Abstract
The carbapenems imipenem and meropenem in combination with clavulanic acid reduced the bacterial burden in Mycobacterium tuberculosis-infected macrophages by 2 logs over 6 days. Despite poor stability in solution and a short half-life in rodents, treatment of chronically infected mice revealed significant reductions of bacterial burden in the lungs and spleens. Our results show that meropenem has activity in two in vivo systems, but stability and pharmacokinetics of long-term administration will offer significant challenges to clinical evaluation.
TEXT
The burden of tuberculosis (TB) continues to rise with significant increases in drug resistance in the developing world (19). Rising rates of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB continue primarily due to inadequate treatment programs, poor laboratory capacity, and the lack of public health infrastructures needed to manage the disease (19). Demonstration of activity against Mycobacterium tuberculosis in vitro does not guarantee in vivo potency because of differences in the microenvironment within which bacteria reside (16). Recently, the combination of meropenem with clavulanic acid (clavulanate) was shown to be active in vitro against Mycobacterium tuberculosis, including against MDR and XDR isolates (9). This suggested the possibility of repurposing carbapenems in combination with β-lactamase inhibitors to treat MDR and XDR TB. β-Lactams were previously thought to be ineffective against M. tuberculosis, primarily due to the endogenous mycobacterial BlaC enzyme which effectively hydrolyzes them; however, clavulanic acid is effective in inactivating this enzyme (8, 9, 14, 18). The in vitro MIC of the meropenem-clavulanate combination was less than 1 μg/ml and resulted in sterilization of aerobically grown cultures within 14 days (9).
Carbapenems are the most potent β-lactams and were developed in the 1980s to enhance resistance to β-lactamases (4, 11). Meropenem is a broad-spectrum carbapenem active against several clinically relevant Gram-positive and Gram-negative aerobes and anaerobes (4). The bactericidal activity of meropenem results from the inhibition of cell wall synthesis through the inactivation of penicillin-binding proteins (4, 20). Carbapenems are not very hydrolytically stable, limiting drug administration to a controlled intravenous infusion (2). Meropenem is FDA approved for the treatment of complicated skin and soft tissue infections, intra-abdominal infections (appendicitis and peritonitis), and bacterial meningitis (1). Clavulanic acid is FDA approved as a β-lactamase inhibitor often administered with amoxicillin (the combination is marketed as Augmentin) to prevent hydrolysis of the active β-lactam (5).
MIC and minimal bactericidal concentration (MBC) values for various carbapenems (meropenem, doripenem, faropenem, ertapenem, and imipenem) in combination with clavulanic acid were determined against M. tuberculosis H37Rv and the virulent Beijing strain used for rabbit infections, HN878 (15). All of these carbapenems were highly active when combined with clavulanic acid, with MICs ranging from 0.23 to 0.84 μM and MBC99s ranging from 0.9 to 3.3 μM for both strains. It was previously established by Cuffini et al. that meropenem penetrates macrophages and achieves intracellular concentrations high enough for active killing of intraphagocytic pathogens like Staphylococcus aureus (3). In addition, plasma protein binding is reported to be only 2% (7); therefore, binding to albumin in fetal bovine serum (FBS) supplemented with Dulbecco's modified Eagle medium (DMEM) is not expected to be a significant factor. Meropenem has been reported to be thermally unstable in aqueous solutions (10, 12, 17); therefore, we determined the stability of meropenem and the other carbapenems at 37°C in water, 7H9 broth medium, and the medium used with the infected macrophages in the susceptibility assay (DMEM) by liquid chromatography (LC)-mass spectrometry (MS) using a Luna NH2 column with a single quadrupole mass-selective detector (Agilent MSD, model G1946DSL). Additional details for this and other experiments can be found in the supplemental material. Meropenem was significantly less stable in DMEM than in either water or 7H9 medium, with a half-life (t1/2) of 8 h in DMEM compared to 38 h in 7H9 and >80 h in water. Other carbapenems had similarly short half-lives, ranging from 3 to 10 h.
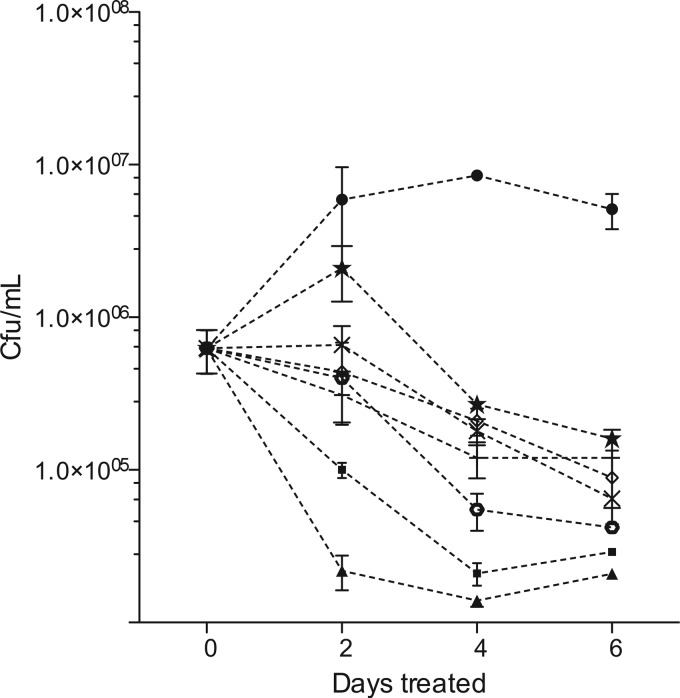
Because of this short half-life, we examined intracellular killing by administering carbapenems to infected macrophages 3 times a day (every 8 h) with medium changes every 2 days. No evidence of cell toxicity was found under these conditions, even at concentrations as high as 400 μg/ml of these carbapenems in combination with clavulanic acid up to 200 μM (data not shown). Murine J774A.1 macrophages were then infected with H37Rv at a multiplicity of 1 to 2 bacilli per cell and subsequently treated with various carbapenems at concentrations equivalent to the known maximum concentrations of drug in serum (Cmax) in humans receiving standard therapeutic doses (50 μg/ml for meropenem, imipenem, and ertapenem, 30 μg/ml for doripenem, and 10 μg/ml for faropenem). Two control drugs, rifampin and isoniazid, were also dosed once a day at the human-equivalent Cmax (5 μg/ml) and also had their medium replaced on days 2 and 4. Treated macrophages were lysed at the indicated times, and serial dilutions were plated for enumeration of CFU (Fig. 1). These data showed significant growth arrest of intracellular H37Rv by 2 days (P = 0.05) and highly significant killing with all carbapenems by 4 and 6 days (P = 0.01 and 0.001 for meropenem, for example, at 4 and 6 days, respectively). At 6 days, the carbapenems demonstrated 1.5- to 2.0-log reductions in bacterial numbers compared to those of untreated controls, with imipenem and meropenem having the largest effect. For comparison, isoniazid and rifampin controls demonstrated a 2-log kill over the same time period.
Fig 1.
Intracellular susceptibility of H37Rv. β-Lactams evaluated in combination with 200 μM clavulanic acid demonstrated killing of intracellular M. tuberculosis. ●, untreated controls; ■, rifampin; ▲, isoniazid; ×, meropenem; ♢, faropenem; +, doripenem; ★; ertapenem; ○, imipenem. Imipenem was most active at killing, with a 2.0-log reduction of bacilli compared to that of untreated controls. By day 2, all of the drug-treated groups were significantly different from the control (P < 0.05, repeated-measures ANOVA). By days 4 and 6, this difference became even more significant (P < 0.01 and P < 0.001, respectively, for all drug treatments compared with the control).
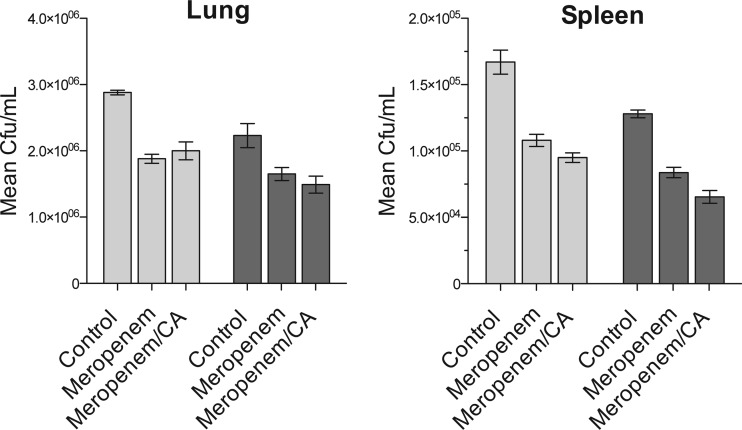
To examine efficacy in an animal model, we infected 30 C57BL/6 mice with M. tuberculosis H37Rv and allowed the infection to progress to a chronic stage. Three months after infection, the mice were divided into three groups of 10 and therapy was initiated. One group was treated with meropenem alone at 300 mg/kg of body weight by subcutaneous injection twice daily, a second group received meropenem at the same dose but was additionally given twice-daily 50-mg/kg oral doses of clavulanic acid, and the final group received vehicle control treatment (phosphate-buffered saline [PBS]). Five mice from each treatment group were sacrificed 2 weeks later, with the remaining five sacrificed at 4 weeks of treatment, and bacterial burdens in both lung and spleen were enumerated. The data in Fig. 2 illustrate that meropenem did, in fact, show activity in this very stringent chronic mouse model. Compared to the control group, both meropenem alone and meropenem in combination with clavulanic acid significantly reduced numbers of CFU in both lung and spleen after 2 weeks of treatment (P = 0.008 and P = 0.002, respectively, in an analysis of variance [ANOVA]), with a further modest reduction in bacterial burden observed after 4 weeks of therapy. Clavulanic acid did not show a statistically significant enhancement of killing in the lungs or spleen at either time point.
Fig 2.
Mouse efficacy of the study. Chronically M. tuberculosis-infected mice were treated with 300 mg/kg meropenem, 300 mg/kg meropenem with 50 mg/kg clavulanic acid (CA), or PBS (control) for 2 (light gray bars) or 4 (dark gray bars) weeks. Treatment with meropenem or meropenem and clavulanic acid for either 2 or 4 weeks reduced the bacterial burdens in the lungs (P = 0.008) and spleen (P = 0.002).
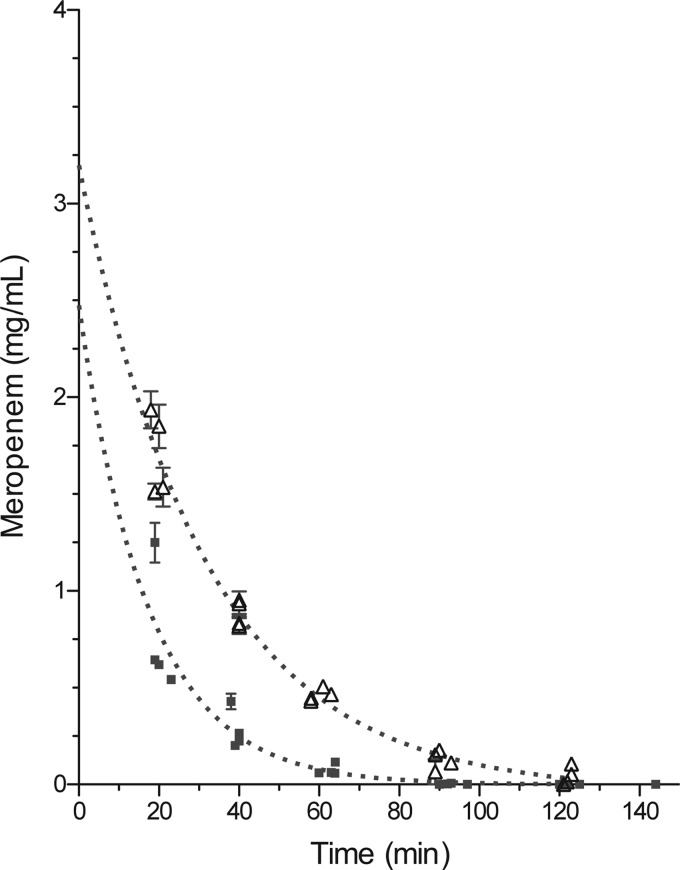
We also planned to conduct an efficacy study in TB-infected New Zealand White rabbits, which develop hypoxic pulmonary lesions (16); therefore, we determined pharmacokinetic (PK) parameters and tolerability in uninfected rabbits for meropenem-clavulanate at doses of 75 mg/kg and 125 mg/kg, respectively. Drugs were delivered sequentially by intravenous (i.v.) bolus in four rabbits. Blood was drawn at scheduled time points, and plasma was obtained for determination of serum concentrations. Data obtained from the four animals were pooled and evaluated using GraphPad Prism 5.0 with a single decay curve and area under the curve (AUC) analyses. The PK plots are representations of pooled data from all four animals (Fig. 3). The mean extrapolated initial concentration after i.v. bolus (C0), t1/2, and AUC were 2.5 mg/ml, 12.1 min, and 15.2 mg-min/ml, respectively, for meropenem-clavulanic acid coadministration. Because of this relatively low exposure, we also repeated this PK study with the addition of cilastatin to prevent hydrolysis of meropenem by renal dihydropeptidase 1 (DHP-1). Treatment of rabbits with cilastatin (75 mg/kg) increased exposure, and the corresponding PK parameters were 3.2 mg/ml, 22.0 min, and 51.5 mg-min/ml for C0, t1/2, and AUC, respectively. Cilastatin therefore increased the exposure by 4 times even though the half-life was not significantly increased. Unfortunately, the increased exposure caused severe diarrhea that resulted in the death of 2 of the 4 animals 48 h after drug exposure. Due to rapid adverse effects in the digestive tract of the rabbit, efficacy in this species was no longer pursued.
Fig 3.
Pharmacokinetic analysis of meropenem-clavulanic acid in New Zealand White rabbits. Plasma concentration as a function of time was plotted using nonlinear single-decay-curve analysis with AUC data analysis. ■, meropenem (75 mg/kg)-clavulanic acid (50 mg/kg); △, meropenem (75 mg/kg)-clavulanic acid (50 mg/kg)-cilastatin (75 mg/kg).
Fukasawa et al. reported that humans have a significantly lower activity of DHP-1 compared to that of other species and that rabbits had a relatively high degree of enzymatic activity (6). Therefore, we hoped that coadministration of cilastatin would substantially reduce renal hydrolysis and help maintain the levels of the active compound in circulation. It is noted that meropenem is considered more stable to DHP-1 hydrolysis than imipenem due to its 1β methyl side chain and the steric hindrance associated with the secondary amine of the C-2 side chain. No information regarding the other carbapenems and DHP-1 hydrolysis in rabbits was identified at this time. Similar complications were reported by Topham et al. for meropenem administered in doses above 125 mg/kg (13). The use of a nonhuman primate model was considered as an alternative; however, according to Topham et al.'s study, cynomolgus monkeys that received seven daily intravenous doses of meropenem or imipenem (180 or 500 mg/kg, respectively) also had episodes of diarrhea in addition to emesis shortly after dosing. Those monkeys also experienced a reduction in body weight of 12.5 to 25%, accompanied by a decreased consumption of food and water, making further therapeutic studies for the treatment of tuberculosis using meropenem or imipenem combinations with clavulanic acid less approachable.
In summary, this report demonstrates that the meropenem-clavulanic acid combination exhibits bactericidal activity in macrophages and modest activity in murine models of disease. Further assessment in animal models is complicated by the comparatively high activity of the renal DHP-1 enzyme in all of the animal models used for experimental chemotherapy of TB. Additional animal studies that utilize controlled infusions of meropenem (as is done clinically for human use) may provide more efficacy information. The human isoform of DHP-1 has comparably lower activity, and these studies suggest that prospective clinical evaluation in humans with chronic, nonresponsive disease is warranted.
Supplementary Material
Footnotes
Published ahead of print 26 March 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Baldwin CM, Lyseng-Williamson KA, Keam SJ. 2008. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs 68:803–838 [DOI] [PubMed] [Google Scholar]
- 2. Bax RP, et al. 1989. The pharmacokinetics of meropenem in volunteers. J. Antimicrob. Chemother. 24(Suppl A):311–320 [DOI] [PubMed] [Google Scholar]
- 3. Cuffini AM, et al. 1993. The entry of meropenem into human macrophages and its immunomodulating activity. J. Antimicrob. Chemother. 32:695–703 [DOI] [PubMed] [Google Scholar]
- 4. Edwards JR. 1995. Meropenem: a microbiological overview. J. Antimicrob. Chemother. 36(Suppl A):1–17 [DOI] [PubMed] [Google Scholar]
- 5. Finlay J, Miller L, Poupard JA. 2003. A review of the antimicrobial activity of clavulanate. J. Antimicrob. Chemother. 52:18–23 [DOI] [PubMed] [Google Scholar]
- 6. Fukasawa M, et al. 1992. Stability of meropenem and effect of 1β-methyl substitution on its stability in the presence of renal dehydropeptidase I. Antimicrob. Agents Chemother. 36:1577–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrison MP, et al. 1989. The disposition and metabolism of meropenem in laboratory animals and man. J. Antimicrob. Chemother. 24(Suppl A):265–277 [DOI] [PubMed] [Google Scholar]
- 8. Hugonnet JE, Blanchard JS. 2007. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry 46:11998–12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mendez AS, Dalomo J, Steppe M, Schapoval EE. 2006. Stability and degradation kinetics of meropenem in powder for injection and reconstituted sample. J. Pharm. Biomed. Anal. 41:1363–1366 [DOI] [PubMed] [Google Scholar]
- 11. Nicolau DP. 2008. Carbapenems: a potent class of antibiotics. Expert Opin. Pharmacother. 9:23–37 [DOI] [PubMed] [Google Scholar]
- 12. Takeuchi Y, Sunagawa M, Isobe Y, Hamazume Y, Noguchi T. 1995. Stability of a 1 beta-methylcarbapenem antibiotic, meropenem (SM-7338) in aqueous solution. Chem. Pharm. Bull. (Tokyo) 43:689–692 [DOI] [PubMed] [Google Scholar]
- 13. Topham JC, Murgatroyd LB, Jones DV, Goonetilleke UR, Wright J. 1989. Safety evaluation of meropenem in animals: studies on the kidney. J. Antimicrob. Chemother. 24(Suppl A):287–306 [DOI] [PubMed] [Google Scholar]
- 14. Tremblay LW, Hugonnet JE, Blanchard JS. 2008. Structure of the covalent adduct formed between Mycobacterium tuberculosis beta-lactamase and clavulanate. Biochemistry 47:5312–5316 [DOI] [PubMed] [Google Scholar]
- 15. Tsenova L, et al. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J. Infect. Dis. 192:98–106 [DOI] [PubMed] [Google Scholar]
- 16. Via LE, et al. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Viaene E, Chanteux H, Servais H, Mingeot-Leclercq MP, Tulkens PM. 2002. Comparative stability studies of antipseudomonal beta-lactams for potential administration through portable elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated syringes (intensive care units). Antimicrob. Agents Chemother. 46:2327–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang F, Cassidy C, Sacchettini JC. 2006. Crystal structure and activity studies of the Mycobacterium tuberculosis beta-lactamase reveal its critical role in resistance to beta-lactam antibiotics. Antimicrob. Agents Chemother. 50:2762–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. WHO 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. World Health Organization, Geneva, Switzerland [Google Scholar]
- 20. Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HM. 1995. Meropenem. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs 50:73–101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.