Abstract
PA-824 is a novel nitroimidazo-oxazine under evaluation as an antituberculosis agent. A dose-ranging randomized study was conducted to evaluate the safety, tolerability, pharmacokinetics, and early bactericidal activity of PA-824 in drug-sensitive, sputum smear-positive adult pulmonary-tuberculosis patients to find the lowest dose giving optimal bactericidal activity (EBA). Fifteen patients per cohort received oral PA-824 in doses of 50 mg, 100 mg, 150 mg, or 200 mg per kg body weight per day for 14 days. Eight subjects received once-daily standard antituberculosis treatment with isoniazid, rifampin, pyrazinamide, and ethambutol (HRZE) as a positive control. The primary efficacy endpoint was the mean rate of decline in log CFU of Mycobacterium tuberculosis in sputum incubated on agar plates from serial overnight sputum collections, expressed as log10 CFU/day/ml sputum (± standard deviation). The mean 14-day EBA of HRZE was consistent with previous studies (0.177 ± 0.042), and that of PA-824 at 50 mg, 100 mg, 150 mg, and 200 mg was 0.063 ± 0.058, 0.091 ± 0.073, 0.078 ± 0.074, and 0.112 ± 0.070, respectively. Although the study was not powered for testing the difference between arms, there was a trend toward significance, indicating a lower EBA at the 50-mg dose. Serum PA-824 levels were approximately dose proportional with respect to the area under the time-concentration curve. All doses were safe and well tolerated with no dose-limiting adverse events or clinically significant QTc changes. A dose of 100 mg to 200 mg PA-824 daily appears to be safe and efficacious and will be further evaluated as a component of novel antituberculosis regimens for drug-sensitive and drug-resistant tuberculosis.
INTRODUCTION
PA-824 is a new chemical entity and a member of a class of compounds known as nitroimidazo-oxazines, with significant antituberculosis activity and a unique mechanism of action (11). Preclinical and clinical studies suggest that PA-824 may assist in shortening tuberculosis (TB) treatment regimens and contribute to the management of drug-resistant TB (2, 9, 10). An earlier dose-ranging study of the early bactericidal activity (EBA) of PA-824 was conducted over 14 days in sputum smear-positive pulmonary-TB patients using PA-824 doses of 200 mg, 600 mg, 1,000 mg, and 1,200 mg/kg body weight. The data showed PA-824 to be well tolerated over the dose range evaluated, but despite increasing exposure with increasing doses, the resulting EBAs were equivalent for all four doses tested, with a mean daily fall in CFU of Mycobacterium tuberculosis of 0.098 log10 CFU/day/ml sputum (2). In view of the lack of EBA dose response, lower doses of 50 mg, 100 mg, 150 mg, and 200 mg PA-824 were selected for further study. Evaluation of the EBA of PA-824 at these lower doses over 14 days, accompanied by evaluation of pharmacokinetics (PK) and safety endpoints, was expected to identify both a minimally efficacious dose and the lowest dose safely inducing maximal bactericidal efficacy to inform the choice of dose for later-stage clinical development. These lower doses were expected to result in lower total drug exposure, a greater safety margin for unexpected adverse events (AEs), and, possibly, improved tolerability.
MATERIALS AND METHODS
Patients.
The study was carried out at two study centers in Cape Town, South Africa (Task Applied Science, Intercare Hospital, Bellville, and Centre for Tuberculosis Research Innovation, University of Cape Town Lung Institute), and in accordance with the principles set out in the Declaration of Helsinki and good clinical practice guidelines. Eligibility for study inclusion required study patients, male or female, aged 18 to 64 years (inclusive) and weighing between 40 kg and 90 kg (inclusive) to have newly diagnosed, previously untreated, sputum smear-positive pulmonary TB (at least 1+ on the International Union Against TB and Lung Disease/WHO scale) (8) and to be able to produce at least 10 ml of sputum overnight. Excluded were HIV-positive patients with a CD4 cell count of <300/μl, and those with any serious underlying medical condition that might endanger their safety during the study or make study endpoints difficult to interpret. Also excluded were patients with lens abnormalities and those excreting bacilli resistant to rifampin and/or isoniazid (GenoType MTBDRplus; Hain Lifesciences, Nehren, Germany); recent isoniazid intake was excluded by urine testing (BBLTaxo INH test strips; Becton Dickinson, Johannesburg, South Africa). All participants gave written informed consent.
Procedures.
The patients were randomized centrally to receive blinded PA-824 doses of 50 mg, 100 mg, 150 mg, or 200 mg once daily in groups of 15 or, as a positive control, standard antituberculosis chemotherapy in the form of weight-adjusted Rifafour e275 tablets (Sanofi-Aventis, Midrand, South Africa) containing isoniazid (H), rifampin (R), pyrazinamide (Z), and ethambutol (E) (HRZE) given to a randomized but unblinded cohort of 8 patients. The patients were hospitalized for the duration of study drug intake and assessed daily for vital signs and AEs. All drug administration was supervised. Intensive profiling of plasma PA-824 concentrations was conducted after the first dose on day 1 and the last dose on day 14 (predose and 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 12, and 16 h after dosing). Predose trough PK plasma samples were drawn daily from days 2 to 13. Sputum was collected for 16 h overnight for two nights before drug intake, daily from days 1 to 4, and every alternate day until day 14. Full blood count, coagulation studies, serum chemistry, urinalysis, and 12-lead electrocardiograms (ECGs) were performed prior to investigational drug intake and at regular intervals during and 2 weeks after the last investigational drug intake. A specialist ophthalmologic assessment took place prior to the first dose and 180 days thereafter. Following discharge from the hospital, the patients were referred to their local TB clinics for a complete course of standard antituberculosis chemotherapy.
Microbiology.
Samples were stored and transported under refrigerated conditions to the Department of Medical Biochemistry, Faculty of Health Sciences, Stellenbosch University, Cape Town, South Africa, where all microbiology was done centrally. CFU counts were performed as described previously (5, 6). Sputum smears, CFU counts, time to culture positivity (TTP) measurements, and susceptibility testing for first-line drugs were performed following standardized protocols. For CFU counting, sputum was homogenized by magnetic stirring. An equal volume of dithiothreitol (1:20 dilution; Sputasol, Oxoid, Cambridge, United Kingdom) was added to a maximum of 10 ml of homogenized sputum, vortexed for 20 s, and left to digest at room temperature for 20 min; 1 ml of this mixture was used to prepare a range of 10-fold dilutions from 100 to 10−5. From each dilution, 100 μl was plated in quadruplicate on 7H11 agar plates (Becton Dickinson) that contained 200 units/ml of polymyxin B, 10 μg/ml of amphotericin B, 100 μg/ml of ticarcillin, and 10 μg/ml of trimethoprim (Selectatab; Mast, Merseyside, United Kingdom). CFU were counted after 3 to 4 weeks of incubation at 37°C at the dilution that yielded 20 to 200 visible colonies. TTP was measured in a standard liquid culture system (Bactec MGIT 960; Becton Dickinson) (3, 4). For TTP, homogenized sputum was decontaminated (MycoPrep; Becton Dickinson), centrifuged, and resuspended, and 0.5 ml was used for incubation in duplicate. Cultures from the baseline and the last overnight sputum collection were used for susceptibility testing for first-line drugs (MGIT SIRE kit; Becton Dickinson), and the MIC of PA-824 was determined for all isolated strains using the agar proportion method with concentrations from 0.1 μg/ml to 3.2 μg/ml. Identification of M. tuberculosis to the species level was performed by PCR (12).
Pharmacological analyses.
PA-824 serum concentrations were determined by a validated high-performance liquid chromatography method (7). The SAS System for Windows (version 9.2) was employed for statistical calculations. The PK analysis was produced using WinNonlin, version 5.2 and the SAS system version 9.2. The software used for the PK-QTc statistical analysis was S-Plus, version 8. The PK profile was assessed from participants' individual plasma concentrations by applying a noncompartmental approach. For PK/pharmacodynamic (PD) evaluations of time above MIC (TMIC), values of 0.1 were used to calculate the TMIC wherever the recorded result was <0.1. For the EBA(0-14) and EBA(2-14) correlations, the maximum concentration of drug in serum (Cmax) and area under the time-concentration curve from 0 to 24 h (AUC0-24) variables at steady state were used (day 14). For the EBA(0-2) correlations, AUC, minimum concentration of drug in serum (Cmin), and Cmax values for day 1 were used.
Statistics.
The sample size of 15 patients per PA-824 group was in accordance with other phase II studies of a similar design and allowed for the possibility of up to three dropouts per treatment group, which, taking into account previous experience at these centers, represented a conservative estimate. For CFU counting, the mean of a maximum of four CFU counts at each time point was calculated, and the EBA over different time intervals during the 14 study days was determined for each individual using the following formula and averaged per treatment group: EBA(CFU) day y − x = (log CFU day y − log CFU day x)/(x − y). TTP was averaged from a maximum of two TTP readings at each time point, and EBA(TTP) was calculated in analogous fashion to EBA(CFU). EBA could be described with linear, bilinear, or nonlinear regression over time, depending on which method best fit the data. The study was not powered for difference testing, so only exploratory comparisons of the PA-824 dosages with one-way analysis of variance (ANOVA) were attempted, and no comparison was made with the bactericidal activity of the standard treatment group.
RESULTS
Subjects.
The demographic characteristics of the patients enrolled in the different treatment groups are summarized in Table 1 and Fig. 1. There were no significant differences in the demographic and anthropometric characteristics of the five treatment groups.
Table 1.
Demographic, anthropometric, and diagnostic features of participants
| Parametera | Value |
||||
|---|---|---|---|---|---|
| PA-824 |
HRZE | ||||
| 50 mg | 100 mg | 150 mg | 200 mg | ||
| No. of participants | 15 | 15 | 15 | 16 | 8 |
| Age, mean (yr ± SD) | 30.5 ± 9.7 | 29.6 ± 9.3 | 26.9 ± 8.9 | 25.3 ± 6.2 | 33.3 ± 14.1 |
| BMI, mean (kg/m2 ± SD) | 19.23 ± 2.804 | 19.32 ± 3.604 | 19.00 ± 2.048 | 19.57 ± 2.221 | 20.35 ± 2.170 |
| Wt, mean (kg ± SD) | 53.53 ± 7.359 | 55.82 ± 9.899 | 51.58 ± 6.812 | 52.17 ± 7.968 | 58.17 ± 6.724 |
| Male [n (%)] | 7 (46.7) | 8 (53.3) | 7 (46.7) | 7 (43.8) | 6 (75.0) |
| HIV positive [n (%)] | 0 | 2 (13.3) | 1 (6.7) | 2 (13.3) | 1 (12.5) |
| CD4 (mean no./cmm ± SD) | 753 ± 305 | 589 ± 235 | 691 ± 225 | 605 ± 233 | 563 ± 136 |
| Baseline sputum CFU (log CFU/ml sputum ± SD) | 6.1 ± 0.6 | 5.8 ± 0.9 | 6.0 ± 0.7 | 6.1 ± 1.2 | 6.1 ± 1.0 |
| Baseline TTP (h ± SD) | 100 ± 11 | 115 ± 32 | 103 ± 20 | 104 ± 36 | 108 ± 26 |
BMI, body mass index.
Fig 1.
Patient disposition. In the 50-mg group, 1 patient was withdrawn due to spontaneous pneumothorax requiring tube thoracostomy, which was judged by the investigator not to be drug related. In the 50-mg and 100-mg PA-824 and the HRZE groups, one patient each was lost to follow-up between the 1-month and the 6-month follow-up visits.
Efficacy.
The EBA of PA-824 evaluated by the fall in CFU per ml sputum and the prolongation of TTP is summarized in Table 2 and illustrated in Fig. 2A and B. The EBA of HRZE as a positive control showed the expected magnitude and biphasic pattern. PA-824 demonstrated substantial EBA (days 0 to 14) at all doses. Exploratory analyses demonstrated that the mean EBAs at the four PA-824 dose levels did not differ significantly from each other. However, there was a trend toward significance, indicating that the 50-mg dose may have lower EBA than the higher doses taken together, whether measured by the fall in CFU counts or by prolongation in TTP (P = 0.0605 for CFU and P = 0.0511 for TTP; EBA days 0 to 14; bilinear regression). All isolates were confirmed as M. tuberculosis, and the majority had a PA-824 MIC of <0.1 μg/ml before and after completion of drug intake; the isolate from a single patient in each of the 50-mg and 100-mg PA-824 groups and from 2 patients in the 200-mg PA-824 group had an increase in MIC from <0.1 μg/ml to >0.4 μg/ml in this period.
Table 2.
Estimation of bactericidal activity during treatment days 0 to 14, 0 to 2, and 2 to 14 in patients receiving PA-824 or HRZEa
| Method | Study period (days) | Bactericidal activity per day (SD) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PA-824 |
HRZE |
||||||||||
| n | 50 mg | n | 100 mg | n | 150 mg | n | 200 mg | n | Activity | ||
| Counting of log10 CFU of M. tuberculosis per ml sputum on solid medium | 0–14 | 12 | 0.063 (0.058) | 15 | 0.091 (0.073) | 14 | 0.078 (0.074) | 14 | 0.112 (0.070) | 8 | 0.177 (0.042) |
| 0–2 | 14 | 0.093 (0.211) | 15 | 0.111 (0.332) | 15 | −0.009 (0.290) | 14 | 0.160 (0.255) | 8 | 0.470 (0.316) | |
| 2–14 | 12 | 0.059 (0.060) | 15 | 0.088 (0.085) | 14 | 0.096 (0.098) | 14 | 0.104 (0.083) | 8 | 0.128 (0.070) | |
| Determination of prolongation of TTP (h) in liquid culture medium | 0–14 | 13 | 2.621 (2.534) | 14 | 4.969 (3.644) | 15 | 4.633 (3.687) | 16 | 4.640 (3.447) | 8 | 13.364 (3.979) |
| 0–2 | 15 | 1.483 (8.153) | 14 | −1.345 (8.586) | 15 | 4.867 (12.755) | 13 | 3.096 (8.202) | 8 | 37.016 (5.639) | |
| 2–14 | 13 | 2.958 (2.652) | 15 | 5.744 (3.973) | 15 | 4.594 (5.035) | 13 | 5.391 (3.608) | 8 | 9.422 (4.367) | |
Note that EBA (days 0 to 14) can deviate from the arithmetic sum of EBA (days 0 to 2) and EBA (days 2 to 14) due to missing data points.
Fig 2.
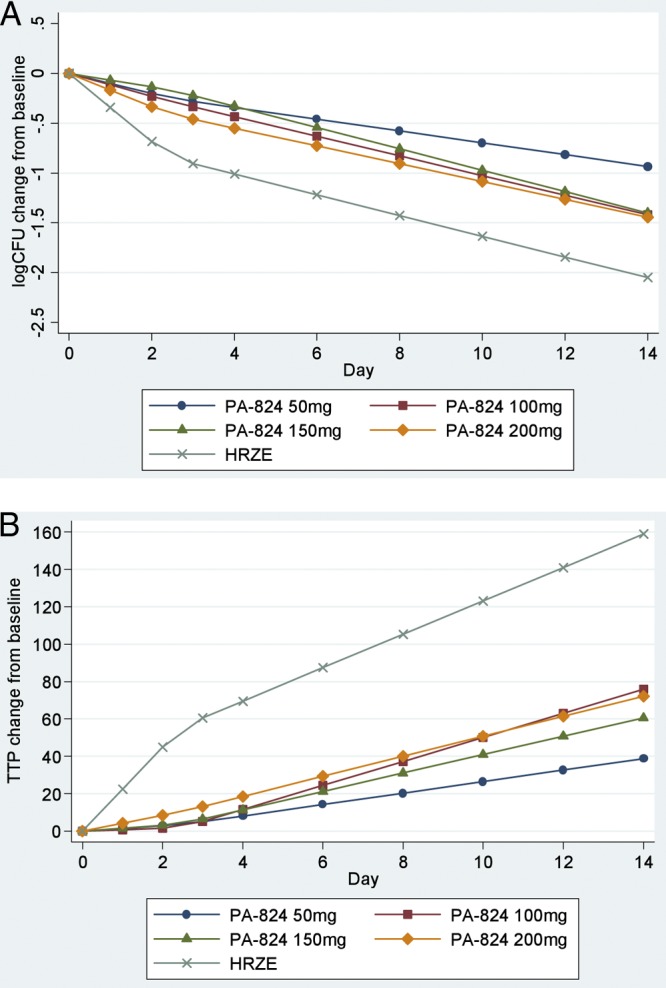
Bilinear regression of log10 CFU (A) and TTP (B) on the day of treatment. Shown is bilinear regression with an inflection point of 2.5 days, illustrating the early bactericidal activity of PA-824 measured by the decline in CFU of M. tuberculosis (A) and by the increase in time to positivity (B) in liquid culture following treatment with PA-824 doses of 50 mg, 100 mg, 150 mg, and 200 mg and Rifafour e275 (HRZE).
Pharmacokinetics.
PA-824 exhibited characteristics consistent with linear PK over the dose range studied. Figure 3 illustrates the arithmetic mean PA-824 concentrations over time for all four PA-824 treatment groups. The shapes of the concentration-time profiles were similar with increasing doses, indicating that neither the absorption nor the elimination kinetics appear to change with the dose. Collectively, these PK data suggest that plasma PA-824 levels increased with the dose in an approximately dose-proportional manner with respect to the AUC. The accumulation ratio (AUC0-24, day 14/AUC0-24, day 1) was approximately 1.8, which is consistent with the reported half-life of PA-824 and once-daily dose administration. The accumulation ratio (AUC0-24, day 14/AUC0-∞, day 1), calculated to determine the time-related linearity of exposure across the dose duration, was approximately 1 for all doses studied, which is consistent with time-invariant PK of PA-824. These findings are qualitatively similar to those obtained in previous studies (7). It should be noted that AUC(0-∞, day 1) is based on an extrapolated AUC, where the extrapolated portion represents on average 40% to 50% of the final estimated value, and therefore should be interpreted with caution. After single-dose administration, the mean Cmax ranged from 465.3 ng/ml (50 mg) to 1,183.0 ng/ml (200 mg) and the mean total exposure (AUC0-∞) from 11.9 μg · h/ml (50 mg) to 38.5 μg · h/ml (200 mg). Steady state was achieved after 5 to 7 days. PA-824 elimination followed a log-linear pattern, with a mean half-life (t1/2) ranging across treatment groups from 16.1 to 23.8 h. Total exposures (AUC) and t1/2 values appeared to be somewhat higher in females than in males. At day 2, all M. tuberculosis isolates had an MIC of <0.1 μg/ml, and the TMIC(0-2) observed was above 98% for all four PA-824 treatment groups. The TMIC(2-14) observed was lowest for the 50-mg PA-824 group (91.45%; standard deviation [SD], 6.114), and increased with the increase in dosage. For the 100-mg PA-824 group, the TMIC(2-14) was 93.42% (SD, 5.885); for the 150-mg PA-824 group, 95.81% (SD, 4.323); and for the 200-mg PA-824 group, 98.82% (SD, 2.218). Similar results were observed for TMIC(0-14). The PK-PD correlations observed, however, were weak, and no overall trends were observed within dose groups. The small number of subjects and significant overlap in exposure across the dose range make it difficult to observe overall trends in these data.
Fig 3.
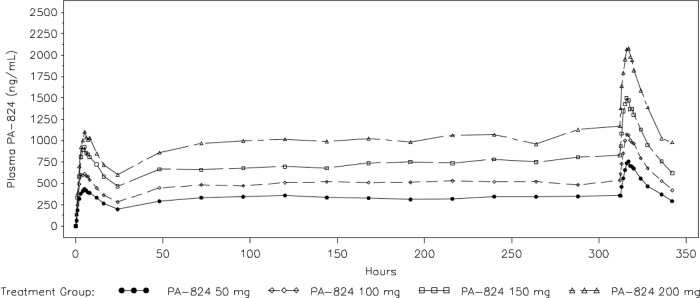
PA-824 plasma concentrations. Shown are arithmetic mean PA-824 plasma concentrations determined intensively over the first and the last 24 h of treatment and predose from day 2 to day 13 in patients receiving doses of 50 mg, 100 mg, 150 mg, and 200 mg. The shapes of the concentration-time profiles were similar with increasing doses, indicating that neither the absorption nor the elimination kinetics appear to change with the dose for all PA-824 groups.
Safety.
PA-824 was well tolerated. There were only two serious AEs: 1 patient in the PA-824 50-mg dosage group developed a spontaneous pneumothorax that necessitated premature withdrawal from the study, and 1 patient receiving PA-824 at 200 mg developed pneumonia, which did not result in drug discontinuation or withdrawal from the study. Neither event was considered drug related. The incidence of AEs varied from 67.5% in the PA-824 50-mg dosage group to 33% in the 100-mg and 150-mg groups, 44% in the 200-mg group, and 50% in the standard-treatment group. A similar percentage of AEs (13 to 20%) in each of the PA-824 groups was considered drug related. Disorders of the skin and subcutaneous tissue (pruritis and macular or papular rashes) were the most common AEs, affecting 6 (33.3%), 2 (13.3%), 1 (6.7%), and 4 (25%) subjects in the 50-mg, 100-mg, 150-mg, and 200-mg groups, respectively, but also 2 (25%) subjects receiving HRZE. In all of the PA-824 groups except the 50-mg group, the mean serum creatinine concentration increased transiently between the pretreatment and posttreatment assessments, as expected from previous studies of PA-824, but remained within normal limits at all time points. This phenomenon has been demonstrated to be reversible and not clinically significant (7). No lens opacity developed in any patient receiving PA-824. A trend toward a decrease in heart rate in all five treatment groups postdose was noted. QT in patients receiving PA-824 was <450 ms at most assessments, and in no patient did it exceed 480 ms; increases from baseline of >60 ms were observed in 1 patient in the PA-824 50- and 100-mg groups and in 3 patients each in the 150-mg and 200-mg dose groups. Similar results were observed for QTcB; however, no change from baseline was >60 ms. QTcF was <450 ms at all visits in all subjects, and the change from baseline was usually <30 ms and exceeded 60 ms in only one patient. The analysis of the plasma PK-dQTcF relationship showed a significant correlation of the plasma concentration with the dQTcF, with a positive slope; a straight-line model predicted a change in QTcF of 9.3 ms for a change in the plasma concentration of 1,022 ng/ml (the observed mean for the 200-mg PA-824 recipients). For the maximum observed concentration of 2,200 ng/ml found following a 200-mg dose, the predicted change is +11.2 ms. The only clinically significant ECG defect reported was in a subject in the PA-824 100-mg group, who developed a left anterior hemiblock that was still present 14 days after cessation of study drug intake.
DISCUSSION
This study of the 14-day EBA of PA-824 found bactericidal activity that, taken in conjunction with the findings of the previous EBA study, shows uniform activity across a wide spectrum of doses ranging from 100 mg to 1,200 mg daily (2). As in previous studies, the wide range of individual patient responses found is reflected in the wide SD of activity in both the CFU-counting and TTP results. Nonetheless, a trend can be discerned toward a somewhat lower PA-824 efficacy at a dose of 50 mg. PA-824 was safe and well tolerated across the doses tested. These findings allow a considerable degree of latitude in the choice of dose to take forward in further studies of PA-824, permitting the use of a dose well above the point at which efficacy appears to decline but at the same time limiting the degree and number of AEs that might be associated with higher doses. A PA-824 dose of 100 mg to 200 mg appears to be the optimal choice for evaluation in future clinical studies. A recent dose fractionation study in mice indicated that PA-824 activity is time dependent and predicted, along with PK modeling of clinical dosages and consistent with the results of the current study, that dosages as low as 100 mg per day of PA-824 would produce a maximum bactericidal effect (1).
Remarkably, the efficacy seen with the 200-mg PA-824 dose and with HRZE was very similar to those seen in the previous “high-dose” PA-824 EBA study performed earlier at the same sites (2). For the same dose groups, the fall in CFU counts per ml sputum and the increase in TTP (days 0 to 14) in the earlier study for PA-824 at 200 mg were 0.106 (SD, 0.049) and 3.818 (SD, 2.327), respectively, and for HRZE they were 0.148 (SD, 0.055) and 9.741 (SD, 5.249), respectively, demonstrating good reproducibility for both methodologies between studies conducted at these sites (2). TTP has recently been shown to discriminate between groups as well as CFU counts do in EBA studies (2). TTP is more sensitive than culture on solid media, technically accessible, and relatively easy to standardize, which makes TTP a promising future biomarker of the early efficacy of potential antituberculosis drugs and regimens.
The PK properties of PA-824 observed in this study are consistent with those observed during previous PA-824 clinical studies (2, 7). Over the PA-824 dose range evaluated, the observed serum creatinine increase was mild, transient, and not clinically relevant, while the mild QTc interval prolongation appears acceptable for pulmonary-TB treatment at a proposed clinical dose of 100 mg to 200 mg.
In conclusion, a dose of 100 mg to 200 mg PA-824 daily appears safe and efficacious, and its further evaluation as a component of novel antituberculosis regimens for drug-sensitive and drug-resistant TB is planned.
ACKNOWLEDGMENTS
This work was supported by the Global Alliance for TB Drug Development, New York, NY.
We thank all participating patients and our site staff, and the Department of Health, Provincial Administration of the Western Cape, and City Health, City of Cape Town, for permission to recruit patients from their clinics.
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Ahmad Z, et al. 2011. PA-824 exhibits time-dependent activity in a murine model of tuberculosis. Antimicrob. Agents Chemother. 55:239–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diacon AH, et al. 2010. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob. Agents Chemother. 54:3402–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diacon AH, et al. 2010. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur. J. Clin. Microbiol. Infect. Dis. 29:1561–1565 [DOI] [PubMed] [Google Scholar]
- 4. Diacon AH, et al. 18 August 2011, posting date Time to liquid culture positivity can substitute for colony counting on agar plates in early bactericidal activity studies of antituberculosis agents. Clin. Microbiol. Infect. [Epub ahead of print.] doi:10.1111/j.1469-0691.2011.03626.x [DOI] [PubMed] [Google Scholar]
- 5. Donald PR, Diacon AH. 2008. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis (Edinburgh) 88(Suppl. 1):S75–S83 [DOI] [PubMed] [Google Scholar]
- 6. Donald PR, et al. 2003. Early bactericidal activity of antituberculosis agents. Expert Rev. Anti Infect. Ther. 1:141–155 [DOI] [PubMed] [Google Scholar]
- 7. Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. 2009. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob. Agents Chemother. 53:3720–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. International Union against Tuberculosis and Lung Disease 2000. Technical guide. Sputum examination for tuberculosis by direct microscopy in low income countries. http://www.theunion.org/component/option,com_guide/Itemid,79/
- 9. Nuermberger E, et al. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52:1522–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singh R, et al. 2008. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 322:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stover CK, et al. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962–966 [DOI] [PubMed] [Google Scholar]
- 12. Warren RM, et al. 2006. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int. J. Tuberc. Lung Dis. 10:818–822 [PubMed] [Google Scholar]



