Abstract
HIV continues to be a problem worldwide. Topical vaginal microbicides represent one option being evaluated to stop the spread of HIV. With drug candidates that have a specific action against HIV now being studied, it is important that, when appropriate and based on the mechanism of action, the drug permeates the tissue so that it can be delivered to specific targets which reside there. Novel formulations of the nucleotide reverse transcriptase inhibitor tenofovir (TFV) and the nonnucleoside reverse transcriptase inhibitor UC781 have been developed and evaluated here. Gels with three distinct rheological properties were prepared. The three gels released both UC781 and TFV under in vitro conditions at concentrations equal to or above the reported 50% effective concentrations (EC50s). The drug concentrations in ectocervical tissues were well in excess of the reported EC50s. The gels maintain ectocervical viability and prevent infection of ectocervical explants after a HIV-1 challenge. This study successfully demonstrates the feasibility of using this novel combination of antiretroviral agents in an aqueous gel as an HIV infection preventative.
INTRODUCTION
HIV/AIDS remains a major global health issue. Within the epidemic, heterosexual transmission represents a major pathway for HIV-1 infection. As a preventative strategy, topical microbicide products designed to be applied vaginally or rectally to prevent sexual transmitted infections, including HIV-1, are being developed. A number of different classes of drug candidates based on the mechanism of action are being developed as microbicide drug products. One specific class which has been widely studied in the field is the HIV-1 reverse transcriptase inhibitors.
Until recently, several microbicide products evaluated in clinical trials failed to show protection against HIV-1 transmission (2, 15, 20, 34, 35). These microbicide products were nonspecific inhibitors of HIV-1. However, tenofovir (TFV), a nucleotide reverse transcriptase inhibitor (NRTI), was shown to be effective in preventing HIV-1 acquisition. Results of a phase IIB trial (CAPRISA 004) showed that 1% TFV vaginal gel administered in a coitally dependent manner can reduce HIV-1 infection by 39% compared to a placebo (1). However, in the ongoing Vaginal and Oral Interventions to Control the Epidemic (VOICE) clinical trial, TFV failed to show efficacy. The TFV oral tablet (Viread) and TFV vaginal gel arms were halted due to poor efficacy compared to the respective placebo products, as reported by the Microbicides Trial Network website (http://www.mtnstopshiv.org/node/3909). It is worth pointing out that in the CAPRISA 004 trial, a coitally dependent dosing regimen was adopted whereas a coitally independent (daily) dosing regimen was implemented in the VOICE trial. Although TFV clinical trials showed progress, the search for more effective microbicides and preventive products is still warranted. Microbicide products containing combinations of anti-HIV-1 agents may provide increased protection against HIV-1 sexual transmission. The benefits of combination microbicides include an increased barrier to infection, overcoming of resistance issues, and reduction of the required dose of each drug used when synergy is present, which could also decrease the potential for toxicity (19). Several combination microbicide products are currently under development (13, 16, 22, 25, 29). A combination product made of cellulose acetate 1,2-benzenedicarboxylate, a polymer that blocks HIV-1 entry by targeting gp120 and gp41, and UC781, a non-NRTI (NNRTI), was tested in vitro and exhibited significant synergistic and complementary effects against HIV-1 infection. This resulted in meaningful dose reductions for each compound (25).
One combination microbicide product being developed is a gel containing UC781 and TFV. TFV use in HIV-1 therapy has been well established. As a microbicide drug candidate, it showed efficacy in preventing HIV-1 infection in vitro and ex vivo (33). TFV alone or in combination with emtricitabine administered vaginally was shown to protect macaques from SHIV infection (30). Clinically, as mentioned earlier, TFV proved a reduction of HIV-1 infection incidence (1). On the other hand, UC781 is the first NNRTI proven to be potent against HIV-1 in in vitro and ex vivo studies (3, 9, 12, 14, 17, 21, 28, 36, 37). In a pig-tailed macaque model, UC781 gel was found to be safe when applied vaginally (31).
In vitro models for testing of anti-HIV-1 activity are important for the screening of drug candidates. However, regarding HIV-1 sexual transmission, in vitro models do not simulate the real-life scenario, where HIV-1 needs to overcome the genital tract innate immunity and epithelial barrier to reach its target cells. Therefore, ex vivo models were used to test the target tissue important for HIV-1 transmission to better evaluate microbicide safety and efficacy. An ex vivo model of nonpolarized human cervical tissue was used to test the anti-HIV-1 activities of several drug candidates, including UC781 (17, 18). In this model, both sides of the tissue are exposed to the drug and the virus. Another ex vivo model has also been used to test the anti-HIV-1 activities of microbicide drug candidates, including TFV and UC781, which provides exposure of the drug product to the mucosal epithelium using polarized tissue, which better recapitulates HIV-1 transmission (12, 33).
Promising data from a nonhuman primate study evaluating TFV gel showed that animals that were protected had drug permeation through the mucosa resulting in plasma drug levels (11). These data suggest that, at least for TFV, there needs to be some level of drug in the tissue for efficacy. In preclinical evaluations of microbicide products, assessment of the ability of drugs to permeate cervicovaginal tissue provides insight into the diffusion of drug candidates into the target tissue in vivo. The abilities of several anti-HIV-1 drugs developed as microbicide products to permeate tissue were previously evaluated in the Franz cell model using human ectocervical tissue, demonstrating drug levels that would achieve protection in vitro (4, 33).
Vaginal gel products have been widely studied for application as delivery platforms for microbicide drug candidates. Several groups have demonstrated that various gel formulations can result in different distribution patterns in the vagina. Additionally, formulation changes can result in a modified drug release profile. The potential for some excipients used in a formulation to create the desired attributes to modify the ability to permeate tissue has also been shown (8, 32). Ultimately, modification of the drug release profile and the drug's ability to permeate tissue will impact gel product safety and efficacy (26). For this reason, it is imperative to assess the impacts of specific formulations on drug release, tissue permeation, safety, and bioactivity.
This study evaluated a series of combination gel products containing TFV and UC781 that were designed to provide the optimal overall coating of vaginal tissues and/or retention within the vaginal tract to deliver the two drug substances. The platform for the gels was created using the algorithm introduced by Mahalingam et al. (26) and expanded by Kiser et al. (24) that takes into account the composition, property, and performance of the gel in the vaginal lumen when diluted with vaginal fluid. TFV and UC781 release rates were determined in in vitro release studies. The apparent tissue permeation coefficients of both UC781 and TFV were determined upon the exposure of excised human ectocervical tissue to the combination products. Finally, the safety and efficacy of the combination products were studied in an ex vivo polarized ectocervical explant model. Collectively, our data show these products to be promising candidates for prevention of HIV-1 infection.
MATERIALS AND METHODS
Materials.
Five combination TFV-UC781 gel products provided by CONRAD were used in these experiments. Combination products were identified as spreading gel at pH 5.2 (SG5.2), intermediate spreading gel at pH 5.2 (ISG5.2), bolus gel at pH 5.2 (BG5.2), spreading gel at pH 4.5 (SG4.5), and bolus gel at pH 4.5 (BG4.5). Placebo gel products (pISG5.2 and pBG4.5) were also provided by CONRAD. The compositions of all of the gels are shown in Table 1. Excipients in the gel formulations included hydroxymethylcellulose, Carbopol 974P, glycerin, methylparaben, propylparben, sodium chloride, sodium hydroxide, hydrochloric acid, and purified water. BG5.2 and BG4.5 are bolus gels and had the largest amounts of Carbopol 974P at 1.73 and 1.93% by weight, respectively. The viscosities of the gels tested were determined in Patrick Kiser's laboratory at the University of Utah and were found to be 77.2 ± 1.4 Pa-s (SG5.2), 234.0 ± 4.4 Pa-s (ISG5.2), 469.8 ± 31.1 Pa-s (BG5.2), 76.6 ± 6.5 Pa-s (SG4.5), and 463.8 ± 53.3 Pa-s (BG4.5).
Table 1.
Compositions of the gel products tested in this study
| Componenta | % by wt |
||||||
|---|---|---|---|---|---|---|---|
| SG5.2 | ISG5.2 | BG5.2 | SG4.5 | BG4.5 | pISG5.2 | pBG4.5 | |
| Active substances | |||||||
| UC781, micronized | 0.1 | 0.1 | 0.1 | 0.25 | 0.25 | ||
| TFV | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||
| Nonactive substances | |||||||
| Hydroxymethylcellulose | 3.0 | 2.5 | 2.0 | 3.0 | 2.0 | 3.2 | 2.0 |
| Carbomer 974P | 0.6 | 1.73 | 1.93 | 0.6 | 1.93 | ||
| Glycerin | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Methylparaben | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Propylparaben | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Sodium hydroxide | QSb to pH 5.2 | QS to pH 5.2 | QS to pH 5.2 | QS to pH 4.5 | QS to pH 4.5 | QS to pH 5.2 | QS to pH 4.5 |
| Hydrochloric acid | |||||||
| Purified water | QS to 100% | QS to 100% | QS to 100% | QS to 100% | QS to 100% | QS to 100% | QS to 100% |
Sodium chloride was used as needed to adjust gel osmolality.
QS, quantum sufficit (add sufficient water to obtain desired quantity).
In vitro release studies.
In in vitro release studies, the low-volume Hanson MicroettePlus system was used. For each drug product, six diffusion cells were set up by filling each cell with receptor fluid (phosphate-buffered saline for TFV studies and 3% SDS in 40% acetonitrile for UC781 studies). A measured volume of the drug product, 100 μl, was placed on a Spectra/Por membrane disc (molecular weight cutoff, 6,000 to 8,000; Spectrum Chemical, Gardena, CA). Sample aliquots were collected after 0, 15, 30, 45, 60, 90, 120, 180, 240, 300, and 360 min and stored in sealed-septum high-performance liquid chromatography (HPLC) vials for analysis of TFV and UC781. The total amount of each drug in each 100-μl volume placed on the membrane was 1,000 μg for TFV and 100 μg for UC781. Aliquots were assayed for TFV and UC781 contents as described below.
Tissue permeation studies.
Human ectocervical cervical tissue was obtained from the University of Pittsburgh Health Sciences Tissue Bank under Institutional Review Board protocol PRO09110431. The tissue permeation experiment was conducted as previously described (4), with some modifications. The receptor compartment consisted of continually stirred tissue culture medium (VEC 100-MM medium from Mat Tek Corporation supplemented with 1% bovine serum albumin). A 100-μl volume of the test gel product was placed in the donor compartment of the Franz cell using a positive-displacement pipette. After 0, 15, 30, 45, 60, 90, 120, 180, 240, 300, and 360 min, 200-μl samples were taken from the receptor compartment and replaced with fresh medium. Aliquots were assayed for TFV and UC781 contents as described below. At the completion of the experiment, the exposed tissue was harvested, washed by vortex in the receptor fluid, and cut in half. Each half was weighed and frozen at −80°C for analysis of the tissue TFV or UC781 level. Apparent tissue permeation coefficients (Papp) were calculated for all of the gels using the equation Papp = slope from the amount-versus-time curve (μg/s)/area exposed (cm2) × donor concentration (μg/ml).
To account for tissue thickness variation between experiments, DK was also plotted for each gel product. DK was obtained using the equation DK = Ph, where D is diffusivity (cm2/s), K is the partition coefficient, P is the tissue permeation coefficient (cm/s), and h is tissue thickness (cm).
Quantitative determination of UC781 and TFV by HPLC.
An HPLC assay was developed to determine the concentration of UC781. This method simultaneously identified UC781 and its degradation products. An Ultimate 3000 system (Dionex) equipped with a photodiode array detector (275 nm) and Chromeleon data-processing software was used. Separations were achieved on a C18 column (4.6 by 150 mm, 3 μm, 120A; Dionex) at 35°C with a flow rate of 1.0 ml/min. The mobile phase was 75% acetonitrile and 25% Milli-Q water. The observed retention time of UC781 was 6 min.
An ultra-HPLC assay was developed to quantitate TFV and its impurities. A Waters Acquity UPLC column equipped with a TUV detector (260 nm) and EMPOWER data-processing software was used. Separations were achieved on an Acquity UPLC BEH C18 column (1.7 μm, 2.1 by 50 mm; Waters) used at ambient temperature. The flow rate was 0.3 ml/min. The mobile phase was a mixture of 90% 10 mM K2HPO4–4 mM t-butylammonium bisulfate (pH 5.7) and 10% methanol. The elution time of TFV was 3.6 min.
Quantitative determination of UC781 and TFV by liquid chromatography tandem mass spectrometry (LC/MS/MS) in human ectocervical tissue.
Tissue samples from the permeability experiments were analyzed at Absorption Systems LP to quantitate TFV. Tissue samples were weighed, mixed with 500 μl of 20% acetonitrile, and sonicated for 60 s in an ice bath. For total TFV analysis, 50 μl of the homogenate was immediately taken and mixed with 150 μl phosphatase in 50 mM ammonium acetate buffer, pH 4.0 (20 U/ml). Samples were incubated in a reciprocal shaking water bath at 38°C for 30 min. A 200-μl volume of an aqueous solution of 0.80 M perchloric acid was mixed with the samples, which were immediately centrifuged at 13,000 rpm for 5 min. A 200-μl volume of supernatant was placed in a vial and mixed with 200 μl 0.45 N sodium hydroxide. Samples were diluted to necessary levels for detection with a homogenized basified solution (HSB) which consisted of 20% acetonitrile, 0.8 M perchloric acid, and 0.45 M sodium hydroxide (1:1:2, vol/vol/vol). After dilution, 100 μl was transferred to a microcentrifuge tube and mixed with 20 μl 50% acetonitrile and 200 μl 100% acetonitrile. A 20-μl volume of internal standard solution (5 μM d6-TFV in 50% acetonitrile) was mixed with the samples. Samples were analyzed by LC/MS/MS. For free TFV and TFV diphosphate (TVF-dp) analyses, 400 μl of 0.8 M perchloric acid was mixed with the remainder of the tissue homogenate, which was immediately centrifuged at 13,000 rpm for 5 min. A 500-μl volume of the supernatant was transferred to a vial and mixed with 500 μl of 0.45 N sodium hydroxide. For analysis of free TFV, samples were diluted with HSB to levels required for detection and the same procedures were done as for the total TFV analysis. For TFV-dp analysis, 10 μl of ammonium hydroxide was placed in a microcentrifuge tube and mixed with 50 μl of a free TFV and TFV-dp sample mixture. A 20-μl volume of a mixture of acetonitrile-water-ammonium hydroxide (50:50:1, vol/vol/vol) was added. A 50-μl volume of 50% acetonitrile was added, and samples were vortexed for 5 s. The internal standard solution was made of 1 μM 2′,3′-dideoxy-3′-thiacytidine-5′-triphosphate (3TCTP) dissolved in a mixture of acetonitrile-water-ammonium hydroxide (50:50:1, vol/vol/vol). A 20-μl volume of the internal standard was mixed with the samples. Samples were then analyzed by LC/MS/MS.
The LC/MS/MS system used by Absorption Systems for evaluation of free TFV and TFV-dp consisted of an Applied Biosystems API 4000 LC/MS/MS apparatus with Perkin-Elmer series 200 micropumps, a CTC Analytics autosampler (HTC PAL System), and a Perkin-Elmer series 200 mixer. A Thermo BioBasic AX HPLC column (50 by 2.1 mm, 5 μm) was used with an in-line frit filter (0.5 μm, 0.062 by 0.065 by 0.2485 mm; Upchurch Scientific) and column at room temperature. The TFV (and TFV-d6, internal standard for TFV) and TFV-dp (and 3TCP, internal standard for TFV-dp) retention times were 2.1 and 2.7 min, respectively, with a 5-min gradient run of a binary mobile-phase system (A, acetonitrile–10 mM ammonium acetate [pH 6.0], 70:30; B, acetonitrile–1 mM ammonium acetate [pH 10.5], 60:40) at a flow rate of 400 μl/min. Autowashes consisted of 2% ammonium hydroxide in water, followed by 50% methanol in water, and the autosampler temperature was ∼5°C. Analytes were detected with a mass spectrometer (Applied Biosystems MDS Sciex API 4000) under a negative-mode multiple-reaction-monitoring (MRM) scan. The mass transitions (m/z) were 286.1 to 133.9 for TFV, 292.1 to 133.8 for TFV-d6, 445.9 to 158.8 for TFV-dp, and 467.9 to 158.6 for 3TCTP.
Tissue samples from the permeability experiments were analyzed at Kar Bioanalytical to quantitate amounts of UC781. Tissue samples were weighed and placed in homogenization microtubes containing 2.8-mm ceramic or 2.38-mm metal beads (Bertin Technologies). A diluent of (1:1 methanol-deionized water) was added to the samples at a 1:5 ratio of tissue to diluent. Samples were homogenized using a Preceylls 24 tissue homogenizer (Bertin Technologies). A 500-μl volume of acetonitrile and 100 μl of the internal standard (15 ng/ml d4-UC781 in acetonitrile) were added to the homogenized samples. This homogenization program was repeated twice. Samples were centrifuged at 13,200 rpm (2 to 8°C) for 10 min. An aliquot of the supernatant was injected for LC/MS/MS analysis of UC781. The LC/MS/MS system consisted of an HPLC separation module (Waters Alliance 2695) connected to a mass spectrometer (Sciex API 3000). A reversed-phase HPLC column (Agilent ZORBAX Eclipse XDB-Phenyl; 75 by 4.6 mm, 3.5 μm) attached to a guard column (Agilent ZORBAX Eclipse XDB-Phenyl; 12.5 by 4.6 mm, 5 μm) was used. The retention time of UC781 and d4-UC781 was 5.8 min with a gradient run of a binary mobile-phase system (A, 10 mM ammonium acetate in methanol-deionized water, 5:95; B, 10 mM ammonium acetate in methanol-ethyl acetate-deionized water, 190:25:10). UC781 and d4-UC781 were detected with a mass spectrometer under a positive-mode MRM scan. The mass transitions (m/z) were 360 to 268.1 for UC781 and 340 to 270.4 for d4-UC781.
Ex vivo safety and anti-HIV activity.
Human ectocervical tissue was acquired from the University of Pittsburgh Health Sciences Tissue Bank as described above. Ectocervical tissue was excised from women undergoing planned therapeutic hysterectomies and placed in L-15 transport medium. The tissues were processed within a few hours after surgery.
Safety testing.
Each gel product was applied to the apical surface of duplicate explants. As a control, no treatment or addition of a 1:5 dilution of 2% nonoxyonol-9 gel was included. After overnight culture, the explants were washed and one of the two duplicates was placed in RPMI containing 250 μg/ml 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) for 4 h. The explants were removed and placed in 1 ml of methanol overnight. The next day, the explants were removed from the methanol and placed on a marked paper towel to dry and be weighed. The methanol dissolved the formazan product produced by live tissue, which resulted in a color change in the methanol that was quantified on a DTX 880. The viability of the treated explants was determined by first correcting the methanol optical density (OD) by the weight of the corresponding explant and then using the formula cOD (corrected OD) of explant ÷ cOD of control × 100 = percent viability. The other explant was fixed in formalin and processed for histology by staining with hematoxylin and eosin.
Efficacy testing.
Each gel product was diluted 1:5 in complete Dulbecco's modified Eagle's medium and added to the apical surfaces of the appropriate explants. Fifteen minutes later, 5 × 104 50% tissue culture infective doses of HIV-1BaL was added to all of the explants, which were then cultured overnight. The explants were washed, and fresh medium was added to the basolateral chamber. Every 3 to 4 days, the medium was collected (saved at −80°C) and fresh medium was added back. At day 21, the explant cultures were stopped and the explants were fixed in formalin and processed for immunohistochemistry to detect HIV-1 p24-expressing cells. The supernatant was tested for HIV-1 replication using the p24gag enzyme-linked immunosorbent assay (ELISA; Perkin-Elmer).
Statistical analysis.
Data were subjected to one-way analysis of variance (ANOVA) to test for significant differences (P < 0.05).
RESULTS
In vitro release studies with SD5.2, ISG5.2, BG5.2, SG4.5, and BG4.5.
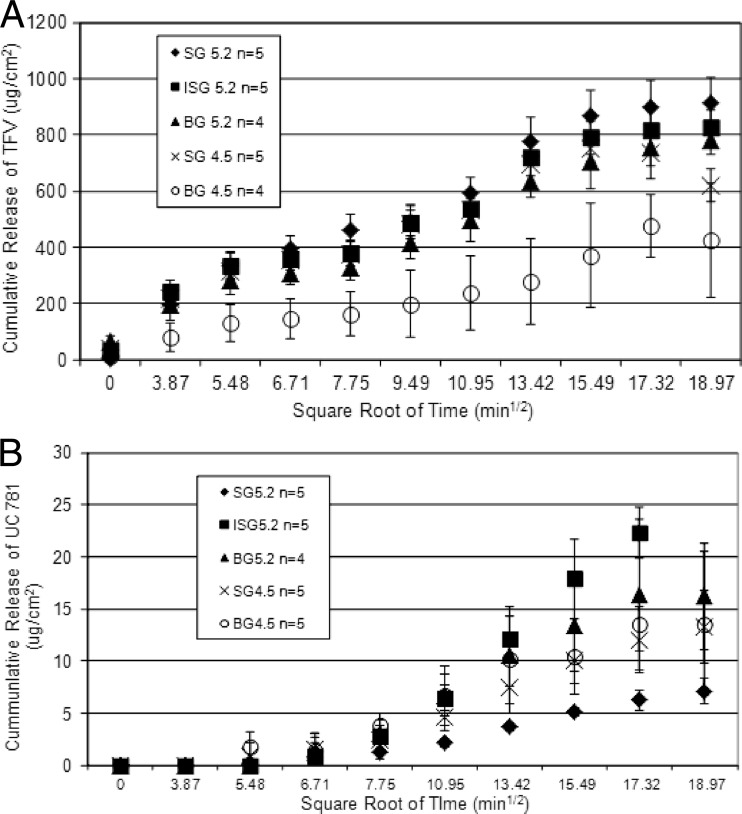
When a drug is delivered vaginally, prior to the drug being available to permeate the tissues of the vagina and cervix, it must be released from the dosage form used. In vitro release studies were conducted to determine the rate of release of the drug substance from a pharmaceutical product. In vitro release data were obtained with the five gel products. The profile in vitro TFV release from the five gel products showed a linear relationship between the cumulative release of TFV and the square root of time (Fig. 1A). Profiles of UC781 release from each gel product showed a linear relationship with the square root of time, beginning with the third time point evaluated (Fig. 1B). All of the products contained 1% TFV, but it should be noted that SG5.2, ISG5.2, and BG5.2 contained a lower UC781 concentration (0.1%) than SG4.5 and BG4.5 (0.25%). No correlations between the drug loading level and the UC781 release profile were observed. The slope of the linear portion of the line (0 to 360 min for TFV, 30 to 300 min for UC781) was used to calculate the drug release rate (Table 2). BG4.5 resulted in the lowest TFV release profile (22.4 μg/cm2/min1/2), while the other gels resulted in the release of similar amounts of TFV, which were almost double that of BG4.5. With respect to UC781 release from the gels, SG5.2 resulted in the lowest UC781 release profile (0.48 μg/cm2/min1/2). The rates of TFV and UC781 release from the five gels seemed not to be affected by gel viscosity. However, the pH had an impact on TFV release but not on UC781 release from the gel. TFV was released faster from gels with the higher pH (5.2). That could be due to the fact that TFV is more soluble at pH 5.2 than at the lower pH (4.5).
Fig 1.
In vitro release data are presented as the cumulative release of TFV (A) or UC781 (B) as a function of the square root of time. Each data point represents the average for six replicates.
Table 2.
Calculated in vitro rates of TFV and UC781 release from each gel product tested in this study
| Drug | Avg release rate in μg/cm2/min1/2(correlation) ± SD |
||||
|---|---|---|---|---|---|
| SG5.2a | ISG5.2a | BG5.2b | SG4.5a | BG4.5a | |
| TFV | 50.32 (0.99) 5.7 | 43.75 (0.99) 3.8 | 43.49 (0.99) 6.6 | 39.95 (0.96) 4.9 | 22.4 (0.96) 11.01 |
| UC781 | 0.48 (0.98) 0.7 | 1.79 (0.99) 0.2 | 1.44 (0.96) 0.4 | 1.06 (0.97) 0.2 | 1.23 (0.96) 0.5 |
n = 5.
n = 4.
Tissue permeability studies with SD5.2, ISG5.2, BG5.2, SG4.5, and BG4.5.
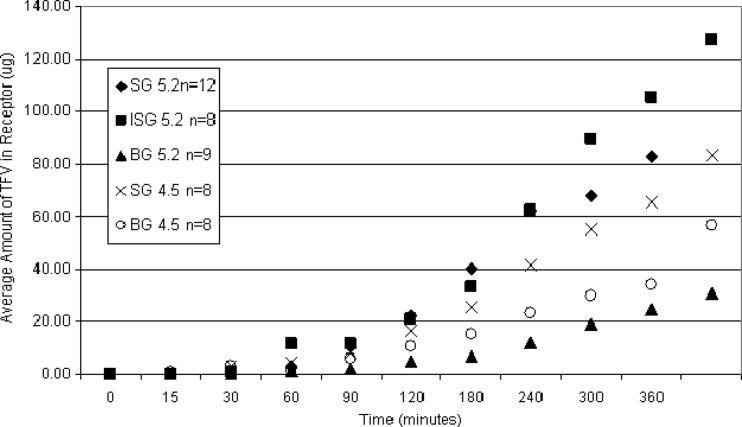
Tissue permeability studies were conducted to evaluate the potential for systemic exposure to TFV or UC781 when the designed gel products are administered vaginally. Furthermore, these studies evaluated the tissue drug concentrations achieved following product exposure, which can directly impact product efficacy. Each gel product was tested in three separate tissue samples. A minimum of duplicate experiments were set up with each tissue sample by the method described above. Experimental samples were quantitated for TFV and UC781. Kinetics of TFV transport was described through analysis of receptor solution drug content over time. Figure 2 illustrates the increase in the amount of TFV measured in the receptor compartment over time. Some intrasubject variability in tissue permeability was observed. For this reason, to compare permeability profiles between gel products, averaged data from multiple experimental setups were used. In all tissues studies, by the 60-min time point, microgram levels of TFV were seen in the receptor compartment, with the minimum amount of 2.4 μg resulting from BG5.2. Following a 6-h exposure period, the amounts of TFV in the receptor chambers ranged from 31 to 127 μg. This variability in the ability of TFV to permeate tissue correlates with other in vitro studies (33), as well as clinical variability in intraindividual plasma TFV levels following exposure to 1% TFV gel.
Fig 2.
Cervical tissue permeation profiles of all of the gel products tested displayed graphically as the amount of TFV (micrograms) analyzed in the receptor compartment as a function of time in minutes. Data points presented are average values of the number of replicates tested with each gel.
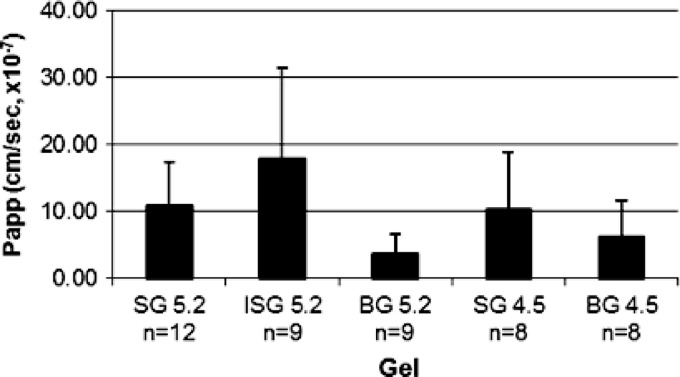
Similar profiles of the ability of TFV to permeate tissue were found with SG5.2 and SG4.5, with calculated apparent tissue permeation coefficients of approximately 10 × 10−7 cm/s for both gels. The apparent tissue permeation coefficients for BG5.2 (3.7 × 10−7 cm/s) and BG4.5 (6.2 × 10−7 cm/s) were lower than that calculated for SG5.2 and SG4.5 (Fig. 3). The greatest tissue permeation of TFV was obtained with ISG5.2, which produced a calculated apparent tissue permeation coefficient of 17.9 × 10−7 cm/s.
Fig 3.
Calculated apparent coefficients (Papp) of the ability of TFV in each gel product to permeate tissue. Values are presented as the average ± the standard error of the mean.
The TFV tissue permeation coefficients of the gel products tested can be ranked as follows: ISG5.2 > SG4.5 ∼ SG5.2 > BG4.5 > BG5.2. The TFV membrane tissue permeation coefficients obtained are on the same order of magnitude as those published for water diffusion into human skin layers. Additionally, the amounts of total TFV, free TFV, and TFV-dp present in tissue after a 6-h period of exposure to each gel product were determined (Table 3). In all experiments, the amount of TFV-dp recovered from tissue was 3 orders of magnitude smaller than that of free TFV. SG5.2 and ISG5.2 resulted in the greatest total TFV delivery to tissue, approximately 400 μg/g, whereas exposure to BG4.5 resulted in 115 μg/g TFV remaining in tissue at the 6-h time point.
Table 3.
TFV concentrations found in tissuea
| Gel (n) | Avg concn (μg/g) ± SD |
||
|---|---|---|---|
| Total TFV | Free TFV | TFV-dp | |
| SG5.2 (13) | 423.5 ± 243.1 | 273.7 ± 0.0 | 0.143 ± 0.00 |
| ISG5.2 (11) | 403.8 ± 236.2 | 402.7 ± 201.0 | 1.085 ± 1.04 |
| BG5.2 (7) | 208.7 ± 149.6 | 214.0 ± 122.4 | 0.401 ± 0.14 |
| SG4.5 (8) | 253.8 ± 172.8 | 216.9 ± 150.8 | 0.446 ± 0.11 |
| BG4.5 (8) | 115.3 ± 68.2 | 136.1 ± 78.5 | 0.462 ± 0.08 |
The total amount of TFV and the levels of free TFV and TFV-dp are presented.
To account for tissue thickness variation between experiments, DK was also plotted for each gel product. Accounting for tissue thickness did not impact the rank order for the gel formulations regarding permeability parameters (Table 4). Since the partition coefficient (K) cannot easily be experimentally determined, comparisons of the combined DK parameter were made. (Since K is a dimensionless value, the rank order of the diffusivity of the gel products is the same as the tissue permeation coefficients above.)
Table 4.
TFV diffusivity parameters calculated from Papp for each gel studied
| Gel (n) | Avg DK for TFV (cm2/s, 10−7) ± SD |
|---|---|
| SG5.2 (12) | 1.03 ± 0.62 |
| ISG5.2 (9) | 1.50 ± 1.15 |
| BG5.2 (9) | 0.28 ± 0.22 |
| SG4.5 (8) | 0.98 ± 0.74 |
| BG4.5 (8) | 0.53 ± 0.49 |
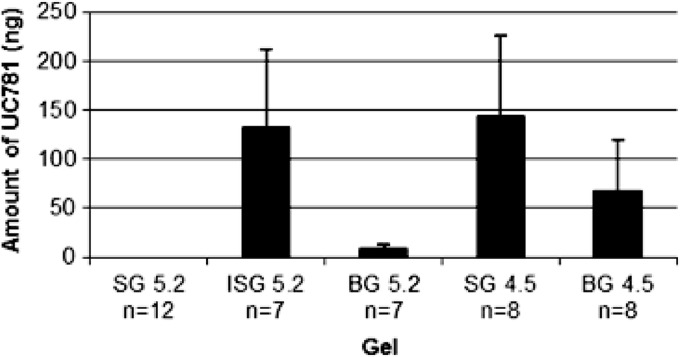
For UC781 tissue permeation, the amount of UC781 in the receptor compartment was below the limit of detection by the UC781 HPLC method described (which is 1.0 ng). No detectable levels at any time point were seen in the receptor compartment. For this reason, LC/MS/MS analysis of selected receptor compartment samples was conducted by Absorption Systems LP (Exton, PA). LC/MS/MS evaluation of the samples obtained from the receptor compartment at the 6-h time point indicated that SG4.5 resulted in the greatest amount of UC781 crossing the tissue barrier (35.93 ± 58 ng/ml) by the 6-h time point, and SG5.2 produced no detectable amounts of UC781 at this time point. The UC781 levels in the receptor compartment at the 6-h time point for the combination gel products are shown in Fig. 4. Given the variability of the data obtained, it was difficult to compare and contrast individual formulations. However, it was noted that although no UC781 could be quantitated at the 2- or 3-h time point for ISG5.2 and BG5.2, by the 6-h time point, a sufficient amount of UC781 had crossed the tissue barrier to result in levels similar to or above the reported 50% effective concentration (EC50) (3 ng/ml, ∼9 nM) of UC781 (28.5 ± 42 and 2.9 ± 3.4 ng/ml, respectively) (5, 6, 7). In summary, gels ISG5.2, BG5.2, SG4.5, and BG4.5 all resulted in levels of UC781 in the receptor chamber which exceed the EC50 of UC781 by the 6-h time point. The tissue drug levels produced by exposure to SD5.2, ISG5.2, BG5.2, SG4.5, and BG4.5 were also determined. Following a 6-h exposure period, 2 to 5 μg of UC781 per g of tissue was found with ISG5.2, BG5.2 SG4.5, and BG4.5. However, SG5.2 resulted in a tissue drug concentration nearly 15-fold higher (72 μg/g) (Fig. 5). These data suggest that a greater amount of drug (both TFV and UC781) was present in tissue after a 6-h exposure to SG5.2 than after exposure to the other gels tested.
Fig 4.
Amounts of UC781 detected in the receptor compartment following a 6-h exposure to gel products determined by LC/MS/MS. Values are presented as the average ± the standard error of the mean.
Fig 5.
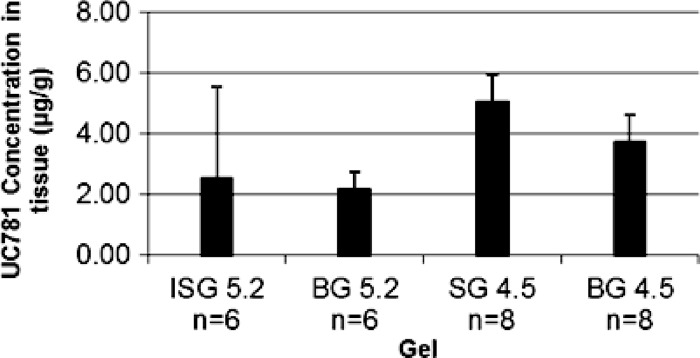
Average drug concentrations in tissues harvested following a 6-h exposure to gel products. Values are presented as the average ± the standard error of the mean. There is no statistically significant difference in mean values among the four gels (P > 0.05, one-way ANOVA). Note that SG5.2 data were omitted from the graph for illustrative purposes. The UC781 concentration in tissue after a 6-h exposure to gel SG5.2 was about 72 μg/g.
Safety and efficacy of TFV-UC781 combination gels in polarized ectocervical explants.
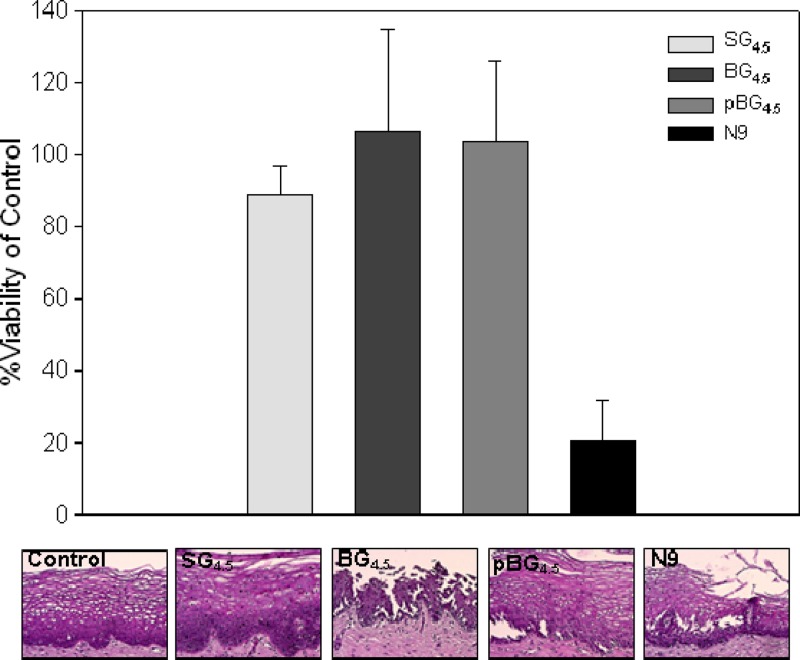
The higher drug content gels, SG4.5 and bolus gels BG4.5 and pBG4.5, were tested for their effects on ectocervical viability. All three gels retained explant viability, as demonstrated by >80% viability in an MTT assay (Fig. 6). The N9 control treatment produced significantly lower viability (P = 0.002; paired Student t test) than the other treatments. Histology showed fracture of the epithelium in explants exposed to BG4.5 and pBG4.5. The epithelium of explants exposed to SG4.5 remained intact. These data suggest that while none of the gels reduce tissue viability, the bolus gels affected the epithelium.
Fig 6.
Ectocervical viability after exposure to UC781-TFV combination gels. Polarized explants were set up in duplicate and exposed to UC781-TFV combination gels or a 2% nonoxynol-9 (N9) gel for 24 h. A no-treatment control was used to base viability. Viability was measured in one of the duplicated explants with the MTT assay (average ± standard deviation). The other explant was used for histology. The data represent three independent tissues. The histology images are representative of one of those tissues.
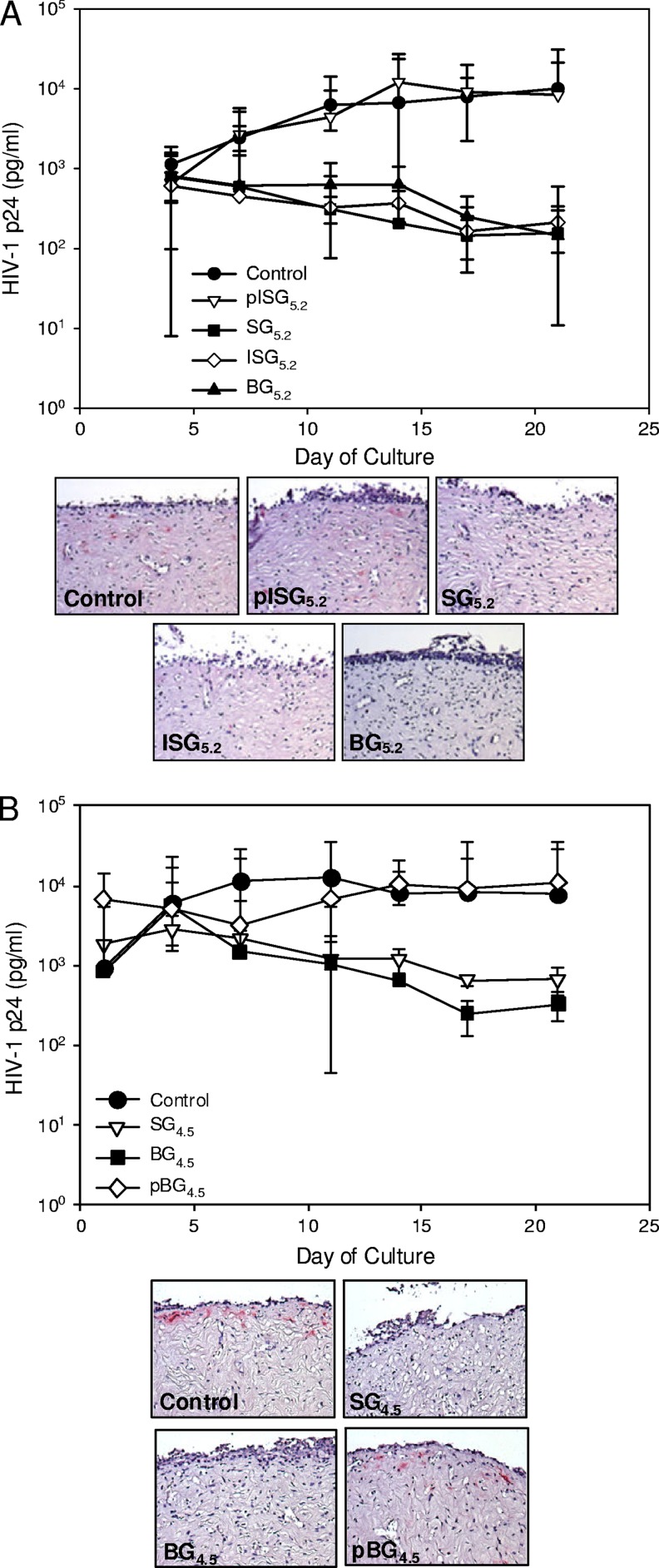
All of the gels were tested for efficacy in polarized ectocervical explants. Gels SG5.2, ISG5.2, and BG5.2 and gels SG4.5 and BG4.5 reduced viral replication by more than 1 log10 (Fig. 7A and B). These treated explants had no HIV-1-expressing cells (represented by pink cells in the tissue; Fig. 7A and B), in contrast to the control explants. Explants treated with placebo gels pISG5.2 and pBG4.5 did not show reduce HIV-1 replication, demonstrated infected cells, and resembled the control explants. Because of the qualitative nature of the polarized explant cultures and the excess amount of drug in the gels, it was not possible to rank the UC781-TFV gels on the basis of the ability to block HIV-1 infection.
Fig 7.
Polarized ectocervical explants are protected from HIV-1 infection by UC781-TFV combination gels pSG5.2, SG5.2, ISG5.2, and BG5.2 (A) and SG4.5, BG4.5, and pSG4.5 (B). Explants were set up in duplicate. The gels were diluted 1:5 in medium and applied 15 min prior to the application of HIV-1. After culture, the explants were washed and followed for 21 days for HIV-1 replication, which was measured by p24gag ELISA and is shown as the median ± the 95% confidence interval of three independent tissues. At the study endpoint, immunohistochemistry was performed to detect cells expressing p24 (pink). The data presented are from one of the three independent tissues.
DISCUSSION
Excipients are required to formulate gels with desired product attributes, including distribution, stability, and bioactivity. Each gel formulation developed is unique with respect to its excipient and product attribute profiles. SG, ISG, and BG gels were created to identify the optimal overall coating of vaginal tissues and/or retention in the vaginal lumen to deliver the two test drugs. Further description of the gel product panel design used here has been provided by Kiser and colleagues (24, 26). The role of some excipients in the enhancement of the ability to permeate tissue has been established (8). Additionally, product attributes such as rheological properties can impact distribution in the vaginal vault (23). Product attributes can also impact drug release, which in turn can potentially have an effect on tissue permeation profiles and ultimately product bioactivity and efficacy.
In vitro release studies were conducted to better understand the impact of formulation differences on the resulting TFV and UC781 release from the combination gel product series. The major factors varied across formulations were the UC781 drug loading level, product viscosity (modified by altering polymer concentrations), and a slight pH change. All of the gel products contained the same amount of TFV (1%). The UC781 concentration was 0.1% in SD5.2, ISG5.2, and BG5.2 and 0.25% in SD4.5 and BG4.5. The pH of these higher-concentration gels was made slightly lower, 4.5 as opposed to 5.2, in an attempt to make gels closer to the natural pH of the female genital tract. BG5.2 and BG4.5 were of higher viscosity due to the increased level of Carbopol 974P in these formulations and are referred to as bolus gels (24). The other gels had lower viscosity and represented spreading-type gel formulations. Increasing the concentration of UC781 in the gel product did not result in an increase in the drug release rate. The lowest TFV release rate was that of the BG4.5 formulation. This may be due to the increased viscosity of this formulation. However, BG5.2, which also had a high viscosity, did not show a similar decreased TFV release rate. This difference may be due to the slight pH difference between the two formulations. TFV solubility is pH dependent and would be higher in the pH 5.2 product. This increased solubility may have resulted in greater TFV release from BG5.2 than from BG4.5. The rate of UC781 release from all of the gel products was found to be lower than that of TFV. This observation is likely due not only to the 4- to 10-fold lower dosing level of UC781 than of TFV but also to the hydrophobic nature of the candidate drug UC781. Additionally, UC781 was present in the gel as a dispersed micronized form, whereas TFV was dissolved.
The in vitro release data allow us to infer the amount of drug released by the product. All of the gels tested resulted in levels of both TFV and UC781 sufficient for bioactivity based on the previously reported EC50s. The cumulative TFV release from the combination gel of approximately 55.1 μg/ml occurred over the 6 h of the assay. By 30 min, the level of TFV released from the gel reached 21.3 μg/ml, which is 37 times greater than the EC50 (0.57 μg/ml, ∼2 μM) needed to block HIV-1 replication in vitro. The cumulative UC781 release from the combination gel of approximately 2.4 μg/ml occurred over the 6 h of the assay. By 30 min, the level of UC781 released from the gel reached 0.1 μg/ml, which is much greater than the EC50 (3 ng/ml, ∼9 nM) needed to block HIV-1 replication in vitro (5, 6, 7).
Once the drug is efficiently released from the gel product, it is available to permeate the target tissues of the vagina and cervix. Tissue permeation studies demonstrated several trends with respect to the abilities TFV and UC781 to permeate tissue when delivered to the vagina with this series of formulated gel products. Data on the ability of TFV to permeate tissue was compared within two groups; group 1 included SD5.2, ISG5.2, and BG5.2, and the second group included SG4.5 and BG4.5. This comparison shows that the ability of TFV to permeate tissue can be rank ordered as follows: ISG5.2 > SG5.2 > BG5.2. Comparison of tissue drug levels following a 6-h exposure period shows that ISG5.2 resulted in greater tissue TFV levels than either SG5.2 or BG5.2. Regarding SG4.5 and BG4.5, gel SG4.5 was shown to produce a better tissue permeation profile and a higher tissue drug level than those obtained with BG4.5. Furthermore, there are similarities between the TFV tissue permeation profiles obtained with SG5.2 and SG4.5 and also with BG5.2 and BG4.5. This was confirmed through comparison of the permeability and diffusion coefficient data. This similarity suggests that the slight pH modification did not result in alteration of the tissue permeation profile of TFV. By correlating TFV in vitro release data and tissue TFV levels, one can see that not all of the gels show a correlation between drug release and tissue drug levels. This could be due to the inherent variability of the tissue samples from different patients. The thickness of the epithelium is a determinant of permeability because the epithelium is one of the barriers to permeation. Both of the media used in the permeability and in vitro studies are aqueous, and since TFV has high aqueous solubility, it is not expected that a difference between the media had an effect.
Studies of tissue permeation by UC781 showed that very small amounts of UC781 were identified in the receptor in most of the permeability assessments conducted, indicating a minimal probability of systemic exposure after the topical application of any of the combination gel products studied. Given that this product is designed to be a topical microbicide, this is a desired attribute. For topical microbicides, it is desirable to limit the amount of systemic exposure of the user to the antiretroviral agent, which may result in toxicity or resistance to future anti-HIV therapy. Comparing these low levels indicates that ISG5.2 and SG4.5 may result in slightly higher levels of UC781 transport to the receptor chamber. Permeability studies of SD5.2, ISG5.2, BG5.2, SG4.5, and BG4.5 also showed that a considerable amount of UC781 is present in tissue at levels which have shown efficacy in in vitro evaluations. The in vitro UC781 release results did not correlate with the tissue UC781 levels in the permeability studies. This observation could be due to the hydrophobicity of UC781 in addition to inherent tissue variability. As a hydrophobic compound, UC781 solubility in the medium used is critical. Because the in vitro release study medium could not be used in the permeability study, a different aqueous medium was used for the permeability study. That could well have resulted in different patterns of drug release from the gels in the permeability study. Additionally, because of its hydrophobicity, UC781 is expected to accumulate in tissue with little or no full tissue permeation. That could lead, depending on the route of permeation, to saturation of the permeation pathway, so a higher drug concentration on the tissue surface (faster release or higher loading) would not then affect or correlate with drug levels in the tissue.
UC781 in the lower-dose gels, SG5.2, ISG5.2, and ISG5.2, along with the higher-dose gels, SG4.5 and BG4.5, was effective at preventing HIV-1 infection in polarized ectocervical explants. These data are supported by the testing of the single-entity gels, demonstrating potent activity in polarized ectocervical explants (12, 33). Our group has evaluated a number of formulations containing either TFV or UC781 alone in both ectocervical and colorectal explants and has found all to inhibit infection (data not shown). It should be noted that the results obtained with the polarized explant system are qualitative rather than quantitative. However, the locations of activity of these two molecules are different. TFV must be phosphorylated intracellularly, while UC781 can stay in the outer epithelium and penetrate the virus directly. Given this difference between the locations of action, the data presented indicate the efficacy of both TFV and UC781.
Safety testing of the higher-dosage gels demonstrated a better profile for SG4.5 than for the two bolus gels. The viscous nature of the bolus gels may have affected the epithelium of the explants during the washing period. Despite the epithelial damage, SD4.5 and BG4.5 were effective at preventing HIV-1 infection in polarized ectocervical explants. It should be noted that the original vaginal 1% TFV gel formulation demonstrated epithelial fracture of ectocervical tissue in vitro due to its hyperosmolar formulation (33). Despite this, the product was acceptable to women using the gel, no adverse events were reported in clinical trials (HPTN 050, 059) (10, 27), and the gel was shown to be modestly protective (1).
Conclusion.
In this study, a series of semisolid formulations designed for the combined delivery of UC781 and TFV were evaluated with respect to their impact on a drug's ability to permeate tissue, its toxicity, and its in vitro bioactivity. It was found that TFV and UC781 were successfully released from all of the gel products evaluated. With all of the gels, TFV was found to cross the tissue barrier. However, the rate at which this occurred varied among the gel products tested, with the most rapid tissue diffusion being obtained with ISG5.2. Little to no UC781 was able to cross the tissue barrier, indicating limited potential for systemic exposure to UC781 when it is vaginally delivered by any of the gel products tested. However, it was found that amounts of UC781 which exceeded the reported EC50 of this compound were detected in tissue following gel exposure, with the greatest amounts resulting from exposure to SG5.2. None of the combination gels resulted in tissue toxicity, and activity of all of the combination gel products in the series against HIV-1 was observed. The findings of these studies are the first for a combination gel product that was strategically formulated. The data generated provide an algorithm to cross compare future combination products.
ACKNOWLEDGMENTS
We acknowledge Dezhong Liu at Absorption Systems, Inc., for providing bioanalytical support. CONRAD provided all of the products used for evaluation in these studies. CONRAD acknowledges the work of Patrick Kiser's microbicide laboratory at the University of Utah for preparing the gels evaluated in this study. We thank Lindsay (Mock) Rude and the University of Pittsburgh Health Sciences Tissue Bank for obtaining the ectocervical tissues and the patients for their willingness to participate in this research.
This work was funded through a cooperative agreement (GPO-A-00-08-00005-00) with the United States Agency for International Development (USAID).
The views expressed here do not necessarily reflect those of USAID.
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Abdool Karim Q, et al. 2010. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329:1168–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdool Karim SS, et al. 2011. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS 25:957–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abner SR, et al. 2005. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J. Infect. Dis. 192:1545–1556 [DOI] [PubMed] [Google Scholar]
- 4. Akil A, et al. 2011. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv. Transl. Res. 1:209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balzarini J, et al. 1996. Highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol. Pharmacol. 50:394–401 [PubMed] [Google Scholar]
- 6. Balzarini J, et al. 1998. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 12:1129–1138 [DOI] [PubMed] [Google Scholar]
- 7. Barnard J, Borkow G, Parniak MA. 1997. The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry 36:7786–7792 [DOI] [PubMed] [Google Scholar]
- 8. Bauerová K, Matusova D, Kassai Z. 2001. Chemical enhancers for transdermal drug transport. Eur. J. Drug Metab. Pharmacokinet. 26:85–94 [DOI] [PubMed] [Google Scholar]
- 9. Borkow G, Salomon H, Wainberg MA, Parniak MA. 2002. Attenuated infectivity of HIV type 1 from epithelial cells pretreated with a tight-binding nonnucleoside reverse transcriptase inhibitor. AIDS Res. Hum. Retroviruses 18:711–714 [DOI] [PubMed] [Google Scholar]
- 10. Carballo-Diéguez A, et al. 2007. Acceptability of tenofovir gel as a vaginal microbicide by US male participants in a phase I clinical trial (HPTN 050). AIDS Care 19:1026–1031 [DOI] [PubMed] [Google Scholar]
- 11. Cranage M, et al. 2008. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med. 5:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cummins JE, Jr, et al. 2007. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob. Agents Chemother. 51:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Cruz OJ, Uckun FM. 2007. Mucosal safety of PHI-443 and stampidine as a combination microbicide to prevent genital transmission of HIV-1. Fertil. Steril. 88(4 Suppl.):1197–1206 [DOI] [PubMed] [Google Scholar]
- 14. Dezzutti CS, et al. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feldblum PJ, et al. 2008. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One 3:e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernández-Romero JA, et al. 2007. Carrageenan/MIV-150 (PC-815), a combination microbicide. Sex. Transm. Dis. 34:9–14 [DOI] [PubMed] [Google Scholar]
- 17. Fletcher P, et al. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 79:11179–11186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fletcher P, et al. 2009. Inhibition of human immunodeficiency virus type 1 infection by the candidate microbicide dapivirine, a nonnucleoside reverse transcriptase inhibitor. Antimicrob. Agents Chemother. 53:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garg AB, Nuttall J, Romano J. 2009. The future of HIV microbicides: challenges and opportunities. Antivir. Chem. Chemother. 19:143–150 [DOI] [PubMed] [Google Scholar]
- 20. Hillier SL, et al. 2005. In vitro and in vivo: the story of nonoxynol 9. J. Acquir. Immune Defic. Syndr. 39:1–8 [DOI] [PubMed] [Google Scholar]
- 21. Hossain MM, Parniak MA. 2006. In vitro microbicidal activity of the nonnucleoside reverse transcriptase inhibitor (NNRTI) UC781 against NNRTI-resistant human immunodeficiency virus type 1. J. Virol. 80:4440–4446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson TJ, Gupta KM, Fabian J, Albright TH, Kiser PF. 2010. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 39:203–212 [DOI] [PubMed] [Google Scholar]
- 23. Kieweg SL, Katz DF. 2007. Interpreting properties of microbicide drug delivery gels: analyzing deployment kinetics due to squeezing. J. Pharm. Sci. 96:835–850 [DOI] [PubMed] [Google Scholar]
- 24. Kiser PF, et al. 2012. Design of tenofovir/UC781 combination microbicide vaginal gels. J. Pharm. Sci. 101:1852–1864 [DOI] [PubMed] [Google Scholar]
- 25. Liu S, Lu H, Neurath AR, Jiang S. 2005. Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 49:1830–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahalingam A, et al. 2010. Design of a semisolid vaginal microbicide gel by relating composition to properties and performance. Pharm. Res. 27:2478–2491 [DOI] [PubMed] [Google Scholar]
- 27. Mayer KH, et al. 2006. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS 20:543–551 [DOI] [PubMed] [Google Scholar]
- 28. Motakis D, Parniak MA. 2002. A tight-binding mode of inhibition is essential for anti-human immunodeficiency virus type 1 virucidal activity of nonnucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:1851–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pal R, et al. 2009. Characterization of vaginal transmission of a simian human immunodeficiency virus (SHIV) encoding the reverse transcriptase gene from HIV-1 in Chinese rhesus macaques. Virology 386:102–108 [DOI] [PubMed] [Google Scholar]
- 30. Parikh UM, et al. 2009. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 83:10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patton DL, et al. 2007. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob. Agents Chemother. 51:1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rasool BK, Aziz US, Sarheed O, Rasool AA. 2011. Design and evaluation of a bioadhesive film for transdermal delivery of propranolol hydrochloride. Acta Pharm. 61:271–282 [DOI] [PubMed] [Google Scholar]
- 33. Rohan LC, et al. 2010. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One 5:e9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skoler-Karpoff S, et al. 2008. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet 372:1977–1987 [DOI] [PubMed] [Google Scholar]
- 35. Van Damme L, et al. 2008. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 359:463–472 [DOI] [PubMed] [Google Scholar]
- 36. Yang H, Parniak MA, Isaacs CE, Hillier SL, Rohan LC. 2008. Characterization of cyclodextrin inclusion complexes of the anti-HIV non-nucleoside reverse transcriptase inhibitor UC781. AAPS J. 10:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zussman A, Lara L, Lara HH, Bentwich Z, Borkow G. 2003. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS 17:653–661 [DOI] [PubMed] [Google Scholar]