Abstract
We compared the activity of dicloxacillin with that of vancomycin against 15 oxacillin-susceptible, methicillin-resistant Staphylococcus aureus (OS-MRSA) clinical isolates. By population analyses, we found that 6 OS-MRSA isolates were able to grow in the presence of up to 8 μg/ml dicloxacillin and 9 isolates were able to grow in 12 to >32 μg/ml dicloxacillin; all isolates grew in up to 2 μg/ml vancomycin. Both drugs exhibited similar bactericidal activities. In experimental infections, the therapeutic efficacy of dicloxacillin was significant (P < 0.05 versus untreated controls) in 10 OS-MRSA isolates and vancomycin was effective (P < 0.05) against 12 isolates; dicloxacillin had an efficacy that was comparable to that of vancomycin (P > 0.05) in 8 isolates. The favorable response to dicloxacillin treatment might suggest that antistaphylococcal penicillins could be used against OS-MRSA infections.
TEXT
Staphylococcus aureus isolates that carry and express the mecA gene are considered methicillin resistant (MRSA) but may exhibit oxacillin MICs ranging from the susceptible range (≤2 μg/ml) to >1,000 μg/ml (8, 16, 20). It was generally believed that most mecA-positive S. aureus strains, including those appearing oxacillin susceptible (OS-MRSA), exhibit a degree of oxacillin heteroresistance and the use of β-lactams might lead to treatment failure. However, OS-MRSA isolates with no oxacillin heteroresistance (truly oxacillin susceptible) also appeared (9).
In a previous study, we reported that the activity of oxacillin against four OS-MRSA isolates was intermediate between that against mecA-negative S. aureus and highly resistant MRSA isolates (9). It was subsequently found that the OS-MRSA isolates of that study harbored specific mutations in their Fem proteins that probably conferred atypical oxacillin responsiveness (6). To further investigate these preliminary observations, we tested and report herein the in vitro and in vivo activities of oxacillin compared with those of vancomycin (treatment of choice for most MRSA infections) against a larger collection of OS-MRSA. The aim of this study was to investigate whether antistaphylococcal β-lactams, which were previously shown to exhibit superior activity than vancomycin against methicillin-susceptible S. aureus (MSSA) (11), retain activity against MRSA isolates that appear phenotypically susceptible to oxacillin. To the best of our knowledge, the activity of β-lactams has not been tested against OS-MRSA isolates.
Bacterial strains and susceptibility testing.
Fifteen vancomycin-susceptible OS-MRSA clinical isolates, collected during 2006 and 2007, were studied. A high-level MRSA isolate (isolate 7263; oxacillin MIC, 256 μg/ml) and the mecA-negative strain S. aureus ATCC 29213 were included as controls. Isolates were stored at −80°C in brain heart infusion broth with 15% glycerol before testing. MIC testing of oxacillin and vancomycin was performed by agar dilution according to CLSI guidelines (4).
Detection of PBP2a and the mecA gene and MLST.
The study isolates were tested for the mecA gene by PCR (12) and for PBP2a production by the Slidex MRSA agglutination test (bioMérieux, Marcy l'Etoile, France). Pulsed-field gel electrophoresis (PFGE) was performed as previously described (21), and banding patterns were compared visually. All isolates were tested for the Panton-Valentine leukocidin (PVL)-encoding genes lukS-lukF (14). Multilocus sequence typing (MLST) was also performed (http://www.mlst.net) (5).
Population analysis assays.
Isolates were tested by population analyses (PAs) for dicloxacillin and vancomycin. Approximately 108 CFU were spread on Mueller-Hinton (MH) agar plates (2% NaCl) containing 0.125 to 32 μg/ml dicloxacillin or vancomycin (9). Colonies were counted after 48 h of growth at 35°C. Analyses were performed in triplicate, and mean CFU counts were plotted on a semilogarithmic graph.
Time-kill assays.
Time-killing curves were performed in triplicate by inoculating approximately 106 CFU into MH broth containing 20 μg/ml dicloxacillin or 10 μg/ml vancomycin (9, 19). Aliquots were removed at 0, 6, 24, and 48 h postinoculation at 35ο C and plated on MH agar plates for CFU enumeration. Bactericidal activity was defined as a ≥3-log10 reduction, and bacteriostatic activity was defined as the maintenance of, or a <3-log10 reduction of, the total number of CFU/ml in the original inoculum (15).
Murine infection model.
An experimental murine thigh infection model was used to test the in vivo activity of dicloxacillin versus that of vancomycin. Animal studies were approved by the Greek Veterinary Authorities and conformed to the Protocol on the Protection and Welfare of Animals. Six-week-old, specific-pathogen-free, female BALB/c mice (Harlan, Indianapolis, IN) weighing 23 to 27 g were used (7). Mice were rendered neutropenic by injecting cyclophosphamide intraperitoneally on day 4 (150 mg/kg) and day 1 (100 mg/kg) preinoculation (1, 12). Thigh infections were performed in triplicate by injecting approximately 106 CFU, and the mice were treated with either dicloxacillin at 500 mg/kg/12 h intraperitoneally or vancomycin at 180 mg/kg/12 h subcutaneously (7, 12, 18) or they were left untreated; animals were euthanized after 24 h. Thigh muscles were aseptically excised, homogenized, serially diluted and plated on antibiotic-free plates for CFU enumeration. Thigh CFU titer was expressed as log 10 CFU/thigh muscle. A t test was used for statistical analysis using Minitab software (version 13.31); a P value of ≤0.05 was considered statistically significant.
Results.
All 15 OS-MRSA isolates carried the mecA gene and produced PBP2a; MICs of oxacillin and vancomycin were 0.25 to 1 μg/ml and ≤1 μg/ml, respectively. Two unrelated PFGE types were identified, with the predominant type including 14 isolates and exhibiting three subtypes that differed by 1 or 2 bands from each other. Fourteen isolates were PVL positive and one was PVL negative. MLST results showed that 13 isolates belonged to ST80, one isolate to ST728, and one to ST30. Characteristics of isolates are shown in Table 1.
Table 1.
Characteristics of the study isolatesa
| Isolate | PVL | MLST type | PFGE type | OXA MIC (μg/ml) | VAN MIC (μg/ml) | Δlog10 CFU reduction at: |
|||
|---|---|---|---|---|---|---|---|---|---|
| 6 h in dicloxacillin time-kill assays | 24 h in dicloxacillin time-kill assays | 6 h in vancomycin time-kill assays | 24 h in vancomycin time-kill assays | ||||||
| 446 | + | 80 | Ia | 0.5 | 0.5 | 3.8 | 4.2 | 3.8 | 5.8 |
| 959 | + | 80 | Ia | 0.25 | 2 | 4.6 | 5.8 | 3.5 | 5.1 |
| 970 | + | 728 | Ib | 0.25 | 1 | 4.9 | 5.9 | 3.5 | 4.7 |
| 1117 | + | 80 | Ib | 0.5 | 1 | 2.4 | 4.4 | 3.4 | 4.9 |
| 1512 | + | 30 | Ic | 0.25 | 1 | 5.4 | 5.4 | 4.4 | 5.9 |
| 1546 | + | 80 | Ic | 0.25 | 1 | 5 | 5.8 | 3.6 | 5.0 |
| 2629 | + | 80 | Ib | 0.25 | 1 | 4.9 | 6 | 3.8 | 6 |
| 4324 | + | 80 | Ib | 0.25 | 1 | 3.6 | 4.7 | 3.7 | 3.8 |
| 5014 | − | 80 | II | 0.5 | 1 | 4.2 | 5.7 | 2.7 | 4.2 |
| 5505 | + | 80 | Ib | 1 | 1 | 3.6 | 4.4 | 3 | 5.4 |
| 6036 | + | 80 | Ia | 0.5 | 1 | 5.1 | 6 | 3.8 | 5.8 |
| 6601 | + | 80 | Ib | 0.25 | 1 | 5 | 5.4 | 4 | 5.2 |
| 7059 | + | 80 | Ib | 0.25 | 1 | 4.9 | 5.0 | 3.9 | 6 |
| 9131 | + | 80 | Ib | 0.25 | 1 | 3.5 | 4.3 | 3.9 | 4.7 |
| 9833 | + | 80 | Ib | 0.5 | 1 | 2.7 | 4.8 | 3.3 | 5.5 |
| ATCC 29213 | − | ND | III | 0.25 | 1 | 5 | 6 | 3.5 | 6 |
| 7263 | + | ND | IV | 256 | 1 | 2 | 2 | 4.5 | 5.8 |
OXA, oxacillin; VAN, vancomycin; ND, not determined.
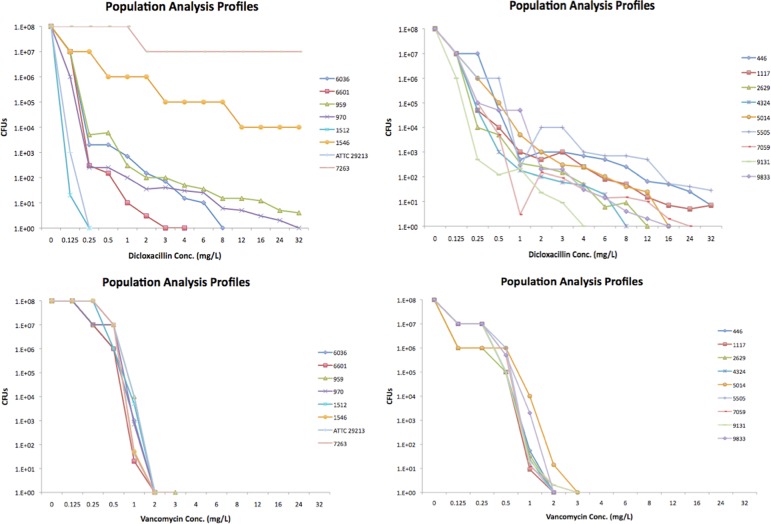
PAs showed that 6 OS-MRSA isolates grew at up to 8 μg/ml oxacillin and 9 isolates at oxacillin concentrations of 12 to >32 μg/ml; all isolates were clearly susceptible to vancomycin. Results of the PAs are presented in Fig. 1.
Fig 1.
Population analysis assays of the 15 OS-MRSA study isolates and the control isolates using dicloxacillin and vancomycin.
Time-killing kinetics showed a ≥3 log 10 reduction of CFU/ml, indicating efficient bactericidal activity of dicloxacillin and vancomycin at 24 h for all OS-MRSA. In 10 isolates, the bactericidal activity of dicloxacillin after 6 h of incubation was higher than that of vancomycin. Overall, vancomycin exhibited low killing activity, eliminating most bacterial populations at 24 to 48 h compared with dicloxacillin, which eliminated most populations at 6 to 24 h in 10 isolates and at 24 to 48 h in 5 isolates. The mecA-negative control ATCC 29213 was rapidly killed, and the highly resistant MRSA control remained unaffected by dicloxacillin. The results of the bactericidal assays at 6 and 24 h are shown in Table 1.
The therapeutic efficacy of dicloxacillin, reflected by comparing the number of colonies grown from thighs of treated animals with the number in untreated animals, was significant (P < 0.05) in 10 of the 15 OS-MRSA isolates. Similarly, vancomycin was effective (P < 0.05 versus untreated controls) against infections caused by 12 OS-MRSA isolates. Interestingly, animals infected by 3 isolates, where vancomycin did not have significant efficacy, responded favorably to dicloxacillin treatment. When directly comparing the efficacy of dicloxacillin with that of vancomycin, a significant difference was not observed (P > 0.05) in 8 OS-MRSA infections, while vancomycin was significantly more effective (P < 0.05) in the remaining 7 isolates. Vancomycin treated significantly more efficiently than dicloxacillin the infections caused by the high-level MRSA control isolate, while the susceptible ATCC 29213 control responded slightly better to dicloxacillin than vancomycin. The results of the experimental infections are shown in Table 2. It should be noted that colonies yielded by infected thighs were tested again and found to carry and express the mecA gene.
Table 2.
Therapeutic efficacies in murine infections of dicloxacillin and vancomycin versus untreated controls and vancomycin versus dicloxacillina
| Isolate | Avg log CFU ± SD per thigh muscle in untreated controls | Avg log CFU ± SD per thigh muscle in DCX treatment | DCX treatment efficiency (P value) | Avg log CFU ± SD per thigh muscle in VAN treatment | VAN treatment efficiency (P value) | DCX vs VAN activity in mouse thigh infections (P value) |
|---|---|---|---|---|---|---|
| 446 | 7.6 ± 0.4 | 5.9 ± 0.4 | 0.016 | 6.3 ± 0.4 | 0.058 | 0.776 |
| 959 | 7.6 ± 0.3 | 6.9 ± 1.9 | 0.502 | 3.6 ± 1.8 | 0.011 | 0.009 |
| 970 | 8.7 ± 0.2 | 6.0 ± 0.6 | 0.037 | 5.8 ± 1.9 | 0.021 | 0.815 |
| 1117 | 7.9 ± 0.5 | 6.7 ± 0.4 | 0.062 | 6.3 ± 0.4 | 0.003 | 0.157 |
| 1512 | 8.7 ± 0.2 | 6.7 ± 0.6 | 0.034 | 4.3 ± 0.4 | 0.007 | 0.007 |
| 1546 | 8.9 ± 0.3 | 6.7 ± 0.8 | 0.208 | 3.9 ± 0.5 | 0.006 | 0.009 |
| 2629 | 8.3 ± 0.7 | 6.3 ± 1.1 | 0.058 | 3.4 ± 0.4 | <0.001 | 0.015 |
| 4324 | 8.8 ± 0.2 | 4.1 ± 0.01 | 0.001 | 3.6 ± 0.4 | <0.001 | 0.078 |
| 5014 | 7.2 ± 0.4 | 6.8 ± 0.8 | 0.488 | 4.0 ± 0.5 | 0.001 | 0.023 |
| 5505 | 7.7 ± 0.4 | 4.0 ± 1.1 | 0.04 | 3.5 ± 1.1 | <0.001 | 0.981 |
| 6036 | 8.8 ± 0.1 | 6.7 ± 0.3 | 0.033 | 5.7 ± 0.3 | 0.025 | 0.014 |
| 6601 | 8.8 ± 0.4 | 7.4 ± 0.3 | 0.015 | 4.2 ± 0.8 | 0.003 | 0.005 |
| 7059 | 8.9 ± 0.2 | 5.0 ± 1.9 | 0.026 | 3.2 ± 0.3 | <0.001 | 0.208 |
| 9131 | 8.7 ± 0.1 | 3.4 ± 0.4 | 0.001 | 3.3 ± 0.4 | <0.001 | 0.273 |
| 9833 | 8.9 ± 0.2 | 6.2 ± 1.4 | 0.035 | 5.1 ± 1.6 | 0.020 | 0.476 |
| ATCC 29213 | 8.7 ± 0.1 | 3.6 ± 0.6 | 0.001 | 3.8 ± 0.5 | <0.001 | 0.771 |
| 7263 | 8.6 ± 0.1 | 8.2 ± 0.2 | 0.33 | 3.3 ± 0.5 | <0.001 | <0.001 |
P value of <0.05 represents significant difference in CFU grown from infected thighs; DCX, dicloxacillin; VAN, vancomycin.
Discussion.
OS-MRSA clinical isolates have been increasingly reported in several countries (2, 8, 17) and in distant Greek regions (3, 9, 19). It has been shown previously that OS-MRSA may respond to oxacillin (9), possibly providing treatment alternatives. Recently, it has also been implied that some mutant MRSA isolates with relatively low oxacillin MICs may respond to oxacillin treatment in murine infections (10). However, it is generally believed that heterogeneous MRSA isolates may become homogeneously resistant under oxacillin exposure, resulting in treatment failure. In that respect, it has been suggested that oxacillin activity against such isolates should be compared with that of vancomycin, as most clinicians who knowingly encounter such strains would utilize vancomycin (9).
In the current study, we directly compared the in vitro and in vivo activities of dicloxacillin versus vancomycin against 15 OS-MRSA clinical isolates. In time-kill assays vancomycin exhibited lower bactericidal activity than dicloxacillin in most isolates. It has to be noted that vancomycin was also previously shown to kill MSSA less rapidly than antistaphylococcal penicillins (20). This low in vitro bactericidal activity of vancomycin probably caused the suboptimal results observed in the treatment of serious S. aureus infections (13, 20). It has also been reported that the bactericidal activity of β-lactams against MSSA may be superior to that of vancomycin (11). In that respect, it is not surprising that our mecA-carrying isolates, which are functionally oxacillin susceptible, responded sufficiently to oxacillin treatment. In particular, dicloxacillin successfully treated 66.7% of mouse infection due to OS-MRSA isolates, similar to vancomycin, which succeeded against 75% of the isolates, while dicloxacillin was efficient in infections where vancomycin failed. Furthermore, the direct comparison of the activities of dicloxacillin and vancomycin showed that, interestingly, dicloxacillin was similarly efficient with vancomycin in more than half of the OS-MRSA isolates tested.
Overall, our findings suggest that the use of antistaphylococcal penicillins could still be considered when treating OS-MRSA infections. Should OS-MRSA be more common in the future, this observation could have significant implications for the treatment of the respective infections.
ACKNOWLEDGMENTS
This study was partially funded by the project “Biofilm and infections” of the Research Committee of the University of Thessaly.
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Andes D, van Ogtrop ML, Peng J, Craig WA. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen FJ, Hiramatsu K, Huang IW, Wang CH, Lauderdale TL. 2009. Panton-Valentine leukocidin (PVL)-positive methicillin-susceptible and resistant Staphylococcus aureus in Taiwan: identification of oxacillin-susceptible mecA-positive methicillin-resistant S. aureus. Diagn. Microbiol. Infect. Dis. 65:351–357 [DOI] [PubMed] [Google Scholar]
- 3. Chini S, et al. 2008. Clonal evolution and antibiotic resistance profile of methicillin-resistant Staphylococcus aureus in Greece during a five-year period, abstr C2-231, p 156. Abstr. 48th Annu. Intersci. Conference Antimicrob. Agents Chemother. (ICAAC) American Society for Microbiology, Washington, DC [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed. Approved standard M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giannouli S, et al. 2010. Detection of mutations in the FemXAB protein family in oxacillin-susceptible mecA-positive Staphylococcus aureus clinical isolates. J. Antimicrob. Chemother. 65:626–633 [DOI] [PubMed] [Google Scholar]
- 7. Hegde SS, Reyes N, Skinner R, Difuntorum S. 2008. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J. Antimicrob. Chemother. 61:169–172 [DOI] [PubMed] [Google Scholar]
- 8. Hososaka Y, et al. 2007. Characterization of oxacillin-susceptible mecA-positive Staphylococcus aureus: a new type of MRSA. J. Infect. Chemother. 13:79–86 [DOI] [PubMed] [Google Scholar]
- 9. Ikonomidis A, et al. 2008. Investigation of oxacillin efficacy among oxacillin-susceptible, mecA positive, Staphylococcus aureus clinical isolates by population analyses, bactericidal assays and experimental thigh infections. Antimicrob. Agents Chemother. 52:3905–390818694946 [Google Scholar]
- 10. Jo DS, Montgomery CP, Yin S, Boyle-Vavra S, Daum RS. 2011. Improved oxacillin treatment outcomes in experimental skin and lung infection by methicillin-resistant Staphylococcus aureus containing a vraSR operon deletion. Antimicrob. Agents Chemother. 55:2818–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joukhadar C, Pillai S, Wennersten C, Moellering RC, Jr, Eliopoulos GM. 2010. Lack of bactericidal antagonism or synergism in vitro between oxacillin and vancomycin against methicillin-susceptible strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 54:773–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LaPlante KL, Leonard SN, Andes DR, Craig WA, Rybak MJ. 2008. Activities of clindamycin, daptomycin, doxycycline, linezolid, trimethoprim-sulfamethoxazole, and vancomycin against community-associated methicillin-resistant Staphylococcus aureus with inducible clindamycin resistance in murine thigh infection and in vitro pharmacodynamic models. Antimicrob. Agents Chemother. 52:2156–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine DP, Fromm BS, Reddy BR. 1991. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann. Intern. Med. 115:674–680 [DOI] [PubMed] [Google Scholar]
- 14. Lina G, et al. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128–1132 [DOI] [PubMed] [Google Scholar]
- 15. Pournaras S, et al. 2011. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int. J. Antimicrob. Agents 37:244–247 [DOI] [PubMed] [Google Scholar]
- 16. Rohrer S, Maki H, Berger-Bachi B. 2003. What makes resistance to methicillin heterogeneous? J. Med. Microbiol. 52:605–607 [DOI] [PubMed] [Google Scholar]
- 17. Saeed K, Dryden M, Parnaby R. 2010. Oxacillin-susceptible MRSA, the emerging MRSA clone in the UK? J. Hosp. Infect. 76:267–268 [DOI] [PubMed] [Google Scholar]
- 18. Sakiniene E, Collins LV. 2002. Combined antibiotic and free radical trap treatment is effective at combating Staphylococcus aureus-induced septic arthritis. Arthritis Res. 4:196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sakoulas G, et al. 2001. Methicillin-resistant Staphylococcus aureus: comparison of susceptibility testing methods and analysis of mecA-positive susceptible strains. J. Clin. Microbiol. 39:3946–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Small PM, Chambers HF. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Belkum A, et al. 1998. Assessment of resolution and intercenter reproducibility of results of genotyping Staphylococcus aureus by pulsed-field gel electrophoresis of SmaI macrorestriction fragments: a multicenter study. J. Clin. Microbiol. 36:1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]