 3 days of ferroquine in two dose-escalation, double-blind, randomized, placebo-controlled clinical trials. In addition, a food interaction study was performed in a subsample of participants (n = 16). The studies were carried out in Lambaréné, Gabon. After single-dose administration of ferroquine, dose linearity was demonstrated in a dose range of 400 to 1,200 mg for maximum mean blood concentrations ([Cmax] 82 to 270 ng/ml) and in a dose range of 400 to 1,600 mg for overall exposure to ferroquine (area under the concentration-time curve [AUC], 13,100 to 49,200 ng · h/ml). Overall mean estimate for blood apparent terminal half-life of ferroquine was 16 days and 31 days for its active and major metabolite desmethylferroquine (SSR97213). In the 3-day repeated-dose study, Cmax and overall cumulated exposure to ferroquine (AUCcum) increased in proportion with the dose from day 1 to day 3 between 400 and 800 mg. No major food effect on ferroquine pharmacokinetics was observed after single administration of 100 mg of ferroquine except for a slight delay of time to maximum blood concentration (tmax) by approximately 3 h. The pharmacokinetics of ferroquine and its active main metabolite are characterized by sustained levels in blood, and the properties of ferroquine as a partner drug in antimalarial combination therapy should be evaluated.
INTRODUCTION
The continuing development and spread of specific drug resistance against currently used antimalarials limits the projected life span of nearly all antimalarial drugs and drug combinations. Artemisinin combination therapy (ACT) has become the accepted standard of care for uncomplicated falciparum malaria worldwide. Despite high cure rates of these first-line treatments in sub-Saharan Africa, the clinical efficacy of artemisinin derivatives and partner drugs is threatened by the potential spread of specific drug resistance (26, 41, 44). New compounds and drug combinations are therefore urgently needed to avoid a dangerous shortage of potential future treatment options. Ferroquine (FQ), a ferrocenic analogue of chloroquine, is one of the most promising new candidate drugs in the antimalarial pipeline (4–7, 14).
Preclinical and first clinical experience with ferroquine.
High efficacy of ferroquine has been demonstrated against chloroquine-susceptible and -resistant P. falciparum strains in preclinical in vitro and in vivo studies (1, 13). Detailed analysis of in vitro drug activity of ferroquine did not show a relevant potential for cross-resistance between ferroquine and chloroquine or any other currently used antimalarial (21). First-in-human data of a phase Ia trial including 42 healthy Caucasian subjects confirmed a favorable pharmacokinetic (PK) profile of ferroquine in humans (unpublished data) as shown by an increase in exposure with a dose increase up to 800 mg and a long apparent terminal half-life. These data served as the basis for further clinical development. Here, we report results obtained in three clinical phase I trials assessing the pharmacokinetics of ferroquine in Gabonese volunteers. The aim of these studies was to assess the safety, tolerability, the maximal tolerated dose, and the pharmacokinetic profile of ferroquine. Safety and tolerability data have been reported recently (29), and the manuscript describes the pharmacokinetic characteristics of ferroquine when given as a single dose and as repeated doses (once daily over 3 days) and the evaluation of potential food interaction with ferroquine pharmacokinetics.
MATERIALS AND METHODS
Study design and study drug administration.
Three phase I clinical trials were conducted from April 2005 to June 2006 at the Medical Research Unit of the Albert Schweitzer Hospital in Lambaréné, Gabon (38). The epidemiological situation there is characterized by a stable transmission of Plasmodium falciparum throughout the year, with a nearly 100% predominance of chloroquine-resistant field strains since 1995 (34). Unlike the situation in other regions, such as Malawi (25), reappearance of chloroquine-sensitive strains after replacement of monotherapy by artemisinin combination treatments according to national guidelines of 2003 has been evidenced to be at best marginal until the present (20, 27).
Study protocols and study conduct complied with the recommendations of the Declaration of Helsinki (1964) and applicable amendments, with updated guidelines on Good Clinical Practice as defined by the International Conference on Harmonization and the WHO and with all national laws and regulations of Gabon. The Ethics Committee of the International Foundation of the Albert Schweitzer Hospital approved the study protocol and the additional documents. Written informed consent according to applicable standards was obtained from all volunteers prior to screening.
Study details have been described previously (29). Participants were young adult male African volunteers with asymptomatic P. falciparum infection. Characteristics of the study population are given in Table 1. Two studies were designed as single-center, sequential, ascending-dose, double-blind, randomized, placebo-controlled trials with administration of ferroquine either as a single ascending dose using 100-mg capsules (single-dose trial) or as once-daily repeated ascending doses using 100-mg and 200-mg capsules (repeated-dose trial). In the single-dose study five sequential groups were scheduled to receive ascending oral doses from 400 mg up to 1,600 mg, whereas four groups were scheduled to receive ascending oral doses of ferroquine ranging from 400 mg to 1,000 mg in the repeated-dose study. In each of the two trials eight participants per group were randomly allocated to receive either ferroquine or placebo in a 3:1 ratio. The third trial, a food interaction study, aimed to evaluate the potential of food interaction on ferroquine pharmacokinetics. This study had an open-label, fixed-dose study design, and 16 volunteers were allocated to two sequential groups of eight participants receiving a dose of 100 mg of ferroquine under either fasted or high-fat diet condition. Since all participants received the same treatment, no provision for blinding was taken. Allocation to each group was performed in the order of recruitment and according to a randomization list provided by the sponsor's biostatistics department. Only the main investigator, who was not involved in recruitment, had access to this list.
Table 1.
Demographic characteristics of participants in phase I trials of ferroquine
| Parametera | Value for the parameter by study and dose (mean ± SD [range]) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single-dose study |
Multiple-dose study |
||||||||||
| Placebo (n = 10)c | Placebo (n = 7) | FQ (mg) |
|||||||||
| 400 (n = 6) | 800 (n = 6) | 1,200 (n = 6) | 1,400 (n = 6) | 1,600 (n = 6) | 400 (n = 6) | 600 (n = 6) | 800 (n = 6) | 1,000 (n = 1) | |||
| Age (yr) | 30.5 ± 9.4 (18–44) | 32.0 ± 4.4 (27–37) | 31.2 ± 6.1 (22–38) | 30.5 ± 8.8 (20–42) | 24.3 ± 6.3 (18–34) | 26.5 ± 4.0 (19–30) | 23.6 ± 5.3 (18–33) | 30.2 ± 6.2 (25–40) | 26.2 ± 6.3 (18–35) | 24.5 ± 5.8 (19–32) | NAb |
| Height (cm) | 167.3 ± 8.7 (147–176) | 168.8 ± 7.0 (160–176) | 168.7 ± 3.9 (163–174) | 166.2 ± 7.4 (154–174) | 172.7 ± 6.7 (163–183) | 164.3 ± 5.0 (157–172) | 168.6 ± 6.5 (160–178) | 167.5 ± 5.8 (158–173) | 166.5 ± 10.5 (150–181) | 170.8 ± 9.9 (155–184) | NA |
| Weight (kg) | 66.0 ± 7.8 (55–78) | 62.8 ± 9.3 (50–71) | 64.7 ± 3.6 (58–59) | 62.7 ± 8.5 (52–75) | 65.2 ± 3.5 (61–70) | 60.6 ± 5.2 (57–71) | 63.0 ± 2.6 (59–66) | 64.8 ± 8.3 (55–75) | 59.8 ± 10.1 (50–74) | 61.3 ± 5.0 (56–70) | NA |
| BMI (kg/m2) | 23.6 ± 2.4 (20.1–27.6) | 21.9 ± 1.9 (19.5–24.3) | 22.8 ± 1.6 (19.8–24.5) | 22.7 ± 1.8 (19.6–24.8) | 21.9 ± 1.3 (19.7–23.0) | 22.5 ± 0.9 (21.9–24.0) | 22.2 ± 1.6 (20.5–25.0) | 23.3 ± 1.7 (21.3–25.3) | 21.5 ± 2.0 (19.2–24.5) | 21.1 ± 1.2 (19.8–23.3) | NA |
BMI, body mass index.
NA, not available.
n, number of participants.
Main inclusion criteria for these three studies were identical and were defined as the following: (i) provision of written informed consent, (ii) African male adult aged between 18 and 45 years and a body weight between 50 and 90 kg, (iii) P. falciparum mono-infection determined by either microscopy, rapid diagnostic test, or PCR, (iv) absence of clinical symptoms of malaria, (v) and no sign of any other disease as evidenced by electrocardiogram (ECG) recording, clinical chemistry, and vital signs. Exclusion criteria were the following: (i) history or presence of any clinically relevant disease, (ii) vaccination, (iii) recent use of an antimalarial drug or any other medication except for paracetamol, (iv) alcohol or substance abuse including heavy smoking, and (v) serologic evidence of hepatitis B surface antigen or presence of hepatitis C antibodies or anti-human immunodeficiency virus type 1 or 2 antibodies.
FQ metabolism.
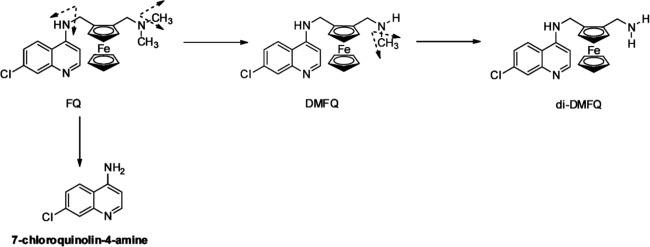
Ferroquine is metabolized by different cytochromes (CYP2C9, CYP3A, and CYP2D6); the relative contribution of each isoform involved in the overall hepatic metabolic clearance is below 50%. Based on current knowledge, ferroquine is metabolized mainly in two metabolites, N-desmethyl-ferroquine (DMFQ) and N-di-desmethyl-ferroquine (di-DMFQ) (Fig. 1). Both metabolites are active against chloroquine-susceptible P. falciparum strains, but only DMFQ showed significant activity against chloroquine-resistant P. falciparum strains (3, 11). For DMFQ a substantial contribution to overall intrinsic activity of ferroquine can therefore be assumed. In contrast, di-DMFQ is of minor pharmacodynamic and pharmacokinetic importance (11) (see Fig. 3 and 4). A pathway of ferroquine degradation results in the formation of 7-chloroquinolin-4-amine, which has no antimalarial activity and also forms in chloroquine metabolism (17) (Fig. 1). This report focuses therefore mainly on ferroquine and its major active metabolite, DMFQ (SSR97213).
Fig 1.
Pathways of ferroquine metabolism. FQ is metabolized via a major dealkylation pathway (>50% of global FQ metabolism) into N-desmethyl-ferroquine (DMFQ) and then into N-di-desmethyl-ferroquine (di-DMFQ). Another pathway results in the formation of 7-chloroquinolin-4-amine. Cytochromes identified to be involved in FQ metabolism are CYP2C9, CYP3A, and CYP2D6.
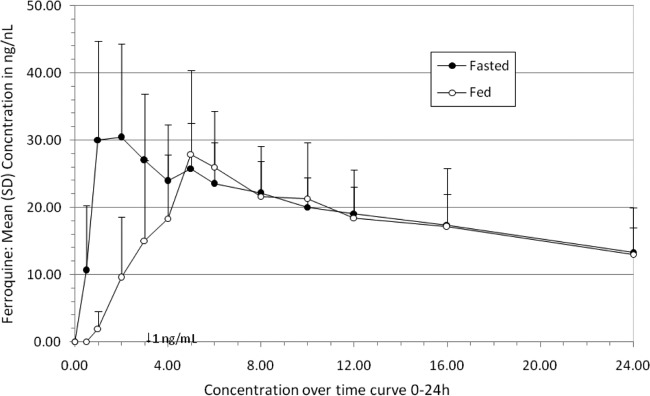
Fig 3.
Food interaction study: mean blood concentration-time profile over a 24-h period after administration of 100 mg of ferroquine as an oral single dose under fasted and fed conditions.
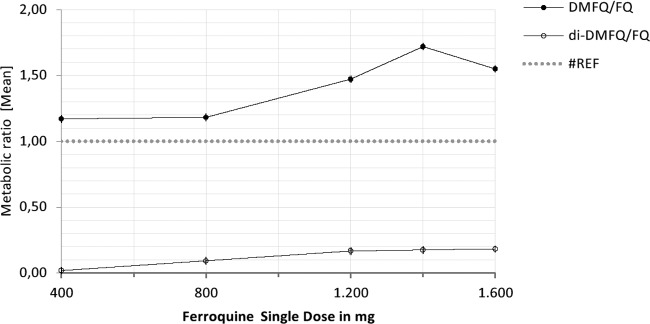
Fig 4.
Metabolite/parent compound ratio for the two main metabolites of FQ, DMFQ and di-DMFQ, after administration of a single dose of FQ in a dose range from 400 mg to 1,600 mg. Values are geometric mean.
Pharmacokinetic analysis.
Standard pharmacokinetic parameters (maximum mean blood concentration [Cmax], time between drug administration and first measurable blood concentration [tlag], time to maximum blood concentration [tmax], AUC calculated using the trapezoidal method from time zero [t0] to the last significant [greater than the limit of quantification {>LOQ}] blood concentration (Clast) time point [AUClast], [refers to the single-dose trial] AUC from 0 to 24 h [AUC0–24], AUC, AUC extrapolated to ∞, AUC0–72, AUC from day 1 to day 3, calculated using the trapezoidal method from t0 to t(Clast) [AUCcumlast] [refers to the repeated-dose trial], AUC calculated from t0 and extrapolated to ∞ [AUCcum], and apparent terminal half-life [t1/2z]) (30) of ferroquine and its metabolite DMFQ were assessed from venous blood sampling using noncompartmental analyses. Blood sampling was performed before study drug administration and hourly thereafter until 6 h postdosing. Subsequent samples were drawn every 2 h until 12 h postdosing, then at 16, 24, 36, 48, 72, 96, 144, 216, and 336 h postdosing, and finally once weekly until the end of follow-up. The total follow-up period was 8 weeks in the single- and repeated-dose studies and 4 weeks in the food interaction trial.
Blood concentrations of ferroquine and its metabolites were determined from venous blood samples. The assay was performed using high-performance liquid chromatography with tandem mass spectrometry (LC-MS/MS) after a liquid-liquid phase extraction. Precision and accuracy of the lower limit of quantification (LLOQ) as lowest validated concentration (1.0 ng/ml) was found to be within 20%; therefore the method was validated within a range from 1 to 125 ng/ml using stable isotope (SI) labeling for the tested compounds. The method has been proven to be specific and selective, with process efficiency close to 70% and without any matrix effect. Accuracy and precision values within day and between days were <20% at LLOQ and <15% at all other tested concentrations. The stability of test compounds was investigated under various storage conditions. Ferroquine and its metabolites were found to be stable in blood after three freeze-thaw cycles for at least 24 h at room temperature, 4 h at 37°C, 29 months at −80°C, and 72 h in processed samples.
Statistical methods.
Dose proportionality for Cmax and total drug exposure (area-under-the-curve parameters) was evaluated using the log-transformed power model. Estimates with 90% confidence intervals (CIs) for parameter increases associated with an r-fold (where r is the ratio of the highest to lowest dose) increase in dose were computed and were converted to a dose-normalized scale. Comparisons of apparent terminal half-lives among dose levels were analyzed by linear fixed-effect models with a fixed term for dose using log(t1/2z). Differences between doses were tested for significance with P values computed within the mixed-model framework on the log-transformed t1/2z.
Accumulation was assessed for log-transformed Cmax and AUC0-24 of ferroquine and its metabolite DMFQ by using a linear mixed-effect model with fixed terms for dose, day, and the dose-by-day interaction and a random term for within-dose patients. Within-patient and total-patient standard deviations of log-transformed Cmax and AUC0-24 values of ferroquine and its metabolite DMFQ were calculated. For Cmax and AUCs, estimates and 90% confidence intervals (CIs) for the ratios of food conditions (fed versus fasted) were obtained by computing estimates and 90% CIs for the differences between food condition means within the fixed-model framework and converting the results to ratios by antilog transformation. (Reported values are generally based on the estimates of statistical PK analysis.)
RESULTS
A total of 82 young African adults with asymptomatic P. falciparum infection were randomized and treated in these studies. In total, 40 participants (8 for each dose level) were treated with 400, 800, 1,200, 1,400, and 1,600 mg of ferroquine or placebo in a 3:1 ratio in the single-dose trial. In the repeated-dose trial, 24 participants (8 for each dose level) received either ferroquine at dose levels of 400, 600, and 800 mg or placebo.
Only two participants, one in the ferroquine arm and one in the placebo arm, were randomized in the 1,000-mg dose group. The study and further recruitment of patients were discontinued at that stage due to asymptomatic, morphological changes of T waves in ECGs observed at the 800- and 1,000-mg dose levels (29). In the food interaction trial, 16 participants (8 in each randomization arm) were allocated to receive 100 mg of ferroquine either under fasted conditions or immediately after a high-fat breakfast consisting of three eggs, 100 g of butter, and four slices of white bread.
Pharmacokinetic characteristics. (i) Single-dose trial.
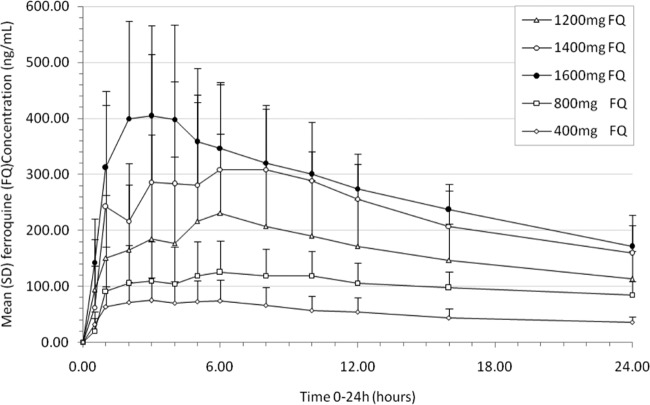
Ferroquine was rapidly absorbed, with tmax observed between 3.5 and 6.5 h after study drug administration (median values by dose). No tlag (time between drug administration and first measurable blood concentration) was observed after drug administration. Mean Cmax (maximum blood concentration) values increased from 82 to 487 ng/ml in the 400- to 1,600-mg dose groups (Table 2 and Fig. 2). Cmax increased in proportion with the dose in the range between 400 and 1,200 mg: a 3-fold increase in administered dose resulted in a 3.2-fold increase in Cmax (90% CI, 1.8- to 5.6-fold). A supradose-proportional increase was observed in the overall dose range. AUClast and AUC increased in proportion with dose in the range of 400 to 1,600 mg: a 4-fold increase in dose resulted in a 3.8-fold increase in AUClast (90% CI, 2.79- to 5.07-fold) and in a 3.63-fold increase in AUC (90% CI, 2.70- to 4.89-fold). There was no significant dose effect on t1/2z (apparent terminal half-life): the overall mean was 15.9 days (95% CI, 14.7 to 17.1 days).
Table 2.
Pharmacokinetic characteristics of FQ and its active main metabolite, DMFQ, after a single dose
| PK parameter and compound | Value for the parameter at the indicated FQ dose (mean ± SD [CV])a |
||||
|---|---|---|---|---|---|
| 400 mg | 800 mg | 1,200 mg | 1,400 mg | 1,600 mg | |
| tmax (h)b | |||||
| FQ | 6.00 (1.08–48.00) | 6.50 (1.00–16.00) | 5.52 (1.00–12.00) | 4.01 (1.00–8.00) | 3.50 (2.00–10.00) |
| DMFQ | 9.00 (3.00–36.03) | 8.00 (2.00–36.00) | 5.52 (2.00–12.00) | 4.01 (1.07–36.05) | 5.01 (2.00–16.00) |
| Cmax (ng/ml) | |||||
| FQ | 81.7 ± 38.4 (47) | 151 ± 61.6 (41) | 270 ± 189 (70) | 406 ± 189 (47) | 487 ± 115 (24) |
| DMFQ | 38.3 ± 24 (63) | 69.3 ± 32.9 (47) | 171 ± 167 (97) | 233 ± 107 (46) | 250 ± 80.7 (32) |
| AUC0–24 (ng · h/ml) | |||||
| FQ | 1,290 ± 556 (43) | 2,420 ± 848 (35) | 3,890 ± 2,190 (56) | 5,590 ± 2,130 (38) | 6,550 ± 1,360 (21) |
| DMFQ | 657 ± 436 (66) | 1,250 ± 660 (53) | 2,810 ± 2,540 (90) | 3,910 ± 1,680 (43) | 3,860 ± 944 (24) |
| AUC (ng · h/ml) | |||||
| FQ | 13,100 ± 2,740 (21) | 19,200 ± 5,950 (31) | 29,300 ± 11,300 (39) | 44,200 ± 17,900 (40) | 49,200 ± 13,900 (28) |
| DMFQ | 25,400 ± 358 (1) | 28,500 ± 10,000 (35) | 69,600 ± 59,800 (86) | 84,500 ± 27,100 (32) | 83,700 ± 18,000 (22) |
| t1/2z (days) | |||||
| FQ | 16.5 ± 3.47 (21) | 16.0 ± 2.03 (13) | 17.7 ± 5.06 (29) | 15.8 ± 2.58 (16) | 14.7 ± 2.81 (19) |
| DMFQ | 42.1 ± 28.6 (68) | 34.4 ± 20.3 (59) | 38.7 ± 16.8 (43) | 31.8 ± 8.41 (26) | 25.0 ± 3.51 (14) |
CV, coefficient of variation expressed as a percentage. Values were tabulated simultaneously.
Values for tmax are median (range).
Fig 2.
Ferroquine single-dose study mean (standard deviation) blood concentration-time profile at doses of 400, 800, 1,200, 1,400, and 1,600 mg over a 24-h period.
The total variability (as measured by the coefficient of variation expressed as a percentage) observed for exposure parameters of ferroquine was moderate to high for Cmax (ranging from 24 to 70%) and low to moderate for AUC (ranging from 21 to 40%). Low total variability was observed for t1/2z and ranged from 13 to 29%.
The active main metabolite, DMFQ, was detected from the first time point, thus indicating its rapid formation (Fig. 3). From 400 to 1,600 mg, mean Cmaxs of DMFQ increased from 38 to 250 ng/ml. Due to evidence of lack of fit, for Cmax and AUClast no statistical analysis was performed in the overall dose range. Cmax increased in proportion with dose in the range of 400 to 1,200 mg: a 3-fold increase in dose resulted in a 4.41-fold increase in Cmax (90% CI, 2.06- to 9.44-fold). AUClast increased dose proportionally in the range of 400 to 1,200 mg: a 3-fold increase in dose resulted in a 2.63-fold increase in AUClast (90% CI, 1.54-fold to 4.48-fold). There was no significant dose effect on t1/2z: the overall mean was 31.4 days (95% CI, 26.8 to 36.7 days). The metabolite/parent compound exposure ratio (calculated from AUClast) in blood ranged from 117 to 172% for DMFQ in the dose range of 400 to 1,600 mg (Fig. 4).
(ii) Repeated-dose trial.
Twenty-six participants were included in the repeated-dose study. At the respective dose levels maximal blood concentrations of ferroquine were observed between 1 and 6 h after drug administration on day 1 and between 2 and 10 h on day 3 (Table 3 and Fig. 5). Within a daily dose range of 400 to 800 mg, mean Cmaxs of ferroquine increased from 143 to 220 ng/ml on day 1 and from 230 to 387 ng/ml on day 3. Within the same dose range, Cmaxs for the active metabolite DMFQ increased from 48.5 to 130 ng/ml on day 1 and from 136 to 325 ng/ml on day 3.
Table 3.
Pharmacokinetic characteristics of FQ and its active main metabolite, DMFQ, under a 3-day regimen
| PK parameter and compound | Value for the parameter at the indicated FQ dose (mg) and day (mean ± SD [CV])a |
|||||
|---|---|---|---|---|---|---|
| 400 |
600 |
800 |
||||
| Day 1 | Day 3 | Day 1 | Day 3 | Day 1 | Day 3 | |
| tmax (h)b | ||||||
| FQ | 1.01 (1.00–12.05) | 2.00 (1.07–6.02) | 4.02 (1.00–6.00) | 7.01 (0.98–96.03) | 6.00 (3.02–8.02) | 10.01 (2.00–12.00) |
| DMFQ | 4.50 (3.00–23.2) | 7.03 (6.00–36.0) | 10.00 (2.03–23.92) | 24.02 (3.02–96.42) | 6.01 (2.00–12.00) | 10.01 (2.00–97.08) |
| Cmax (ng/ml) | ||||||
| FQ | 143 ± 87.1 (61) | 230 ± 86.6 (38) | 166 ± 89.7 (54) | 356 ± 250 (70) | 220 ± 69.8 (32) | 387 ± 105 (27) |
| DMFQ | 48.5 ± 32.2 (66) | 136 ± 79.9 (59) | 45.7 ± 32.4 (71) | 190 ± 133 (70) | 130 ± 44.1 (34) | 325 ± 83.8 (26) |
| AUC0–72 (ng · h/ml) | ||||||
| FQ | 8,850 ± 3,210 (36) | 13,300 ± 8,340 (63) | 17,400 ± 4,770 (27) | |||
| DMFQ | 5,530 ± 3,320 (60) | 6,530 ± 4,590 (70) | 13,000 ± 3,270 (25) | |||
| AUCcum (ng · h/ml) | ||||||
| FQ | 48,800 ± 14,600 (30) | 83,000 ± 39,800 (48) | 96,600 ± 30,600 (32) | |||
| DMFQ | 74,700 ± 20,800 (28) | 136,000 ± 61,000 (45) | 209,000 ± 43,300 (21) | |||
| t1/2z (days) | ||||||
| FQ | 15.4 ± 2.16 (14) | 13.9 ± 1.57 (11) | 15.7 ± 2.65 (17) | |||
| DMFQ | 24.3 ± 6.46 (27) | 28.3 ± 8.17 (29) | 32.6 ± 6.38 (20) | |||
CV, coefficient of variation expressed as a percentage. Values were tabulated simultaneously.
Values for tmax are median (range).
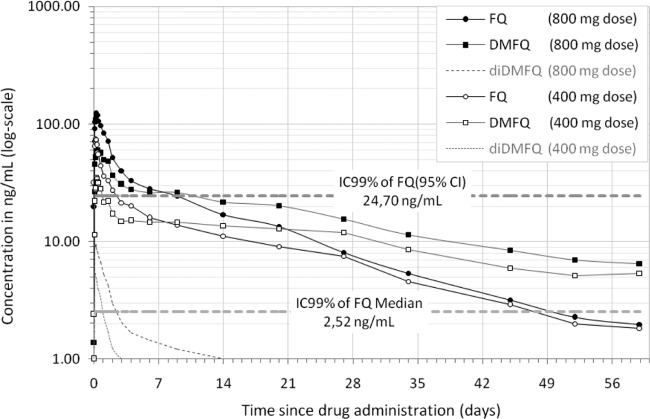
Fig 5.
Pharmacokinetic profile of ferroquine, its active metabolite, DMFQ, and di-DMFQ over 60 days after administration of 400 and 800 mg of ferroquine once daily for 3 days. Ferroquine IC99s obtained from in vitro studies (9) are overlaid.
From 400 to 800 mg of daily ferroquine intake, mean AUC0-24 values of ferroquine increased from 1,730 to 3,490 ng · h/ml and from 4,040 to 7,610 ng · h/ml on days 1 and 3, respectively. In the same dose range, mean AUC0-24 values of DMFQ increased from 920 to 2,100 ng · h/ml and from 2,740 to 6,070 ng · h/ml on days 1 and 3, respectively. Since only one participant received a 3-day repeated oral administration dose of 1,000 mg, no data are shown for this dose level.
Evidence for dose proportionality for ferroquine and for each day was observed for Cmax and AUC0-24 at a daily ferroquine dose of 400 to 800 mg. A 2-fold increase in dose resulted in 1.71- and 1.96-fold increases in Cmax (90% CIs, 1.05- to 2.78-fold and 1.29- to 2.97-fold on days 1 and 3, respectively). A 2-fold increase in dose resulted in 2.01- and 2.11-fold increases in AUC0-24 (90% CIs, 1.35- to 2.99-fold and 1.45- to 3.05-fold on days 1 and 3, respectively). Observed total-patient variability for Cmax was 59.7%. Evidence of dose proportionality for the principal metabolite was observed for Cmax and AUC0-24 on days 1 and 3 within the same dose range, between 400 and 800 mg, of ferroquine when it was administered once daily during 3 days.
The observed within-patient variability of ferroquine for AUC0-24 and Cmax was low across all dose levels, ranging from approximately 13.7% to 24.8%. The corresponding observed total-patient variability was 42.2% and 54.3%, respectively. The potential and extent of accumulation of ferroquine between day 1 and day 3 was evaluated across all dose levels: accumulation ratios of 2.25 and 1.88 (95% CI, 2.04 to 2.50 and 1.59 to 2.23, respectively) were observed for AUC0-24 and Cmax, respectively.
Mean AUC0-72, AUCcumlast, and AUCcum values of ferroquine increased from 8,850 to 17,400 ng · h/ml, 46,000 to 92,400 ng · h/ml, and 48,800 to 96,600 ng · h/ml, respectively, between 400- and 800-mg dose levels. Evidence of dose proportionality was observed for all cumulated drug exposure to ferroquine between 400 and 800 mg with a 2-fold increase in dose resulting in 1.98-, 1.96-, and 1.96-fold increases in AUC0-72, AUCcumlast, and AUCcum, respectively, with associated 90% CIs of 1.31- to 3.00-fold, 1.25- to 3.07-fold, and 1.33- to 2.89-fold, respectively. There was no significant dose effect on t1/2z: overall mean estimate was 14.9 days (95% CI, 13.8 to 16.0 days). The metabolite/parent compound exposure ratio (calculated from AUCcumlast) ranged from 118% to 196% for DMFQ in the dose range of 400 to 800 mg.
Food interaction trial.
A pilot food effect study was initiated in asymptomatic P. falciparum carriers. Based on previous PK results in healthy Caucasian volunteers, AUClast increased in proportion with the dose between 100 and 800 mg. Therefore, 100 mg was selected to investigate a possible food effect.
After a single oral dose administration of 100 mg, food intake of a high-fat breakfast delayed tmax (first time to reach Cmax) of ferroquine from 2.0 h to 5.05 h (Table 4 and Fig. 6). Under fed conditions, 6 out of 8 participants had a tlag, with an observed median value of 0.78 h. Similarly, food intake led to a decrease in Cmax by 11% and a decrease in AUClast by 7%. For DMFQ, similar decreases of Cmax (27%) and AUClast (9%) were observed.
Table 4.
Pharmacokinetic characteristics of ferroquine under fasted and fed conditions after a single dose of 100 mg
| PK parameter | Value for the parameter under the indicated condition (mean ± SD [CV])a |
|
|---|---|---|
| Fasted | Fed | |
| tlag (h)b | 0.00 (0.00–0.50) | 0.78 (0.00–3.02) |
| tmax (h)b | 2.00 (1.03–8.00) | 5.05 (5.00–10.00) |
| Cmax (ng/ml) | 33.8 ± 12.0 (36) | 29.7 ± 10.1 (34) |
| AUCO–24 (ng · h/ml) | 469 ± 119 (25) | 407 ± 170 (42) |
| AUC (ng · h/ml) | 4,420 ± 1,340 (30) | 4,860 ± 2,530 (52) |
| t1/2z (days) | 12.9 ± 3.60 (28) | 14.6 ± 6.56 (45) |
CV, coefficient of variation expressed as a percentage.
Values are median (range).
Fig 6.
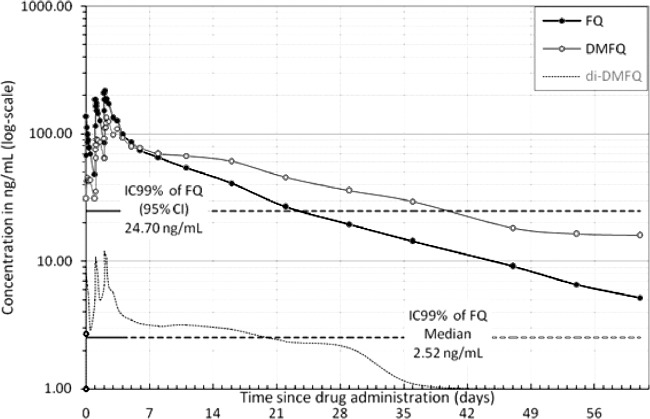
Pharmacokinetic profile of ferroquine and its main active metabolite, DMFQ, and di-DMFQ over 62 days after administration of 400 mg of ferroquine once daily for 3 days. Ferroquine IC99s obtained from in vitro studies (9) are overlaid.
Food intake did not modify bioavailability of ferroquine, and no significant food effect was observed for t1/2z after a single oral administration of 100 mg (12.9 ± 3.60 days versus 14.6 ± 6.56 days for fasted and fed, respectively). Total patient variability for Cmax was not significantly influenced by food conditions. However, the AUClast values of ferroquine and its active metabolite DMFQ were found to be higher under fed than under fasted conditions.
Pharmacodynamic results.
Presence of P. falciparum at baseline was assessed in the reported trials by PCR, rapid test, or microscopy. Due to insufficient quantitative data, no conclusions about parasite clearance could be drawn.
DISCUSSION
In this clinical phase I drug development program, ferroquine pharmacokinetics were characterized in single- and multiple-dose administration. In addition, the potential of food interaction with ferroquine pharmacokinetics was assessed. In single-dose drug administration of ferroquine, data show rapid absorption and dose-linear increases of maximal blood drug concentrations in a dose range between 400 mg and 1,200 mg. Overall maximal blood concentrations show moderate interindividual variability. Similarly, the overall estimate for AUC increases in a dose-proportional manner between 400 and 1,200 mg, with a low to moderate interindividual variability.
The estimate of the apparent terminal half-life of ferroquine was 15.9 days. However, the equally active principal metabolite DMFQ was found to have a longer t1/2z, with an overall mean estimate of 31.4 days; its blood concentration exceeded that of the parent compound approximately from day 5 (day 7 in the repeated-dose trial) onwards.
Ferroquine therefore shows a longer t1/2z than other currently used antimalarials such as lumefantrine (t1/2z ≈ 3 days) (46) or pyronaridine (t1/2z ≈14 to 18 days) (9, 40) and a similar t1/2z as slowly eliminated antimalarials such as mefloquine (t1/2z ≈14 to 21 days) (39, 42, 45) and piperaquine (t1/2z ≈12 to 33 days) (43). DMFQ, the major active metabolite of ferroquine, shows a longer t1/2z than nearly all other antimalarial compounds except chloroquine (t1/2z of >30 days) (23).
The 3-day repeated trial was discontinued after two participants at the 1,000-mg dose level experienced morphological T wave changes. Since only one patient of this group had been treated with ferroquine (29), evaluation could be performed only for a daily oral dose between 400 and 800 mg of ferroquine. For this dose range, a dose-proportional increase in exposure could be shown. Moderate total-patient variability and an accumulation ratio of around 2 after 3 days of dosing were found for ferroquine and its active major metabolite DMFQ.
In the course of the food interaction study, no major food effects on Cmax and AUC could be observed at the dose of 100 mg except for a delay of tmax by approximately 3 h. In comparison to the fasted condition, food intake had no significant influence on the overall exposure parameters Cmax and AUClast. Compared to currently recommended antimalarials, for example, artemether-lumefantrine, this pharmacokinetic property might be a clinically significant advantage since concomitant intake of a high-fat diet is notoriously difficult in acutely ill patients (24).
These pharmacokinetic data, observed in asymptomatic carriers of P. falciparum (12), compare well with previous results obtained in healthy volunteers. Comparison of main exposure parameters for Caucasian and Gabonese study participants does not show major differences between the two populations (unpublished data). Maximal blood concentrations of ferroquine at the 400- and 800-mg dose levels were similar or slightly higher in the Gabonese than in the Caucasian population (81.7 ± 38.4 ng/ml and 151 ± 61.6 ng/ml versus 74.7 ± 44.7 ng/ml and 136 ± 23.1 ng/ml, respectively). Similarly, AUCs were comparable in both populations: 13,100 ± 2,740 and 19,200 ± 5,950 ng · h/ml in Gabonese and 9,290 ± 1,610 and 19,100 ± 4,530 ng · h/ml in Caucasian participants, respectively. In the study performed in Caucasian volunteers, the in vivo erythrocyte-to-blood ratios of Cmax and AUC for ferroquine and its active metabolite, DMFQ, were approximately 2 at the 400-mg dose level, indicating high affinity to red blood cells (unpublished data).
A comparison of maximum ferroquine blood concentrations in vivo with in vitro drug activity studies on field strains of P. falciparum suggests that mean Cmax levels of ferroquine are well beyond 50% and 99% inhibitory concentration (IC50 and IC99, respectively) levels of in vitro studies conducted so far. Pooled results of growth inhibition studies in Southeast Asia and Africa exhibit a potent activity of ferroquine against both chloroquine-susceptible and -resistant P. falciparum strains (16, 19, 35, 36). One of these studies was performed in Lambaréné on chloroquine-resistant field isolates of P. falciparum and demonstrated median IC50 (4.5 ng/ml [95% CI, 1.4 to 15.6]) and IC99 (13.3 ng/ml [95% CI, 2.6 to 132.0]) values that are 51 times and 17 times, respectively, lower than Cmax values of a 3-day course of 400 mg of ferroquine (21) (Fig. 5).
Moreover, experimental selection pressure supports the expectation that the likelihood for the development of FQ resistance is poor due to the extreme high fitness cost for P. falciparum (10). Although in vitro drug activity data may not directly be extrapolated to the in vivo situation, these results indicate, at least in theory, a strong antimalarial activity.
Over the past decade antimalarial treatment policy has moved from sequential use of mono-therapy to first-line artemisinin combination Treatment (ACT) in order to slow down the evolution of drug resistance against mono-compounds (8, 18, 28, 37). The outstanding success of this strategy (2, 22, 31, 46) could decline in the near future because the currently employed artemisinin derivates are equally threatened by resistance development (15, 32) and because they exhibit particularly short half-lives and may therefore not ideally protect the partner compound in case of reinfection in high-transmission regions. In this background the identification of a partner drug which probably better matches pharmacokinetic properties of ferroquine will be of utmost importance.
In summary, based on in vitro pharmacodynamic data indicating high activity against chloroquine-susceptible and -resistant P. falciparum strains and on PK characteristics, potentially sustained activity in blood with acceptable interindividual variability may be expected at the projected dose levels. Ferroquine therefore constitutes one of the most important new antimalarial compounds, which, together with an appropriate partner drug, may potentially be qualified for the development of a single-dose antimalarial combination treatment. These promising properties, however, need further evaluation in clinical phase II and III studies.
ACKNOWLEDGMENTS
We thank the participants, the chiefs of villages and the inhabitants of Lambaréné and its surroundings. We thank the staff of the Medical Research Unit of the Albert Schweitzer Hospital, particularly Philemon Koumba Koumba, Léonce Massoussa Mbadinga, Tatiana Nymane, Virginie Mouteyi, Brigitte Migombet, Ariane Ntseyi, and Ulrich Davy Kombila.
We declare that we have no conflicts of interest.
This study was funded by the Department of Sanofi-Aventis Research as part of the development program of ferroquine.
C.S., G.M.-N., M.P.D.-B., C.L.O.S., S.I., and B.L. carried out the studies. M.R. and P.G.K. participated in the design of the study and performed statistical analysis. F.M. did the PK analysis; A.F.-A. did the assays. Hélène Albérini, Sanofi-Aventis, D.T.-M., and B.L. conceived the study and participated in its design and coordination. C.S. wrote the paper. All authors read, commented, and approved the final manuscript.
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Atteke C, et al. 2003. In vitro susceptibility to a new antimalarial organometallic analogue, ferroquine, of Plasmodium falciparum isolates from the Haut-Ogooue region of Gabon. J. Antimicrob. Chemother. 51:1021–1024 [DOI] [PubMed] [Google Scholar]
- 2. Baird JK. 2005. Effectiveness of antimalarial drugs. N. Engl. J. Med. 352:1565–1577 [DOI] [PubMed] [Google Scholar]
- 3. Barends M, Jaidee A, Khaohirun N, Singhasivanon P, Nosten F. 2007. In vitro activity of ferroquine (SSR 97193) against Plasmodium falciparum isolates from the Thai-Burmese border. Malar. J. 6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biot C, Glorian G, Maciejewski LA, Brocard JS. 1997. Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene-chloroquine analogue. J. Med. Chem. 40:3715–3718 [DOI] [PubMed] [Google Scholar]
- 5. Biot C, et al. 1999. Synthesis and antimalarial activity in vitro of potential metabolites of ferrochloroquine and related compounds. Bioorg. Med. Chem. 7:2843–2847 [DOI] [PubMed] [Google Scholar]
- 6. Biot C, Dive D. 2010. Bioorganometallic chemistry and malaria. Top. Organomet. Chem. 32:155–193 [Google Scholar]
- 7. Biot C, et al. 2011. The antimalarial ferroquine: from bench to clinic. Parasite 18:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borrmann S, et al. 2004. Fosmidomycin-clindamycin for Plasmodium falciparum infections in African children. J. Infect. Dis. 189:901–908 [DOI] [PubMed] [Google Scholar]
- 9. Chang C, Lin-Hua T, Jantanavivat C. 1992. Studies on a new antimalarial compound: pyronaridine. Trans. R. Soc. Trop. Med. Hyg. 86:7–10 [DOI] [PubMed] [Google Scholar]
- 10. Daher W, et al. 2006. Assessment of Plasmodium falciparum resistance to ferroquine (SSR97193) in field isolates and in W2 strain under pressure. Malar. J. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daher W, et al. 2006. In vitro metabolism of ferroquine (SSR97193) in animal and human hepatic models and antimalarial activity of major metabolites on Plasmodium falciparum. Drug Metab. Dispos. 34:667–682 [DOI] [PubMed] [Google Scholar]
- 12. Dal-Bianco MP, et al. 2007. High prevalence of asymptomatic Plasmodium falciparum infection in gabonese adults. Am. J. Trop. Med. Hyg. 77:939–942 [PubMed] [Google Scholar]
- 13. Delhaes L, et al. 2001. In vitro and in vivo antimalarial activity of ferrochloroquine, a ferrocenyl analogue of chloroquine against chloroquine resistant malaria parasites. Parasitol. Res. 87:239–244 [DOI] [PubMed] [Google Scholar]
- 14. Domarle O, et al. 1998. In vitro antimalarial activity of a new organometallic analog, ferrocene-chloroquine. Antimicrob. Agents Chemother. 42:540–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dondorp AM, et al. 2010. Artemisinin resistance: current status and scenarios for containment. Nat. Rev. Microbiol. 8:272–280 [DOI] [PubMed] [Google Scholar]
- 16. Dubar F, et al. 2011. The antimalarial ferroquine: role of the metal and intramolecular hydrogen bond in activity and resistance. ACS Chem. Biol. 6:275–287 [DOI] [PubMed] [Google Scholar]
- 17. Ducharme J, Farinotti R. 1996. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin. Pharmacokinet. 31:257–274 [DOI] [PubMed] [Google Scholar]
- 18. Grobusch M, et al. 2007. Intermittent preventive treatment against malaria in infants in Gabon–a randomized, double-blind, placebo-controlled trial. J. Infect. Dis. 196:1595–1602 [DOI] [PubMed] [Google Scholar]
- 19. Henry M, et al. 2008. In vitro activity of ferroquine is independent of polymorphisms in transport protein genes implicated in quinoline resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 52:2755–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang H, Joy DA, Furuya T, Su X-Z. 2006. Current understanding of the molecular basis of chloroquine-resistance in Plasmodium falciparum. J. Postgrad. Med. 52:271–276 [PubMed] [Google Scholar]
- 21. Kreidenweiss A, Kremsner PG, Dietz K, Mordmüller B. 2006. In vitro activity of ferroquine (SAR97193) is independent of chloroquine resistance in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 75:1178–1181 [PubMed] [Google Scholar]
- 22. Kremsner PG, Krishna S. 2004. Antimalarial combinations. Lancet 364:285–294 [DOI] [PubMed] [Google Scholar]
- 23. Krishna S, White NJ. 1996. Pharmacokinetics of quinine, chloroquine and amodiaquine. Clin. Pharmacokinet. 30:263–299 [DOI] [PubMed] [Google Scholar]
- 24. Kurth F, et al. 2010. Do paediatric drug formulations of artemisinin combination therapies improve the treatment of children with malaria? A systematic review and meta-analysis. Lancet Infect. Dis. 10:125–132 [DOI] [PubMed] [Google Scholar]
- 25. Laufer M, et al. 2006. Return of chloroquine antimalarial efficacy in Malawi. N. Engl. J. Med. 355:1959–1966 [DOI] [PubMed] [Google Scholar]
- 26. Le Bras J, Durand R. 2003. The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum. Fundam. Clin. Pharmacol. 17:147–153 [DOI] [PubMed] [Google Scholar]
- 27. Lehners NN. 2010. Return of chloroquine sensitive strains of Plasmodium falciparum in Lambaréné, Gabon. PhD thesis Eberhard Karls Universität, Tübingen, Germany [Google Scholar]
- 28. Metzger W, Mordmüller B, Graninger W, Bienzle U, Kremsner PG. 1995. Sulfadoxine/pyrimethamine or chloroquine/clindamycin treatment of Gabonese school children infected with chloroquine resistant malaria. J. Antimicrob. Chemother. 36:723–728 [DOI] [PubMed] [Google Scholar]
- 29. Mombo-Ngoma G, et al. 2011. Phase I randomized dose-ascending placebo-controlled trials of ferroquine—a candidate anti-malarial drug—in adults with asymptomatic Plasmodium falciparum infection. Malar. J. 10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2002. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 55:601–607 [DOI] [PubMed] [Google Scholar]
- 31. Myint HY, et al. 2004. A systematic overview of published antimalarial drug trials. Trans. R. Soc. Trop. Med. Hyg. 98:73–81 [DOI] [PubMed] [Google Scholar]
- 32. Noedl H. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620 [DOI] [PubMed] [Google Scholar]
- 33. Reference deleted.
- 34. Philipps J, Radloff PD, Wernsdorfer W, Kremsner PG. 1998. Follow-up of the susceptibility of Plasmodium falciparum to antimalarials in Gabon. Am. J. Trop. Med. Hyg. 58:612–618 [DOI] [PubMed] [Google Scholar]
- 35. Pradines B, et al. 2001. Ferrocene-chloroquine analogues as antimalarial agents: in vitro activity of ferrochloroquine against 103 Gabonese isolates of Plasmodium falciparum. J. Antimicrob. Chemother. 48:179–184 [DOI] [PubMed] [Google Scholar]
- 36. Pradines B, et al. 2002. In vitro activities of ferrochloroquine against 55 Senegalese isolates of Plasmodium falciparum in comparison with those of standard antimalarial drugs. Trop. Med. Int. Health 7:265–270 [DOI] [PubMed] [Google Scholar]
- 37. Ramharter M, et al. 2005. Artesunate-clindamycin versus quinine-clindamycin in the treatment of Plasmodium falciparum. Clin. Infect. Dis. 40:1777–1784 [DOI] [PubMed] [Google Scholar]
- 38. Ramharter M, et al. 2007. History and perspectives of medical research at the Albert Schweitzer Hospital in Lambaréné, Gabon. Wien. Klin. Wochenschr. 119(Suppl 3):8–12 [DOI] [PubMed] [Google Scholar]
- 39. Ramharter M, et al. 2007. Pharmacokinetics of two paediatric artesunate mefloquine drug formulations in the treatment of uncomplicated falciparum malaria in Gabon. J. Antimicrob. Chemother. 60:1091–1096 [DOI] [PubMed] [Google Scholar]
- 40. Ramharter M, et al. 2008. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J. Infect. Dis. 198:911–919 [DOI] [PubMed] [Google Scholar]
- 41. Ramharter M, Wernsdorfer WH, Kremsner PG. 2004. In vitro activity of quinolines against Plasmodium falciparum in Gabon. Acta Trop. 90:55–60 [DOI] [PubMed] [Google Scholar]
- 42. Simpson JA, et al. 2000. Mefloquine pharmacokinetic-pharmacodynamic models: implications for dosing and resistance. Antimicrob. Agents Chemother. 44:3414–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tarning J, et al. 2005. Pitfalls in estimating piperaquine elimination. Antimicrob. Agents Chemother. 49:5127–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uhlemann AC, Ramharter M, Lell B, Kremsner PG, Krishna S. 2005. Amplification of Plasmodium falciparum multidrug resistance gene 1 in isolates from Gabon. J. Infect. Dis. 192:1830–1835 [DOI] [PubMed] [Google Scholar]
- 45. White NJ. 1996. The treatment of malaria. N. Engl. J. Med. 335:800–806 [DOI] [PubMed] [Google Scholar]
- 46. WHO 2010. Guidelines for the treatment of Malaria, 2nd ed World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2010/en/index.html [Google Scholar]