Abstract
In response to a published concern about the potency and quality of generic vancomycin products, the United States Food and Drug Administration investigated a small sampling of the vancomycin products available in North America with regard to purity, content, and potency. To facilitate identification of impurities, a new liquid chromatography method was developed using high-resolution mass spectrometry in addition to diode array detection to characterize impurities in several commercial products. Furthermore, a microbiological assay was utilized to link the analytical profiles with an in vitro potency. All products tested met the quality specifications outlined in the United States Pharmacopeia (USP) (vancomycin hydrochloride for injection monograph) for impurities and potency (USP, Vancomycin hydrochloride for injection. United States Pharmacopeia and National Formulary, vol USP 34-NF 29, 2011).
INTRODUCTION
Vancomycin is a glycopeptide antibiotic effective against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (13). Originally developed by Eli Lilly and approved for use in 1958 by the FDA, vancomycin commonly is used as a drug of last resort for infections that have developed resistance to other antibiotics, particularly penicillins. Vancomycin is rarely used as a first-line treatment because it has to be administered intravenously, it is less effective for Staphylococcus strains that are not methicillin resistant, and early, less-pure forms exhibited nephrotoxicity and ototoxicity (1, 10, 11). Even today, there are ongoing concerns regarding nephrotoxicity and its relationship to the administered dose of vancomycin (1, 10, 11).
Vancomycin is produced by fermentation of the actinobacterial species Amycolatopsis orientalis (9). It is a secondary metabolite produced from a seven-module, three-protein nonribosomal peptide synthetase that enzymatically assembles the heptapeptide core structure of vancomycin from 5 nonproteogenic amino acids, β-OH-Tyr (position 2), β-OH-Tyr (position 6), 4-OH-PheGly (position 4), 4-OH-PheGly (position 5), and 3,5-(OH)2-PheGly (position 7) and Leu (position 1) and Asn (position 3) (15). This core structure undergoes oxidative cross-linking before final enzymatic tailoring adds a methylation to the amino group of Leu and two successive sugars, glucosyl and vancosaminyl moieties, to produce the final bioactive structure (15). This compound inhibits cell wall synthesis in Gram-positive bacteria, which is its clinical mode of action (9).
When these studies were performed, six manufacturers were marketing vancomycin for injection in the United States. The innovator product from Eli Lilly is no longer manufactured. In a recent article by Vesga et al., the quality and efficacy of generic vancomycin products were assessed and called into question (20). The authors claimed that two of the generic manufacturers' products were inferior to Eli Lilly's innovator product (which the authors had acquired before its removal from the market). The authors postulated that the differences observed were related to a specific impurity, crystalline degradation product (CDP-1) (8). CDP-1 results from the spontaneous deamidation of asparagine in the vancomycin B heptapeptide backbone (8), which is a known process for asparagine-containing peptides (21). CDP-1 exists as a major and minor atropisomer after degradation. This transformation is accelerated by high temperatures and alkaline pH and results in a change in the solubility of the molecule. More specifically, this impurity has been proposed as a functional vancomycin antagonist and may affect vancomycin potency and efficacy.
In response to this report, impurity and potency studies were initiated by the United States Food and Drug Administration (FDA) to characterize the quality of U.S. marketplace vancomycin products. Many methods for the determination and characterization of vancomycin in various matrices exist in the literature (2, 4, 5, 7, 16, 17). Significantly, both the United States Pharmacopeia and British Pharmacopoeia publish methods for the determination of vancomycin purity and potency (3, 19). In order to assess these claims of poor purity and potency, the FDA performed impurity assays according to compendial methods on all six vancomycin products available in the U.S. marketplace, as described in the accompanying paper by Nambiar et al. (14). However, the exact quantification of CDP-1 was not possible due to its coelution with other impurities in the compendial methods. Moreover, the UV traces in the impurity analysis often contained multiple impurities that were not identified in the USP or BP methods. In order to quantify the amount of CDP-1 present as well as to identify some of the additional impurities, the FDA developed an accurate-mass-based ultrahigh-performance liquid chromatography (UHPLC)-UV mass spectrometric (MS) method to assess the quality of these marketplace products. Finally, the microbiological activities of the six vancomycin products and CDP-1 alone were assessed to further elucidate the biological significance of the characterized impurity profiles and particularly the effect of CDP-1. Collectively, these data provide a comprehensive look at the quality of the U.S. marketplace products.
MATERIALS AND METHODS
All chemicals used were from Fisher Scientific. Vancomycin for injection was purchased from the American marketplace. Vancomycin B standards were obtained from USP and Sigma-Aldrich. All MS solvents were Optima liquid chromatography (LC)-MS grade or better. CDP-1 was prepared according to the literature (8, 12) and characterized by accurate-mass LC-MS.
LC-MS acquisition and analysis.
An ultrahigh-pressure liquid chromatographic (UHPLC) method, based on a modification of the work of Diana et al., was used to evaluate vancomycin products (4). Using a Phenomenex Kinetex C18 column (100 by 2.1 mm, 1.7 μm) operating at 40°C, vancomycin B and any impurities were separated using a binary gradient where mobile phase A was 95:5 (vol/vol) H2O-0.2 M ammonium acetate, pH 9, and mobile phase B was 65:30:5 (vol/vol/vol) H2O-methanol-0.2 M ammonium acetate, pH 9. The flow rate was 200 μl/min, and the gradient program ramped from 50% B at 0 min to 70% B at 20 min and from 70% B to 100% B in 0.1 min and then was held at 100% B for 10 min, before reequilibrating at 50% B for 7 min. The dwell volume of this system is approximately 900 μl. The inline diode array was scanned from 200 to 400 nm in 2-nm steps, and the 280-nm-wavelength chromatogram was extracted. The injection volume was 5 μl. Eluent was infused directly into an LTQ-Orbitrap XL with electron transfer dissociation (ETD) through the ion max source operating in positive ion mode. Mass spectra (500 to 1,700 m/z) were collected in the Orbitrap with the resolution set to 30,000 at 400 m/z. Spectra were acquired in profile mode. All data analysis was performed using ThermoFisher's Xcalibur software (2.0.7). Quantification was performed using peak areas derived from extracted wavelength chromatograms (λ = 280 nm) or extracted ion chromatograms (EIC) at the m/z of interest (e.g., m/z 724.71 for vancomycin B and m/z 725.21 for CDP-1).
For validation studies, USP vancomycin was used as received. The method was evaluated with regard to specificity, linearity, accuracy, and precision. Vancomycin stock solutions were prepared using filter-sterilized water (Waters' Synergy 185 MilliQ system) at a concentration of 10 mg/ml. Working solutions were prepared by serial dilution of the stock solution. For both assay and validation, CDP-1 stock solution, 0.1 mg/ml, was prepared by dissolving CDP-1 in mobile phase B. Samples prepared for accuracy studies were prepared using the vancomycin and CDP-1 stock solutions. For assay, vancomycin samples were prepared by dissolving solid products in water and then diluting them to 1.0 mg/ml with water. One product was supplied as a vancomycin solution for injection (nominal concentration, 5 mg/ml), and for analysis, this solution was diluted with water to a concentration of 1.0 mg/ml. For degradation studies, a working solution was prepared using Sigma-Aldrich vancomycin B (a nonpharmaceutical preparation) at 2 mg/ml in water.
Microbiological assay.
The USP method for vancomycin described in “Antibiotics—microbial assays” was used to conduct the cylinder plate potency evaluation of vancomycin HCl products (18). In brief, Difco (Lawrence, KS) antibiotic medium 8 (100 ml) was seeded with 4 ml of a stock spore suspension of Bacillus subtilis (ATCC 6633; Manassas, VA) and added to medium 8-coated petri dishes. The amount of spore suspension seeded was determined by trial to give a linear response with a USP vancomycin standard dilution series (data not shown). One-milligram/milliliter stock solutions of USP vancomycin HCl or vancomycin products were prepared in MilliQ H2O. Final concentrations of 10 μg/ml (products) and USP standard curve samples were prepared in phosphate buffer, pH 4.5. For CDP-1 spiking experiments, CDP-1 was prepared as a 10-mg/ml stock in dimethyl sulfoxide (DMSO) and then diluted in the 1-mg/ml vancomycin solutions. For studies involving CDP-1 alone, CDP-1 was diluted (1:10,000 to 1:40) from a 10-mg/ml CDP-1 DMSO stock into 1 ml of MilliQ water. DMSO was used as a solvent control for the USP vancomycin standard. The median concentration of USP vancomycin (10 μg/ml; 50 μl) was added to 3/6 cylinders on each plate in alternating positions. The remaining cylinders received either USP standard atropisomer or vancomycin product (10 μg/ml) using 3 plates per point. After a 24-h incubation at 37°C, the zones of inhibition were measured from scanned images of each plate using Image J software (NIH, Bethesda, MD). The average USP vancomycin median concentration (10 μg/ml) in each test or standard group (3 dishes; n = 9) and the total assay average USP vancomycin median concentration (30 dishes; n = 90) were used to normalize each test or standard concentration. A standard curve was determined using linear regression analysis in Excel with log USP vancomycin μg/ml on the x axis and corrected diameter (mm) on the y axis. Antibiotic potency was calculated by interpolation from this standard curve and was expressed as a percentage of the reference concentration. Briefly, the expected concentration of a sample is equal to the antilog [(corrected mean diameter − a)/b], where the corrected mean diameter is the corrected mean of a sample's zones of inhibition, a is the intercept of the regression line, and b is the slope of the regression line. The potency is then expressed as the ratio of the expected sample concentration to the reference sample concentration multiplied by 100. Potency for the six American marketplace samples tested neat, spiked with 9% CDP-1, and spiked with 25% CDP-1 was determined. The antimicrobial activity of CDP-1 was also assessed. Significance was determined between neat and CDP-1-spiked vancomycin products using a paired two-tailed t test in Excel (*, P < 0.05; **, P < 0.01).
RESULTS AND DISCUSSION
The UHPLC-MS method was suitably linear, as a strong correlation between instrument response (peak area) and vancomycin B concentration for both UV (R ≥ 0.999) and EIC (R ≥ 0.990) was observed over the range of 0.5 to 1.2 mg/ml, the concentration range in which all products were tested. Accuracy, expressed as the recovery, was 100.1% ± 0.5% and 97.9% ± 3.5% (n = 18) with UV and EIC (at m/z 724.72), respectively. Precision, expressed as the % relative standard deviation (RSD) of the average recovery, was 0.5% and 3.5% for UV and EIC, respectively. The specificity of the method was defined as baseline resolution between crystalline degradation product atropisomers (CDP-1m and CDP-1M) and vancomycin B. In cases where method optimization did not allow for baseline resolution of other vancomycin impurities, the specificity of the method was provided by the high mass resolution of the mass spectrometer used in these studies.
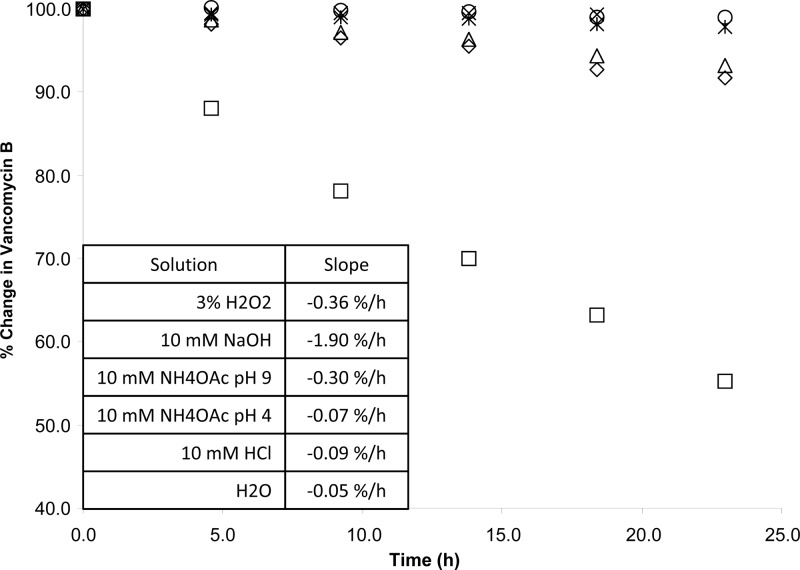
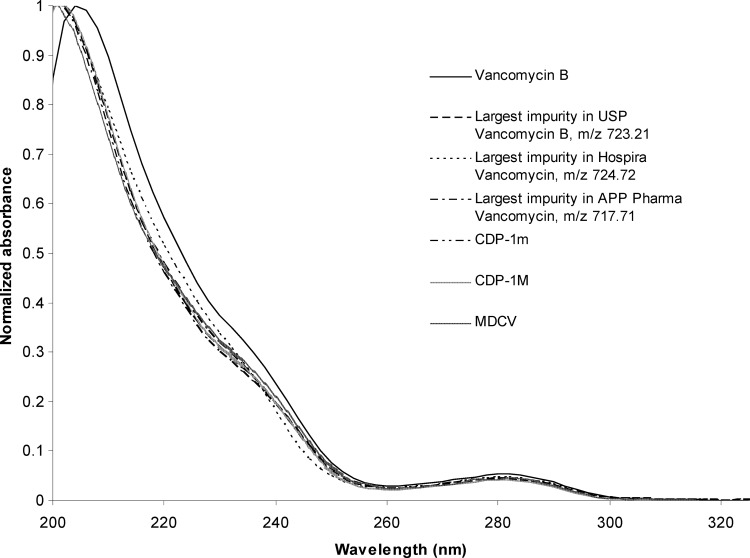
Quantification of CDP-1 was less precise and accurate than that for vancomycin B. CDP-1 recovery was studied over the range of 0.1% to 1.0% (wt/wt) and averaged 128% ± 15% and 96% ± 14% (n = 24) by UV and EIC (at m/z 725.21), respectively. In order to dissolve CDP-1 at a concentration adequate to spike vancomycin B solutions for accuracy studies, mobile phase B, a weakly alkaline solution, was used. While CDP-1 is stable in this solution for at least 5 days at 8°C in 10 mM ammonium acetate, pH 9, vancomycin B is not. CDP-1 is formed from vancomycin B at a rate of approximately 2.7 μg ml−1 h−1 at 8°C in 10 mM ammonium acetate, pH 9 (Fig. 1). As a result, CDP-1 recovery from spiked vancomycin B solutions was high at low concentrations using UV detection. Because the performance of the method for the quantification of CDP-1 by external standard was less accurate than desirable, the method of normalized area percentages was adopted to estimate impurities. Implicit in any normalized area percent assay is the assumption that any impurity detected provides the same instrument response as the main analyte of interest. To test this assumption, the UV spectra for vancomycin B and each impurity were normalized (to account for differences in concentration) and compared. The results for major impurities (i.e., impurities present at approximately 1%) are displayed in Fig. 2. Vancomycin B impurities produce UV spectra nearly identical to vancomycin B. As a result, vancomycin impurities with the same core aromatic structure were assumed to provide nearly identical instrument responses when analyzed using UV detection.
Fig 1.
Degradation of vancomycin B in various solvents. ♢, 3% H2O2; ×, 10 mM ammonium acetate, pH 4; △, 10 mM ammonium acetate, pH 9; *, 10 mM HCl; ○, H2O; □, 10 mM NaOH.
Fig 2.
Normalized UV spectra of vancomycin B and some representative major (area % > 1) impurities.
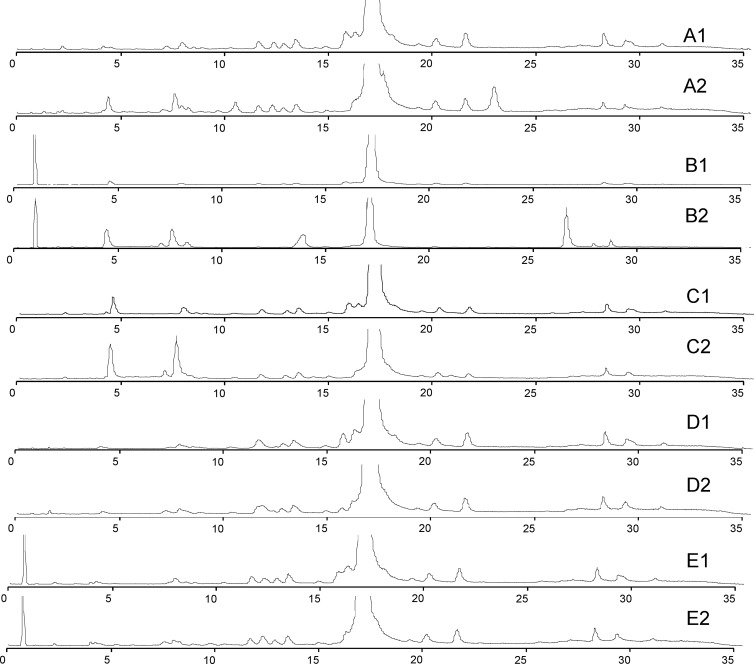
For impurity identity tests, a nonpharmaceutical vancomycin sample was forcibly degraded under a variety of conditions and analyzed in order to determine what types of impurities and/or degradation products might be present in pharmaceutical preparations (Fig. 3). Twenty unique masses were attributed by exact mass shifts (<4 ppm) from vancomycin B or CDP-1, and their identities are shown in Table 1. These assignments were confirmed by tandem MS data. Vancomycin and its derivatives or degradation products had a characteristic fragmentation pattern where loss of the vancoaminyl sugar was followed by the loss of the glucosyl sugar, leaving the core structure behind. In all cases, any mass shift was associated with modification of the core structure.
Fig 3.
Solution degradation of vancomycin B. (A1 and A2) VM B in 3% H2O2 fresh and at 24 h, respectively. (B1 and B2) VM B in 10 mM NaOH fresh and at 24 h, respectively. (C1 and C2) VM B in 0.2 M ammonium acetate (pH 9) fresh and at 24 h, respectively. (D1 and D2) VM B in 0.2 ammonium acetate (pH 4) fresh and at 24 h, respectively. (E1 and E2) VM B in 10 mM HCl fresh and at 24 h, respectively.
Table 1.
Identities of vancomycin impurities
| Compound | Abbreviation | Formula | Exact mass | (M + H+)/z, +1 | (M + 2H+)/z, +2 |
|---|---|---|---|---|---|
| Aglucovancomycin | AGLUV | C53H52Cl2N8O17 | 1,142.28275 | 1,143.29058 | NDa |
| Desvancosaminylvancomycin | DESV | C59H62Cl2N8O22 | 1,304.33557 | 1,305.3434 | ND |
| Deamidated desvancosaminylvancomycin | DESV* | C59H61Cl2N7O23 | 1,305.31959 | 1,306.32741 | 653.66762 |
| Oxidized desvancosaminylvancomycin | Ox-DESV | C59H62Cl2N8O23 | 1,320.33048 | 1,321.33831 | 661.17307 |
| Deamidated oxidized desvancosaminylvancomycin | Ox-DESV* | C59H61Cl2N7O24 | 1,321.3145 | 1,322.32233 | 661.66508 |
| Didechlorovancomycin | DDCV | C66H77N9O24 | 1,379.50814 | 1,380.51597 | 690.7619 |
| Monodechlorovancomycin 1 or 2 | MDCV | C66H76ClN9O24 | 1,413.46917 | 1,414.477 | 707.74241 |
| Deamidated monodechlorovancomycin 1 or 2 | MDCV* | C66H75ClN8O25 | 1,414.45319 | 1,415.46101 | 708.23442 |
| CDP intermediate | CDPi | C66H72Cl2N8O24 | 1,430.40365 | 1,431.41148 | 716.20965 |
| Vancomycin B − CH3 + H | VM B − Me | C65H73Cl2N9O24 | 1,433.41455 | 1,434.42237 | 717.7151 |
| Deamidated vancomycin B − CH3 + H | VM B − Me* | C65H72Cl2N8O25 | 1,434.39857 | 1,435.38258 | 718.20711 |
| CDP − CH3 +H | CDP − Me | C65H72Cl2N8O25 | 1,434.39857 | 1,435.40639 | 718.20711 |
| Vancomycin B | VM B | C66H75Cl2N9O24 | 1,447.4302 | 1,448.43803 | 724.72293 |
| CDPm | CDP | C66H74Cl2N8O25 | 1,448.41422 | 1,449.42205 | 725.21494 |
| CDPM | CDP | C66H74Cl2N8O25 | 1,448.41422 | 1,449.42205 | 725.21494 |
| Oxidized vancomycin B − CH3 + H | Ox-VM B − Me | C65H73Cl2N9O25 | 1,449.40946 | 1,450.41729 | 725.71256 |
| Vancomycin B + CH3 − H | VM B + Me | C67H77Cl2N9O24 | 1,461.44585 | 1,462.45368 | 731.73075 |
| Vancomycin B + Cl − H | CDP + Me | C67H76Cl2N8O25 | 1,462.42987 | 1,463.4377 | 732.22276 |
| Oxidized vancomycin B | Ox-VM B | C66H75Cl2N9O25 | 1,463.42511 | 1,464.43294 | 732.72038 |
| Vancomycin B + Cl − H | VM B + Cl | C66H74Cl3N9O24 | 1,481.39123 | 1,482.39905 | 741.70344 |
| Unknown 1 | Unknown 1 | 1,481.37827 | 1,482.38554 | 741.69641 | |
| Unknown 2 | Unknown 2 | 1,465.38337 | 1,466.39064 | 733.69896 | |
| Unknown 3 | Unknown 3 | 1,473.44275 | 1,474.45002 | 737.72865 | |
| Unknown 4 | Unknown 4 | 1,467.39691 | 1,468.40418 | 734.70573 | |
| Unknown 5 | Unknown 5 | 1,579.46797 | 1,580.47524 | 790.74126 | |
| Unknown 6 | Unknown 6 | 1,431.43297 | 1,432.44024 | 716.72376 | |
| Unknown 7 | Unknown 7 | 1,445.41189 | 1,446.41916 | 723.71322 |
ND, not detected.
The forced degradation of (nonpharmaceutical) vancomycin revealed that in all cases, once vancomycin goes into aqueous solution, CDP-1 is formed, and monodechlorovancomycin (MDCV) disappears with time. The rate at which CDP-1 is formed is dependent on the solution pH, and formation is fastest in alkaline solutions. Furthermore, in addition to CDP-1, other impurities are formed and some impurities which are initially present disappear. The degradation of vancomycin B appears to be pseudo-first-order with respect to the sample solvent.
The products from the six manufacturers were analyzed with the validated method, and the results are shown in Table 2. All products met USP specifications of not more than 9% of any one impurity and not less than 80% vancomycin B in agreement with the previous analysis by the USP and British Pharmacopoeia methods, as described in the accompanying paper by Nambiar et al. (14). Variability of the impurity profiles was observed between manufacturers, including the crystalline degradation product, CDP-1, which coeluted with another impurity in some products, making precise quantitation difficult by UV. CDP-1 was typically not the most abundant impurity for freshly prepared solutions. In the marketplace samples studied, the largest single impurities were between 0.95% and 1.24% and total impurities were between 4.68% and 9.37%, well within the USP specifications of <9% and <20%, respectively. CDP-1 was determined to be less than 2% (wt/wt) in all cases.
Table 2.
Summary of results for U.S. marketplace vancomycin samples
| Parameter | Vancomycin assay result |
||||||
|---|---|---|---|---|---|---|---|
| USP | Hospira | Bioniche | Akorn Strides | APP Pharma | Sandoz | Baxter | |
| Vancomycin B assay value (%, wt/wt)a | NDc | 100.8 | 111.4 | 109.8 | 107.0 | 109.9 | 94.9 |
| CDP-1 total (%, wt/wt) | ND | 0.48 | 0.20 | 0.25 | 0.15 | 1.21 | 0.06 |
| Largest impurity | Unknown | VM B isomer | VM B isomer | VM B isomer | VM B − Me | VM B isomer | Unknown |
| m/z of largest impurity (z = 2) | 723.71266 | 724.72104 | 724.72104 | 724.72104 | 717.71280 | 724.72104 | 723.71266 |
| Total impurities (area %)b | 5.43 | 9.37 | 4.79 | 5.15 | 5.03 | 4.68 | 5.53 |
| CDP-1 total (area %)b | 0.05 | 0.28 | 0.19 | 0.16 | 0.19 | 0.40 | 0.09 |
| Largest impurity (area %)b | 1.23 | 1.03 | 0.97 | 0.95 | 1.12 | 0.95 | 1.24 |
| Area % of VM Bb | 94.6 | 90.6 | 95.2 | 94.9 | 95.0 | 95.3 | 94.5 |
When assayed quantitatively against a USP standard.
Normalized area percent.
ND, not determined.
For the U.S. marketplace samples, impurities were identified by exact mass matching to the forced degradation identifications (Table 1). In addition to the five previously described impurities in the compendial methods, monodechlorovancomycin (MDCV), N-demethylvancomycin B (VM B − Me), aglucovancomycin (AGLUV), desvancosaminylvancomycin B (DESV), and CDP-1, other impurities with masses corresponding to an addition of a methyl group (VM B + Me), addition of chlorine (VM B + Cl), and the deamidation succinimide intermediate (CDPi) were also measured (Table 3). Given that the enzymes involved in synthesis of nonribosomal peptides (i.e., vancomycin) do not have perfect fidelity (6) or are oxidative in nature, these compounds appear to be derivatives or degradation products of vancomycin B.
Table 3.
Impurity distributions of 6 U.S.-marketed vancomycin formulationsa
| Avg UV RT | Avg BPC RT | Identity by MS | m/z, z = 2 | UV area % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| USP | Hospira | Bioniche | Akorn Strides | APP Pharma | Sandoz | Baxter | ||||
| 1.6 | ND | 0.09 | 0.02 | 0.04 | ||||||
| 1.9 | ND | 0.02 | ||||||||
| 2.4 | 2.5 | Unknown 1 | 741.69641 | 0.70 | 0.02 | 0.12 | 0.08 | 0.13 | ||
| 4.1 | 4.2 | CDP-1m | 725.21282 | 0.02 | 0.18 | 0.11 | 0.09 | 0.11 | 0.31 | 0.03 |
| 6.8 | 7.0 | CDP-1M | 725.21295 | 0.03 | 0.10 | 0.08 | 0.07 | 0.08 | 0.09 | 0.06 |
| 7.4 | 7.5 | VM B − Me | 717.71326 | 0.03 | 0.19 | 0.03 | 0.09 | 0.52 | 0.02 | |
| 7.6 | 7.7 | CDP | 725.21322 | 0.40 | 0.12 | 0.11 | 0.12 | 0.14 | ||
| 7.8 | Unknown 2 | 733.69896 | 0.05 | 0.41 | 0.08 | 0.10 | 0.16 | 0.07 | 0.18 | |
| 8.4 | 8.5 | VM B − Me | 717.71315 | 0.12 | 0.03 | 0.02 | 0.03 | 0.07 | ||
| 8.8 | ND | 0.02 | ||||||||
| 9.2 | 9.3 | VM B − Me | 717.71293 | 0.06 | 0.24 | 0.14 | ||||
| 9.9 | 10.1 | VM B − Me | 717.71250 | 0.02 | 0.06 | 0.03 | ||||
| 10.6 | 10.7 | VM B − Me | 717.71280 | 0.38 | 0.29 | 0.11 | 0.12 | 1.12 | 0.05 | 1.07 |
| 11.0 | 11.1 | CDPi | 716.20750 | 0.09 | 0.29 | 0.18 | 0.40 | 0.20 | 0.30 | |
| 11.7 | 11.8 | MDCV | 707.74020 | 0.06 | 0.16 | 0.05 | 0.07 | 0.10 | 0.16 | 0.05 |
| 12.2 | 12.3 | VM B + Me | 731.72869 | 0.18 | 0.52 | 0.17 | 0.09 | 0.25 | 0.02 | 0.38 |
| 13.0 | 13.1 | Unknown 3 | 790.74126 | 0.08 | 0.02 | |||||
| 13.6 | 13.7 | VM B | 724.72006 | 0.21 | 0.18 | 0.22 | 0.14 | 0.18 | 0.10 | |
| 14.3 | 14.4 | Unknown 4 | 734.70573 | 0.96 | 0.72 | 0.18 | 0.15 | 0.14 | 0.07 | |
| 14.8 | ND | 0.42 | 0.33 | 0.36 | ||||||
| 15.9 | 16.0 | VM B | 724.72133 | 94.58 | 90.64 | 95.19 | 94.87 | 94.99 | 95.33 | 94.49 |
| 18.4 | 18.5 | VM B isomer | 724.72084 | 0.03 | 0.48 | 0.03 | 0.07 | 0.02 | 0.18 | 0.10 |
| 18.6 | ND | 0.09 | ||||||||
| 18.9 | 19.0 | VM B isomer | 724.72145 | 0.19 | 0.58 | 0.58 | 0.65 | 0.27 | 0.49 | 0.19 |
| 19.4 | ND | 0.05 | ||||||||
| 20.2 | 20.4 | VM B isomer | 724.72104 | 0.63 | 1.03 | 0.97 | 0.95 | 0.85 | 0.95 | 0.32 |
| 24.3 | 24.4 | VM B + Cl | 742.70065 | 0.18 | 0.51 | 0.39 | 0.25 | 0.19 | 0.11 | 0.13 |
| 25.8 | ND | 0.02 | 0.03 | |||||||
| 26.3 | ND | 0.04 | 0.05 | 0.04 | 0.04 | 0.05 | 0.05 | |||
| 26.7 | ND | 0.03 | 0.02 | 0.06 | 0.02 | 0.02 | ||||
| 26.8 | 27.0 | Unknown 5 | 737.72865 | 0.04 | ||||||
| 27.1 | 27.2 | VM B + Me | 731.72916 | 0.51 | 1.00 | 0.57 | 0.48 | 0.09 | 0.10 | 0.19 |
| 27.2 | ND | 0.08 | ||||||||
| 27.7 | 27.9 | Unknown 6 | 716.72376 | 0.68 | 0.56 | 0.22 | 0.30 | 0.25 | ||
| 28.3 | 28.5 | Unknown 7 | 723.71322 | 1.23 | 0.39 | 0.46 | 0.15 | 0.21 | 1.24 | |
| 28.8 | ND | 0.02 | ||||||||
| 29.5 | ND | 0.05 | 0.06 | |||||||
| 31.0 | ND | 0.01 | 0.02 | |||||||
| 31.4 | ND | 0.23 | 0.05 | 0.06 | 0.07 | 0.08 | 0.08 | |||
| 32.3 | AGLUV | ND | 0.09 | 0.15 | 0.21 | 0.18 | 0.23 | 0.04 | ||
| 34.2 | ND | 0.06 | 0.05 | |||||||
Values for the largest impurities found in a product are shown in bold. ND, not determined; RT, retention time; BPC, base peak chromatogram.
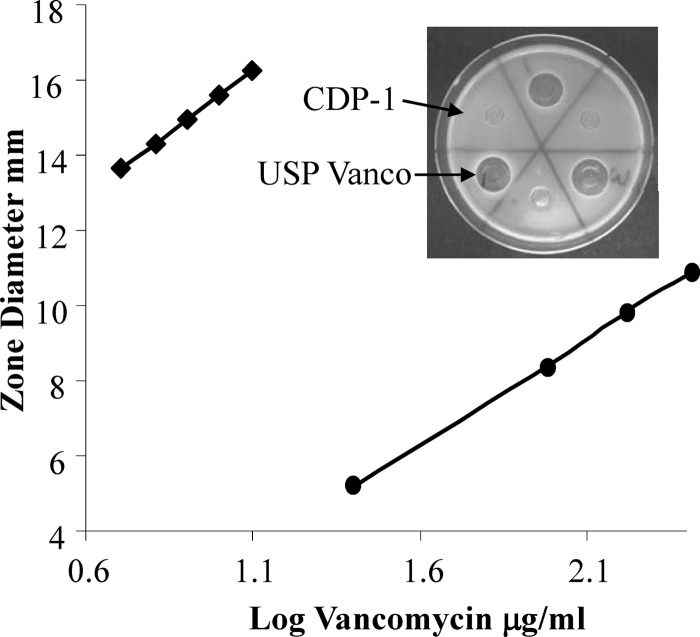
The mean antimicrobial potency values from the vancomycin products ranged from 97 to 112%, meeting the USP acceptance criteria for vancomycin HCl products (90 to 115%) as shown in Table 4. Vancomycin samples spiked with 9% CDP-1 (wt/wt), the maximum impurity limit, had mean potency values ranging from 98% to 105%. All of the products spiked with 9% CDP-1 met the USP acceptance criteria, and only one product had a statistically significant potency difference between the neat sample and the spiked sample. Vancomycin samples spiked with 25% CDP-1 (wt/wt) had mean potency values ranging from 83% to 96%. Two of the 25% CDP-1-spiked products failed to meet the USP acceptance criteria, and five of the products had significantly different average potencies in comparison of the neat samples to the CDP-1-spiked samples. CDP-1 (25 to 250 μg/ml) had a concentration-dependent increase in antimicrobial activity which was limited by solubility (Fig. 4). Nevertheless, CDP-1 at concentrations up to 10 μg/ml, the median concentration of USP vancomycin, had no activity (Fig. 4, inset). At the highest concentration tested, 250 μg/ml, CDP-1 had a kill zone diameter corresponding to less than 5 μg/ml USP vancomycin, the lowest point on the standard curve. These data indicate that CDP-1 has inherent antimicrobial activity but is far less potent than vancomycin.
Table 4.
Summary of antimicrobial potency results for marketplace vancomycin samplesa
| Source | Potency of vancomycin product |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Neat |
Spiked with 9% (wt/wt) CDP-1 |
Spiked with 25% (wt/wt) CDP-1 |
|||||||
| Avg | SD | % RSD | Avgb | SD | % RSD | Avgb | SD | % RSD | |
| Sandoz Incorporated | 106 | 5.9 | 5.6 | 102 | 3.8 | 3.7 | 90* | 2.9 | 3.2 |
| Baxter Health Care Corporation | 97 | 7.3 | 7.6 | 98 | 6.4 | 6.6 | 83 | 7.3 | 8.7 |
| Hospira Inc. | 106 | 9.5 | 8.9 | 105 | 11.9 | 11.4 | 89* | 1.7 | 1.9 |
| APP Pharmaceutical LLC | 108 | 2.9 | 2.7 | 103 | 5.7 | 5.5 | 96** | 1.4 | 1.5 |
| Bioniche Pharma USA LLC | 112 | 5.6 | 5 | 99 | 7.5 | 7.6 | 93** | 6.9 | 7.4 |
| Akorn Strides LLC | 109 | 1.9 | 1.7 | 103* | 2.6 | 2.6 | 92* | 5.1 | 5.6 |
USP potency criterion, 90 to 115%.
Significant difference between neat and CDP-1-spiked samples (*, P < 0.05; **, P < 0.01).
Fig 4.
Antimicrobial activity comparison of USP vancomycin HCl (diamonds) and CDP-1 (circles). USP vancomycin (5 to 12.5 μg/ml) and CDP-1 (25 to 250 μg/ml) were compared using a USP cylinder plate zone-of-inhibition assay according to the USP method. After a 24-h incubation at 37°C, the kill zone diameters were measured from scanned images of each plate using Image J software. USP vancomycin and CDP-1 activities were described by the lines y = 6.77x + 8.83 (R2 = 0.99) and y = 5.84x − 3.25 (R2 = 0.99), respectively. The inset shows a representative plate comparing 10-μg/ml amounts of CDP-1 and USP vancomycin.
Conclusions.
The new UPLC-UV-MS method took advantage of the higher separation efficiency afforded by a column packed with particles less than 2 μm, adopted an eluent composition compatible with mass spectrometric detection, and provided superior selectivity by using a high-mass-resolution (resolving power, >30,000), high-mass-accuracy (3-ppm) Orbitrap mass spectrometer as a detector to provide a quantitative analysis of vancomycin B products as well as to identify the majority of the components in the drug substance.
All of the market samples analyzed contained relatively small amounts of CDP-1, and in all cases, CDP-1 was not the most abundant impurity. Importantly, all the marketplace samples met USP potency criteria even when spiked with the maximum impurity limit of CDP-1 (9%), indicating that CDP-1's role as a functional antagonist is limited. When samples were spiked with 25% CDP-1, there was a decrease in potency compared with the neat samples, suggesting a possibility of weak functional antagonism by CDP-1 at very high concentrations. These data suggest that these products are well within current specifications. The impurities identified are expected from biologically derived products and are well within the limits. Moreover, naturally derived as well as exaggerated levels of CDP-1 have a marginal effect on in vitro potency and therefore are unlikely to change the in vivo efficacy of the products.
ACKNOWLEDGMENTS
We acknowledge the assistance of Tom Colatsky and Lucinda Buhse (FDA) for their helpful advice and discussions.
The findings and conclusions of this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Footnotes
Published ahead of print 27 February 2012
REFERENCES
- 1. Bosso JA, et al. 2011. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob. Agents Chemother. 55:5475–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bozdag S, et al. 2010. In vitro evaluation of gentamicin- and vancomycin-containing minitablets as a replacement for fortified eye drops. Drug Dev. Ind. Pharm. 36:1259–1270 [DOI] [PubMed] [Google Scholar]
- 3. British Pharmacopoeia 2012. Vancomycin intravenous infusion. British pharmacopoeia, vol III TSO, Norwich, United Kingdom [Google Scholar]
- 4. Diana J, Visky D, Hoogmartens J, Van Schepdael A, Adams E. 2006. Investigation of vancomycin and related substances by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 20:685–693 [DOI] [PubMed] [Google Scholar]
- 5. Diana J, Visky D, Roets E, Hoogmartens J. 2003. Development and validation of an improved method for the analysis of vancomycin by liquid chromatography selectivity of reversed-phase columns towards vancomycin components. J. Chromatogr. A 996:115–131 [DOI] [PubMed] [Google Scholar]
- 6. Fischbach MA, Walsh CT. 2006. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 106:3468–3496 [DOI] [PubMed] [Google Scholar]
- 7. Haghedooren E, Diana J, Noszal B, Hoogmartens J, Adams E. 2007. Classification of reversed-phase columns based on their selectivity towards vancomycin compounds. Talanta 71:31–37 [DOI] [PubMed] [Google Scholar]
- 8. Harris CM, Kopecka H, Harris TM. 1983. Vancomycin: structure and transformation to CDP-I. J. Am. Chem. Soc. 105:6915–6922 [Google Scholar]
- 9. Kahne D, Leimkuhler C, Lu W, Walsh C. 2005. Glycopeptide and lipoglycopeptide antibiotics. Chem. Rev. 105:425–448 [DOI] [PubMed] [Google Scholar]
- 10. Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 52:1330–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49:507–514 [DOI] [PubMed] [Google Scholar]
- 12. Marshall FJ. 1965. Structure studies on vancomycin. J. Med. Chem. 8:18–22 [DOI] [PubMed] [Google Scholar]
- 13. Murray BE. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710–721 [DOI] [PubMed] [Google Scholar]
- 14. Nambiar S, et al. 2012. Product quality of parenteral vancomycin products in the United States. Antimicrob. Agents Chemother. 56:2819–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nolan EM, Walsh CT. 2009. How nature morphs peptide scaffolds into antibiotics. Chembiochem 10:34–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plock N, Buerger C, Kloft C. 2005. Successful management of discovered pH dependence in vancomycin recovery studies: novel HPLC method for microdialysis and plasma samples. Biomed. Chromatogr. 19:237–244 [DOI] [PubMed] [Google Scholar]
- 17. Somerville AL, Wright DH, Rotschafer JC. 1999. Implications of vancomycin degradation products on therapeutic drug monitoring in patients with end-stage renal disease. Pharmacotherapy 19:702–707 [DOI] [PubMed] [Google Scholar]
- 18. USP 2011. Antibiotics—microbial assays. United States pharmacopeia and national formulary, vol USP 34-NF 29. USP, Rockville, MD [Google Scholar]
- 19. USP 2011. Vancomycin hydrochloride for injection. United States pharmacopeia and national formulary, vol USP 34-NF 29. USP, Rockville, MD [Google Scholar]
- 20. Vesga O, Agudelo M, Salazar BE, Rodriguez CA, Zuluaga AF. 2010. Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator. Antimicrob. Agents Chemother. 54:3271–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang H, Zubarev RA. 2010. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis 31:1764–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]