Abstract
In vivo pharmacokinetics are often evaluated in only one variation of an infection model, and the resulting exposures are assumed to be similar in each model. We evaluated and compared the effect of lung infection and immune status on the murine pharmacokinetics and pulmonary disposition of tedizolid and linezolid. Both factors resulted in differing blood and pulmonary exposure profiles, with similar trends for tedizolid and linezolid. These data highlight the importance of pharmacokinetic confirmation in each model.
TEXT
Tedizolid (formally torezolid), the active moiety of tedizolid phosphate, is a novel oxazolidinone with activity against Gram-positive pathogens (6, 9), including methicillin-resistant Staphylococcus aureus. Since linezolid is the only FDA-approved oxazolidinone, there has been much interest in comparing the in vitro and in vivo efficacy of these two oxazolidinones against clinically relevant pathogens. When assessing the in vivo pharmacodynamics of antimicrobials, neutropenic and immunocompetent infection models are often used to evaluate the degree of antibacterial activity of a given regimen, as well as the impact of the host immune system on bacterial clearance. Frequently, the drug exposures are evaluated in only one variation of the infection model and it is assumed that the pharmacokinetic profile is similar in each of these potential variations. Without pharmacokinetic confirmation, exposure disparities rather than the host immune system may actually be responsible for any differences in efficacy, leading to inaccurate comparisons between models and/or the compounds under investigation. In this study, we sought to evaluate and compare the effect of lung infection and immune status on the pharmacokinetics and pulmonary disposition of tedizolid and linezolid.
Analytical grade tedizolid phosphate (lot 9AK0017E; Albany Molecular Research, Inc., Albany, NY) and linezolid (lot 0014; Pfizer, Inc., Groton, CT) were used for the in vivo analyses. Immediately prior to each in vivo experiment, each antimicrobial was weighed, reconstituted, and further diluted in appropriate diluents to achieve the desired concentration. Each solution was stored under refrigeration and discarded 24 h after reconstitution. Specific-pathogen-free, female BALB/c mice weighing approximately 20 g each were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN), and utilized throughout these experiments. The study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. Animals were maintained and used in accordance with National Research Council recommendations and provided food and water ad libitum.
The neutropenic pneumonia model has been well described previously (2, 3, 4). Briefly, mice were rendered transiently neutropenic by intraperitoneal injections of cyclophosphamide (Baxter, Deerfield, IL) 250 mg/kg and 100 mg/kg given 4 days and 1 day, respectively, prior to inoculation. Six hours prior to the initiation of antimicrobial therapy, isoflurane-anesthetized mice were held upright and orally inoculated with 0.05 ml of a 107 CFU/ml suspension of S. aureus 156 in 3% mucin (Sigma-Aldrich, St. Louis, MO). Inocula were administered directly into the buccal cavity of the mice, and their nares were blocked to induce aspiration. Mice utilized in the immunocompetent studies underwent the same procedure as neutropenic mice but without the use of cyclophosphamide prior to inoculation with an inoculum of 109 CFU/ml. For the uninfected mice, no procedures were performed prior to dose administration.
Single doses of tedizolid 8.4 mg/kg or linezolid 60 mg/kg were administered to the mice, as these doses have been shown to simulate humanized plasma exposures (1, 7, 8). Blood and bronchoalveolar lavage (BAL) fluid were collected from groups of six mice at 1, 2, 4, 8, and 12 h after the dose for both compounds with the additional time point of 24 h for tedizolid in each of the murine models. Plasma (tedizolid) or serum (linezolid) samples, hereinafter referred to as blood, were separated by centrifugation and stored at −80°C until analysis. Concentrations were analyzed using validated liquid chromatography-tandem mass spectrometry (LC–MS-MS) and high performance liquid chromatography (HPLC) assays for tedizolid and linezolid, respectively. The protein binding values for tedizolid and linezolid were 85% and 30% (1, 5, 8), respectively, and the area under the free-drug concentration-time curve (fAUC) for both regimens was calculated using the trapezoidal rule. Differences in exposures were compared using a one-way analysis of variance test followed by the Tukey multiple-comparison post hoc test. Portions of blood and BAL fluid were tested for their urea concentrations by a commercially available urea assay (TecoDiagnostics, Anaheim CA). The drug concentrations in epithelial lining fluid (ELF) were calculated from the following formula: ELF concentration = BAL fluid concentration × (blood urea concentration/BAL fluid urea concentration) (4, 10).
Pharmacokinetic exposures and relative penetration ratios are presented in Table 1. The concentration-time profiles in blood for each agent for all model conditions are shown in Fig. 1. The blood pharmacokinetic exposures for both tedizolid and linezolid were consistently the highest in the immunocompetent model and the lowest in the uninfected model. Similarly, ELF exposures for both drugs were highest in the immunocompetent animals. Overall, tedizolid had enhanced ELF penetration for all three models in comparison with linezolid. Additionally, the presence of infection improved penetration for tedizolid (penetration ratio, 9.32 to 10.62 for the infected versus 6.13 for the uninfected model), whereas linezolid had enhanced penetration in the uninfected animals (penetration ratio, 0.87 to 1.28 for the infected versus 1.72 for the uninfected model).
Table 1.
Pharmacokinetic and relative penetration ratios for single doses of tedizolid and linezolid in the murine pneumonia model under various conditionsa
| Drug | Model | Blood fAUCb | ELF AUC | ELF penetration ratio |
|---|---|---|---|---|
| Tedizolid | Immunocompetent | 4.7 (0.1)c | 43.9 (2.8)c | 9.34 |
| Neutropenic | 3.35 (0.1)c | 35.6 (0.2)c | 10.63 | |
| Uninfected | 2.77 (0.1)c | 17.0 (0.1)c | 6.14 | |
| Linezolid | Immunocompetent | 115.9 (44.0)d | 155.8 (14.2)d | 1.34 |
| Neutropenic | 53.4 (4.7) | 61.4 (17.9) | 1.15 | |
| Uninfected | 36.3 (0.9) | 62.5 (17.7) | 1.72 |
Dosages were 8.4 mg/kg for tedizolid and 60 mg/kg for linezolid. AUC (area under the concentration-time profile) data are reported as means, with standard deviations in parentheses. ELF, epithelial lining fluid.
For linezolid, the fAUC (area under the free-drug concentration-time profile) from 0 to 12 h was determined, and for tedizolid, the fAUC from 0 to 24 h was determined.
The AUC was significantly different than in other models (P < 0.001).
The AUC in the immunocompetent model was significantly different than in the neutropenic and uninfected models (P < 0.001).
Fig 1.
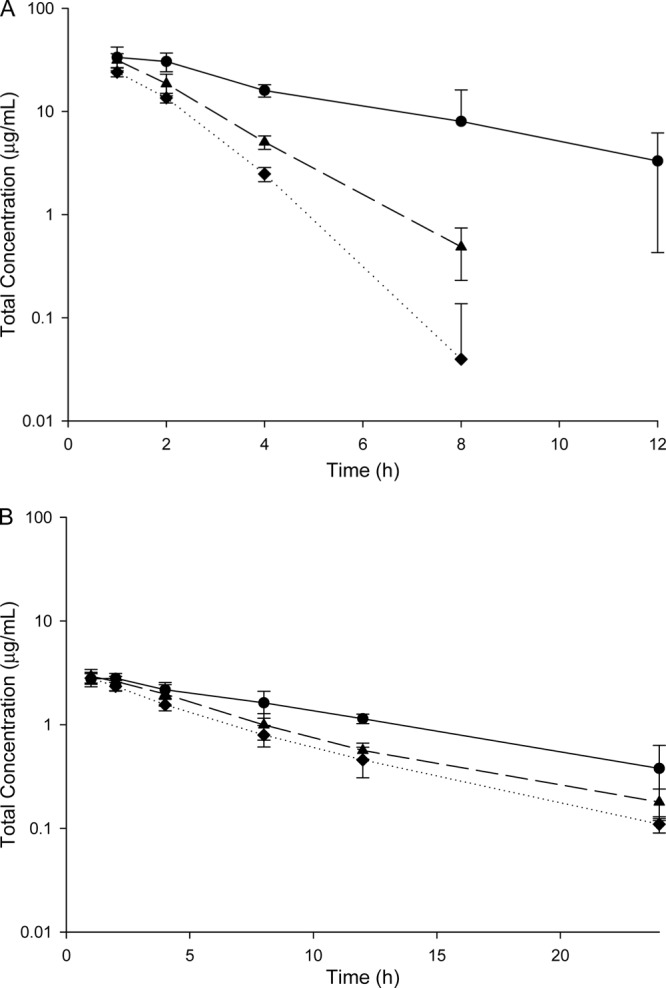
(A) Serum concentration-time profile for a single dose of linezolid 60 mg/kg in the immunocompetent (circles), neutropenic (triangles), and uninfected (diamonds) murine model. (B) Plasma concentration-time profile for a single dose of tedizolid 8.4 mg/kg in the immunocompetent (circles), neutropenic (triangles), and uninfected (diamonds) murine model.
While these dosing regimens of tedizolid and linezolid may achieve similar blood exposures to humans in the neutropenic BALB/c mouse, substantially higher exposures were attained in the immunocompetent model and lower exposures in uninfected mice. For tedizolid, exposures in the immunocompetent and uninfected models were 41% higher and 17% lower than what was observed in the neutropenic model. As for linezolid, more pronounced trends were noted, with exposures increased by 117% in the immunocompetent model and decreased by 32% in the uninfected model.
While it is not evident mechanistically why these exposure differences occurred between the models, the trend was consistent for both agents. As seen in Fig. 1, the differences among the models were seemingly due to a change in the rate of elimination rather than changes in the volume of distribution. If this change in elimination were predicted by the presence of infection, one would expect the immunocompetent and neutropenic models to have similar blood exposures; if it were due to a drug interaction with cyclophosphamide, similar exposures would be expected in immunocompetent and uninfected mice. Since neither of these obvious scenarios occurred, other unidentified factors or a combination of these factors appear responsible for these exposure disparities.
Immune status and the presence of lung infection resulted in discordances in the blood and pulmonary profiles of tedizolid and linezolid. Any assumptions made regarding similar pharmacokinetic exposures and drug disposition between variations of the same model would lead to inaccurate efficacy comparisons if these dosing regimens were employed in these models. These substantial differences in exposures due to the murine model used emphasize the importance of pharmacokinetic confirmation in each murine model utilized.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicus and Christina Sutherland for analytical determination. Additionally, we thank Henry Christensen, Mao Hagihara, Seth Housman, Debora Santini, Pamela Tessier, Lindsay Tuttle, and Dora Wiskirchen for their assistance with the animal experimentation.
This work was supported by Trius Pharmaceuticals (San Diego, CA).
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Andes D, van Ogtrop ML, Peng J, Craig WA. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crandon JL, Kuti JL, Nicolau DP. 2010. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob. Agents Chemother. 54:5115–5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koomanachai P, Crandon JL, Banevicius MA, Peng L, Nicolau DP. 2009. Pharmacodynamic profile of tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob. Agents Chemother. 53:5060–5063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laohavaleeson S, Tessier PR, Nicolau DP. 2008. Pharmacodynamic characterization of ceftobiprole in experimental pneumonia caused by phenotypically diverse Staphylococcus aureus strains. Antimicrob. Agents Chemother. 52:2389–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. La Plante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models 52:2156–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Locke JB, Hilgers M, Shaw KJ. 2009. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and tedizolid (TR-700). Antimicrob. Agents Chemother. 53:5265–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of tedizolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. Antimicrob. Agents Chemother. 55:3453–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pichereau S, et al. 2009. Comparative pharmacodynamics of novel oxazolidinone, tedizolid phosphate (TR-701), against S. aureus in the neutropenic murine pneumonia model, abstr A1-1939. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 9. Prokocimer P, et al. 2011. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral tedizolid phosphate in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 55:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziglam HM, Baldwin DR, Daniels I, Andrew JM, Finch RG. 2002. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 50:1011–1015 [DOI] [PubMed] [Google Scholar]


