Abstract
The treatment of Gram-negative infections is increasingly compromised by the spread of resistance. With few agents currently in development, clinicians are now considering the use of unorthodox combination therapies for multidrug-resistant strains. Here we assessed the in vitro activity of the novel lipoglycopeptide telavancin (TLV) when combined with colistin (COL) versus 13 Gram-negative type strains and 66 clinical isolates. Marked synergy was observed in either checkerboard (fractional inhibitory concentration index [FICI], <0.5; susceptibility breakpoint index [SBPI], >2) or time-kill assays (>2-log reduction in viable counts compared with starting inocula at 24 h) versus the majority of COL-susceptible enterobacteria, Stenotrophomonas maltophilia, and Acinetobacter baumannii isolates, but only limited effects were seen against Pseudomonas aeruginosa or strains with COL resistance. Using an Etest/agar dilution method, the activity of TLV was potentiated by relatively low concentrations of COL (0.25 to 0.75 μg/ml), reducing the MIC of TLV from >32 μg/ml to ≤1 μg/ml for 35% of the clinical isolates. This provides further evidence that glycopeptide-polymyxin combinations may be a useful therapeutic option in the treatment of Gram-negative infections.
INTRODUCTION
Multidrug-resistant (MDR) bacteria represent an enormous challenge to modern health care systems. Although some new agents have been introduced in the last 10 years (e.g., linezolid, daptomycin, and tigecycline) (12, 14), these agents predominantly target Gram-positive bacteria, many of which still remain susceptible to older, conventional drugs (e.g., glycopeptides, macrolides, and fluoroquinolones). The problem is now most acute with respect to Gram-negative infections (7). In hospitals, MDR strains of Acinetobacter baumannii (5) and Pseudomonas aeruginosa (3) are increasingly responsible for infections in the critically ill. In both hospitals and the community, the emergence and rapid spread of extended-spectrum β-lactamases (CTX-M) and carbapenemases (KPC, NDM-1) in Enterobacteriaceae (e.g., Escherichia coli and Klebsiella spp.) threaten the treatment of even mild infections with these organisms (6, 27). With very few options now left, unorthodox combination therapies are increasingly being considered.
Previously, we investigated the activities of the glycopeptides vancomycin (4) and teicoplanin (25) combined with low concentrations of colistin (COL) versus MDR A. baumannii. Potent synergy was observed with both agents in vitro, and the combination was also effective in an invertebrate model of A. baumannii infection (8), a phenomenon thought to be mediated by the ability of colistin to permeabilize the Gram-negative outer membrane to large hydrophobic molecules, which are usually excluded (21).
Telavancin (TLV) is a novel lipoglycopeptide antibiotic, structurally related to vancomycin, that was approved in 2009 in the United States and Canada for the treatment of complicated skin and skin structure infections (cSSSI) caused by susceptible Gram-positive bacteria and was recently approved in Europe for treatment of nosocomial pneumonia, including ventilator-associated pneumonia known or suspected to be caused by methicillin-resistant Staphylococcus aureus (MRSA). Telavancin is highly active against most aerobic and anaerobic Gram-positive bacteria but differs from vancomycin by the addition of a decylaminoethyl side chain (Fig. 1). This lipophilic side chain anchors the molecule in the bacterial cytoplasmic membrane, increasing the affinity for peptidoglycan precursor targets located at the base of the cell wall as well as directly destabilizing the cell membrane (13). Although telavancin has no useful activity against Gram-negative bacteria alone, its activity could potentially be improved if given in combination with other antimicrobial agents. In this study, we assessed the in vitro activity of telavancin when combined with colistin against A. baumannii and other common Gram-negative pathogens.
Fig 1.
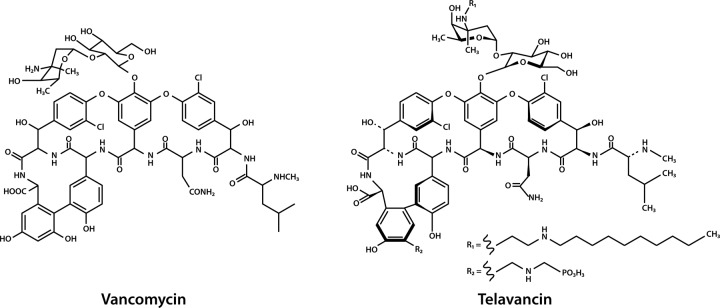
Comparative structures of vancomycin and telavancin. (These structures are in the public domain.)
(This work was presented in part at the European Congress of Clinical Microbiology and Infectious Diseases 2011 in Milan, Italy.)
MATERIALS AND METHODS
Antimicrobial compounds.
Colistin sulfate was obtained from Sigma-Aldrich Ltd. (Poole, Dorset, United Kingdom), and telavancin was obtained from Astellas Pharma Europe (lot no. 07-2460). Stock solutions of 10,000 μg/ml (COL) and 2,000 μg/ml (TLV) were prepared in sterile distilled water and 100% dimethyl sulfoxide (DMSO), respectively, and diluted with Iso-Sensitest broth (Oxoid, Basingstoke, United Kingdom) to working-strength concentrations.
Bacterial strains.
Representative bacterial type strains were obtained from the National Collection of Type Cultures (NCTC; Health Protection Agency, London, United Kingdom) and consisted of A. baumannii (ATCC19606 NCTC 12156), E. coli NCTC 12241, E. coli NCTC 11954, Enterobacter aerogenes NCTC 9735, Enterobacter cloacae NCTC 10005, E. cloacae NCTC 13380, Klebsiella pneumoniae NCTC 13368, Proteus mirabilis NCTC 13376, P. aeruginosa NCTC 27853, Serratia marcescens NCTC 13382, and Stenotrophomonas maltophilia NCTC 10258. The MICs of COL and TLV were determined individually for each isolate by Etest (bioMérieux, Marcy l'Étoile, France) on Iso-Sensitest agar (Oxoid), by broth microtiter dilution and, when grown in 10 ml of Iso-Sensitest broth, with rapid shaking prior to performing time-kill assays.
Telavancin-colistin checkerboard assays.
Synergy between telavancin and colistin was assessed in microtiter plate checkerboard assays. Plates were set up with increasing concentrations of telavancin (0 to 128 μg/ml) in each column and colistin (0 to 8 μg/ml) in each row. Wells were inoculated with 105 CFU/ml of the test organism and incubated at 37°C for 18 h in 5% CO2. Checkerboards containing S. maltophilia as the test organism were incubated at 30°C (10). Plates were assessed for turbidity by eye, and absence of growth in nonturbid wells was confirmed by the addition of 5 μl of alamarBlue reagent (Invitrogen, Paisley, United Kingdom), a redox dye that turns red in the presence of metabolically active bacteria. Fractional inhibitory concentration indices (FICI) were calculated as follows: [(MIC of TLV in combination with COL)/(MIC of TLV alone)] + [(MIC of COL in combination with TLV)/(MIC of COL alone)], and a FICI of ≤0.5 in the well with the lowest FIC was used to define synergy. Synergy was also assessed using the susceptibility breakpoint index (SBPI), which was calculated as follows: [(susceptibility breakpoint of TLV)/(MIC of TLV in combination with COL)] + [(susceptibility breakpoint of COL)/(MIC of COL in combination with TLV)] (15). An SBPI of >2 indicated synergistic activity resulting in a MIC for the combination that was lower than the clinical breakpoint of either drug. The U.S. FDA and EUCAST breakpoint of ≤1 μg/ml for staphylococci was used to define susceptibility of the test organism to telavancin (http://www.astellas.us/docs/us/VIBATIV_PI_Final.pdf).
Time-kill assays.
The bactericidal activity of the TLV-COL combination was assessed over 24 h by performing viable counts of bacteria grown in Iso-Sensitest broth, supplemented with or without COL and/or TLV. TLV was added at a fixed concentration of 20 μg/ml, corresponding to the predicted 12-h steady-state plasma drug concentration after administration of 10 mg/kg of body weight to healthy subjects (19). COL was added at one dilution below (0.5× MIC) the concentration required to prevent growth of the organism in 10 ml of Iso-Sensitest broth after incubation for 24 h with vigorous shaking (250 rpm), up to a maximum concentration of 256 μg/ml. A starting inoculum of approximately 106 CFU/ml prepared from an overnight culture was used, and serial dilutions were plated onto Iso-Sensitest agar plates at 0, 2, 4, 6, and 24 h. Bactericidal activity was defined as a >3-log reduction in the viable count at each time point, and synergy was defined as a >2-log reduction in viable count at 24 h compared to the starting inoculum.
Synergy screen of clinical isolates.
A screen for TLV-COL synergy was also conducted using the 13 type strains and 66 random COL-susceptible Gram-negative clinical isolates. These bacteria consisted of 43 nonfermenters (A. baumannii [n = 35], S. maltophilia [n = 8]) and 23 Enterobacteriaceae (K. pneumoniae [n = 12], E. aerogenes [n = 6], E. coli [n = 4], E. cloacae [n = 1]) obtained from the clinical laboratory at Barts and The London NHS Trust, London, United Kingdom. Isolates were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker UK Limited, Coventry, United Kingdom), and all A. baumannii isolates were confirmed by the species-specific blaOXA-51-like PCR described by Woodford et al. (26). Routine susceptibility testing was conducted using the MicroScan WalkAway system (Siemens, Sacramento, CA). Screening for synergy between the two compounds was performed using an Etest method with COL added to Iso-Sensitest agar plates at fixed subbreakpoint concentrations of 0.25 to 0.75 μg/ml. TLV MIC50 and MIC90 values and mean fold reductions in the MICs of TLV (“sensitization factor”) (23) were calculated on this medium and compared to those obtained on unsupplemented Iso-Sensitest agar.
RESULTS
Synergy between TLV and COL in checkerboard assays.
The 11 bacterial type strains were all highly resistant to TLV, with MICs of >32 μg/ml when determined by Etest and >128 μg/ml in broth microtiter dilution plates. The P. mirabilis NCTC 13376 and S. marcescens NCTC 13382 isolates were both highly resistant to COL (MICs, >256 μg/ml), a property intrinsic to these species, as was one of the E. cloacae type strains (NCTC 10005). The remaining eight organisms were considered susceptible to COL (MICs, <2 μg/ml by Etest) (Table 1). COL MICs were between 2- and 64-fold higher when determined by broth microtiter dilution and up to 256-fold higher in the kinetic assays (Table 1).
Table 1.
Summary of the in vitro activity of telavancin combined with colistin versus bacterial type strains
| Organism | Strain | MIC, in μg/ml (method) |
Synergy testing results |
||||||
|---|---|---|---|---|---|---|---|---|---|
| TLV (Etest) | COL (Etest) | COL (microtiter dilution) | COL (shaken in broth)a | Lowest FICI | SBPI | TLV MIC by Etest (concn of COL in agar)b | Synergy by time-kill? | ||
| A. baumannii | ATCC 19606 | >32 | 0.125 | 1 | 2 | 0.252 | 8 | 0.25 (0.125) | Yes |
| E. coli | NCTC 12241 | >32 | 0.125 | 2 | 16 | 0.141 | 8.5 | 0.25 (0.125) | No |
| E. coli | NCTC 11954 | >32 | 0.5 | 1 | 8 | 0.252 | 8 | 1 (0.125) | Yes |
| E. aerogenes | NCTC 9735 | >32 | 0.25 | 1 | 32 | 0.5 | 6 | 0.125 (0.25) | Yes |
| E. cloacae | NCTC 10005 | >32 | >256c | >8 | >256 | 2 | < 0.13 | >32 (0.75) | No |
| E. cloacae | NCTC 13380 | >32 | 0.125 | 0.25 | 0.5 | 2 | 8 | 1 (0.125) | No |
| K. pneumoniae | NCTC 13368 | >32 | 0.5 | 1 | 8 | 0.258 | 3 | 1 (0.25) | Yes |
| P. mirabilis | NCTC 13376 | >32 | >256c | >8 | >256 | 2 | < 0.13 | >32 (0.75) | No |
| P. aeruginosa | NCTC 27853 | >32 | 0.5 | 1 | 2 | 2 | 2 | >32 (0.125) | Yes |
| S. marcescens | NCTC 13382 | >32 | >256c | >8 | >256 | 2 | < 0.13 | >32 (0.75) | No |
| S. maltophilia | NCTC 10258 | >32 | 0.125 | 8 | 32 | 0.033 | 10 | 1 (0.25) | No |
Concentration of COL required to prevent visible growth in 10 ml of Iso-Sensitest broth with vigorous shaking at 37°C for 24 h.
Highest concentration (in μg/ml) supporting semiconfluent growth on Iso-Sensitest agar.
COL-resistant isolate.
In checkerboard assays, synergy (FICI, ≤0.5) was observed when TLV was combined with COL for all of the COL-susceptible isolates with the exceptions of E. cloacae NCTC 13380 and P. aeruginosa NCTC 27853. The combination had no significant effects on any of the COL-resistant strains. Calculation of the susceptibility breakpoint indices for strains with a TLV-COL FICI of ≤0.5 revealed an SBPI of >2 for each (range, 3 to 10), suggesting that the strength of the interaction observed was consistent with currently accepted TLV and COL pharmacodynamic breakpoints (Table 1).
Time-kill assays.
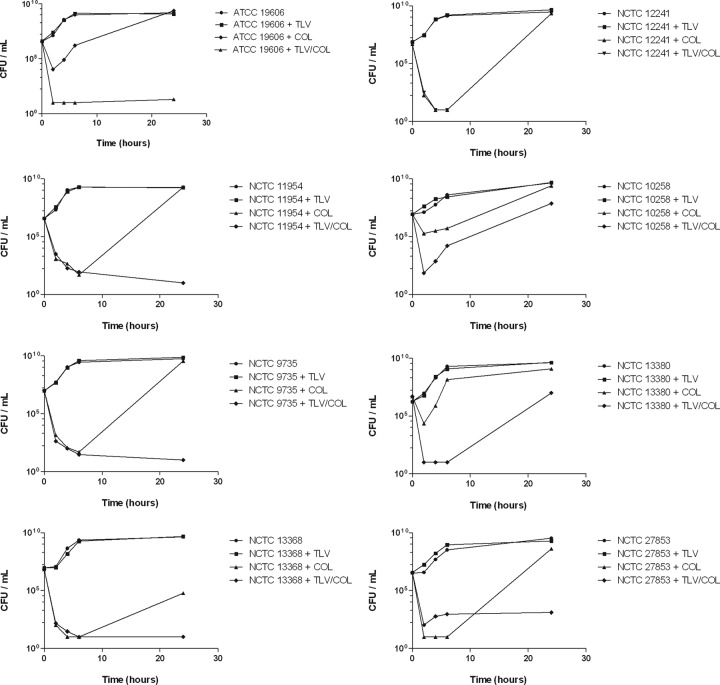
Killing of each of the type strains by TLV (20 μg/ml), COL (0.5× MIC), and TLV in combination with COL was assessed over 24 h using a time-kill methodology. TLV alone had no effect, and viable counts were comparable to untreated controls. A subinhibitory concentration of COL was initially bactericidal against all COL-susceptible isolates, but this was not sustained, and each isolate was able to regrow to a bacterial density higher than the starting inoculum, except for K. pneumoniae NCTC 13368 (Fig. 2). However, the addition of TLV (20 μg/ml) resulted in continued bactericidal activity and synergy (>2-log reduction in CFU/ml) after 24 h for five of the type strains tested (Table 1).
Fig 2.
Time-kill assays with TLV (20 μg/ml), COL (0.5× MIC), and the TLV-COL combination versus COL-susceptible type strains.
Activity of TLV-COL versus clinical isolates.
TLV Etests and COL-supplemented Iso-Sensitest plates were used as a method of easily assessing synergy within the constraints of a routine diagnostic laboratory (Fig. 3). Each of the isolates studied was susceptible to COL (MIC, <2 μg/ml), and all of the A. baumannii isolates were MDR (susceptible only to COL and tigecycline). The mean reduction in the TLV MIC was >5-fold for all of the Gram-negative isolates tested, with 35% rendered susceptible based on the FDA/EUCAST staphylocococcal breakpoint (≤1 μg/ml). MIC50 and MIC90 values and mean fold reductions in the TLV MICs for each species are shown in Table 2.
Fig 3.
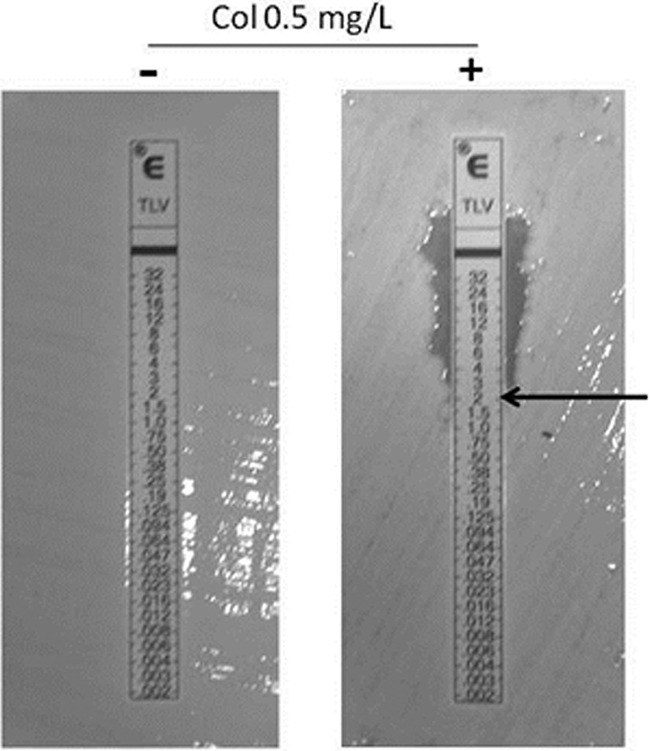
TLV-COL synergy testing by the Etest/agar dilution method. The MICs of TLV versus MDR A. baumanii with (+) and without (−) COL supplementation are shown.
Table 2.
Activity of TLV in combination with COL against clinical isolates
| Species (no. of isolates) | TLV MIC (μg/ml) results on Iso-Sensitest + COLa |
% susceptibleb | ||
|---|---|---|---|---|
| MIC50 | MIC90 | Mean fold reduction | ||
| A. baumannii (35) | 2 | 16 | >4.5 | 20 |
| S. maltophilia (8) | 2 | 2 | >5 | 12.5 |
| Enterobacteriacae (23) | 0.5 | 4 | >6 | 65 |
| All isolates (66) | 2 | 12 | >5 | 35 |
Data were obtained using the highest concentration of COL (0.25 to 0.75 μg/ml) able to support semiconfluent growth on Iso-Sensitest agar for each isolate.
Based on the FDA/EUCAST breakpoint for staphylococci of ≤1 μg/ml.
DISCUSSION
The erosion of effective treatments by resistance, combined with a drug development pipeline that is almost dry, has renewed interest in using unorthodox therapies for Gram-negative infections. In this study we looked at the effects of combining the polymyxin E derivative COL with the novel lipoglycopeptide TLV. Using a number of methods, we demonstrated that in vitro, TLV was highly active when combined with COL against A. baumannii, E. coli, K. pneumoniae, Enterobacter spp., and S. maltophilia type strains and representative clinical isolates. When used together, the drugs were not only synergistic but also bactericidal and prevented the regrowth of COL-exposed bacteria in time-kill assays, with the exception of S. maltophilia NCTC 10258. These data suggest that a TLV-COL combination could be a useful option for the treatment of complicated Gram-negative bacterial infections.
We were unable to demonstrate significant synergy versus any of the isolates exhibiting COL resistance, most likely due to the inability of COL to disrupt the outer membrane of these strains. In P. mirabilis and S. marcescens, resistance to COL is an intrinsic property mediated by modifications to phosphate residues within the lipopolysaccharide (LPS) core region. This alters the net charge on the LPS molecule, which inhibits electrostatic binding of COL to lipid A and its ability to destabilize the membrane (9). Mutations in either the LPS biosynthetic genes or the regulators of two-component systems involved in their expression have also been shown to lead to acquired COL resistance in a number of Gram-negative species (1, 11), making it unlikely that a TLV-COL combination would be active against these isolates as well. A number of other cell-permeabilizing peptides have been shown to be active against COL-resistant organisms, notably mastaparan (24, 28), and it may prove useful to investigate the activities of glycopeptides in combination with these compounds.
The combination was also only found to be synergistic versus P. aeruginosa NCTC 27853 in the time-kill assay, despite the strain appearing to be fully susceptible to COL when we used static methods (Etest or broth microtiter dilution). When polymyxins have been combined with other antimicrobials usually inactive against P. aeruginosa (e.g., macrolides or rifampin), marked synergy has been reported, suggesting that COL is at least capable of permeabilizing the membrane of this organism (22). Interestingly, the NCTC 27853 strain has recently been shown to exhibit a heteroresistant phenotype when subjected to population analysis (2). This could explain our observations in the time-kill assay, whereby COL exposure led to the selection of a COL-resistant subpopulation that was susceptible to the action of TLV. Heteroresistance to COL is increasingly reported in both type strains and clinical isolates. Although we did not undertake a formal population analysis, this appears to be a property of the E. coli NCTC 12241 isolate, as COL resistance was readily selected at 0.5× MIC even when combined with TLV (Fig. 2).
TLV is the latest glycopeptide to be studied in combination with COL. Its antimicrobial activity can clearly be potentiated by COL, not just toward MDR A. baumanii but also a wider range of Gram-negative species. Although these in vitro studies did not evaluate other glycopeptides, the selection of TLV may have provided some practical advantages. In contrast to other glycopeptides, TLV has potent and rapidly bactericidal activities against Gram-positive bacteria and retains activity against vancomycin-intermediate S. aureus strains, which may be advantageous when mixed Gram-positive and Gram-negative infections are suspected. The drug exhibits linear pharmacokinetics when administered as a once-daily infusion (16), and unlike vancomycin and teicoplanin, there is no requirement for therapeutic monitoring of drug levels. The incidence of adverse events, such as pruritus, rashes, and “red man syndrome,” appears to be lower than those reported following administration of vancomycin (20). TLV is associated with renal toxicity, which has been reported more frequently than with vancomycin in a number of clinical trials. As COL administration alone is also associated with significant nephrotoxicity (17), there may be concerns over its use in combination with TLV or other glycopeptides. Nevertheless, it should be stressed that the concentrations of COL required to mediate TLV synergy in vitro are relatively low, which may reduce the risk of developing renal impairment if the agents are given together. A pharmacokinetic study will be needed to assess how best to dose and monitor the two drugs in combination before any firm conclusions can be drawn.
TLV is approved in Europe (although not in the United States or Canada) for the treatment of nosocomial pneumonia, including ventilator-associated pneumonia known or suspected to be caused by MRSA. Accurate identification of the organisms responsible for these conditions is challenging, but Gram-negative organisms are increasingly implicated. Therapy is often empirical and selected to target the most likely organisms involved in individual patients. A TLV-COL combination would cover both Gram-positive and COL-susceptible Gram-negative bacteria, which may be synergistic in some cases. Concerns over TLV-COL nephrotoxicity may also be less important if COL were to be administered in aerosolized form. Nebulized polymyxins have been increasingly used in the treatment of MDR Gram-negative respiratory tract infections in a number of settings (e.g., ventilator-associated pneumonia, chronic cystic fibrosis infections), and there is little evidence to suggest they promote renal impairment when delivered via a nebulizer (18).
A number of other glycopeptides (e.g., dalbavancin, oritavancin) are in development, as well as novel polymyxin derivatives that have been engineered to improve their antimicrobial and permeability effects while reducing nephrotoxicity (23, 24). The potential synergistic effects of these novel glycopeptides and polymyxin derivatives could be assessed to determine the optimum combination to maximize activity against Gram-negative organisms while minimizing potential adverse events.
Although we were able to show synergy against a range of COL-susceptible bacteria, this was still species and strain specific. In view of this, we do not advocate use of COL-glycopeptide combinations without prior in vitro evidence of potential benefit. This may prove to be a problem, as current methods for assessing the strengths of antimicrobial combinations are not practical for most routine diagnostic laboratories. Synergy testing using a time-kill methodology is extremely labor-intensive, as are checkerboard assays if custom-made prediluted plates are not available. Automated platforms are also usually not configured to assess the activities of drugs in combination. Laboratories are likely to be asked to investigate the potential of an unorthodox combination on a case-by-case basis, either due to multidrug resistance or complications with conventional therapies. We suggest that an Etest-based method, such as the one used here, may be the most adaptable methodology to employ in such a setting.
In summary, we have provided further evidence that glycopeptide-COL combinations have useful antimicrobial activities against Gram-negative bacteria. With very few new agents likely to become available for the treatment of MDR Gram-negative bacteria in the next 5 to 10 years, combinations such as these could be considered potential therapies that can help bridge the developmental gap. Further in vivo and clinical studies will be required to support this suggestion.
ACKNOWLEDGMENTS
This work was funded by Astellas Pharma Europe Limited.
Circulation of the manuscript for sponsor review and collation of comments was coordinated by Emily Hutchinson, a medical writer at Envision Scientific Solutions, which is funded by Astellas Pharma Europe Limited.
Footnotes
Published ahead of print 9 April 2012
REFERENCES
- 1. Beceiro A, et al. 2011. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 55:3370–3379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergen PJ, et al. 2011. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 55:5685–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Doyle JS, Buising KL, Thursky KA, Worth LJ, Richards MJ. 2011. Epidemiology of infections acquired in intensive care units. Semin. Respir. Crit. Care Med. 32:115–138 [DOI] [PubMed] [Google Scholar]
- 4. Gordon NC, Png K, Wareham DW. 2010. Potent synergy and sustained bactericidal activity of a vancomycin-colistin combination versus multidrug-resistant strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 54:5316–5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gordon NC, Wareham DW. 2010. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int. J. Antimicrob. Agents 35:219–226 [DOI] [PubMed] [Google Scholar]
- 6. Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin. Infect. Dis. 53:60–67 [DOI] [PubMed] [Google Scholar]
- 7. Ho J, Tambyah PA, Paterson DL. 2010. Multiresistant Gram-negative infections: a global perspective. Curr. Opin. Infect. Dis. 23:546–553 [DOI] [PubMed] [Google Scholar]
- 8. Hornsey M, Wareham DW. 2011. In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 55:3534–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang SS, et al. 2010. Proteus mirabilis pmrI, an RppA-regulated gene necessary for polymyxin B resistance, biofilm formation, and urothelial cell invasion. Antimicrob. Agents Chemother. 54:1564–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. King A. 2001. Recommendations for susceptibility tests on fastidious organisms and those requiring special handling. J. Antimicrob. Chemother. 48(Suppl. 1):77–80 [DOI] [PubMed] [Google Scholar]
- 11. Lee H, Hsu FF, Turk J, Groisman EA. 2004. The PmrA-regulated pmrC gene mediates phosphoethanolamine modification of lipid A and polymyxin resistance in Salmonella enterica. J. Bacteriol. 186:4124–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Livermore DM. 2011. Discovery research: the scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 66:1941–1944 [DOI] [PubMed] [Google Scholar]
- 13. Lunde CS, et al. 2009. Telavancin disrupts the functional integrity of the bacterial membrane through targeted interaction with the cell wall precursor lipid II. Antimicrob. Agents Chemother. 53:3375–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Micek ST. 2007. Alternatives to vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin. Infect. Dis. 45(Suppl. 3):S184–S190 [DOI] [PubMed] [Google Scholar]
- 15. Milne KE, Gould IM. 2010. Combination testing of multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J. Antimicrob. Chemother. 65:82–90 [DOI] [PubMed] [Google Scholar]
- 16. Pankuch GA, Appelbaum PC. 2009. Postantibiotic effects of telavancin against 16 gram-positive organisms. Antimicrob. Agents Chemother. 53:1275–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paul M, et al. 2010. Effectiveness and safety of colistin: prospective comparative cohort study. J. Antimicrob. Chemother. 65:1019–1027 [DOI] [PubMed] [Google Scholar]
- 18. Rattanaumpawan P, Lorsutthitham J, Ungprasert P, Angkasekwinai N, Thamlikitkul V. 2010. Randomized controlled trial of nebulized colistimethate sodium as adjunctive therapy of ventilator-associated pneumonia caused by Gram-negative bacteria. J. Antimicrob. Chemother. 65:2645–2649 [DOI] [PubMed] [Google Scholar]
- 19. Shaw JP, Cheong J, Goldberg MR, Kitt MM. 2010. Mass balance and pharmacokinetics of [14C]telavancin following intravenous administration to healthy male volunteers. Antimicrob. Agents Chemother. 54:3365–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stryjewski ME, et al. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46:1683–1693 [DOI] [PubMed] [Google Scholar]
- 21. Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaara M, et al. 2008. Novel polymyxin derivatives carrying only three positive charges are effective antibacterial agents. Antimicrob. Agents Chemother. 52:3229–3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vaara M, et al. 2010. A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob. Agents Chemother. 54:3341–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vila-Farres X, et al. 2011. In vitro activity of several antimicrobial peptides against colistin-susceptible and colistin-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 18:383–387 [DOI] [PubMed] [Google Scholar]
- 25. Wareham DW, Gordon NC, Hornsey M. 2011. In vitro activity of teicoplanin combined with colistin versus multidrug-resistant strains of Acinetobacter baumannii. J. Antimicrob. Chemother. 66:1047–1051 [DOI] [PubMed] [Google Scholar]
- 26. Woodford N, et al. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 27:351–353 [DOI] [PubMed] [Google Scholar]
- 27. Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35:736–755 [DOI] [PubMed] [Google Scholar]
- 28. Yandek LE, Pokorny A, Almeida PF. 2009. Wasp mastoparans follow the same mechanism as the cell-penetrating peptide transportan 10. Biochemistry 48:7342–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]