LETTER
Proveblue (international patent PCT/FR/2007/001193), which is a methylene blue preparation that complies with the European Pharmacopoeia and contains limited organic impurities and heavy metals of recognized toxicity, has previously been demonstrated to possess in vitro antimalarial activity (at a geometric mean 50% inhibitory concentration [IC50] of 3.62 nM) against 23 Plasmodium falciparum strains that are resistant to various other antimalarials (11). No significant association was found between Proveblue IC50s and polymorphisms in the genes that are involved in quinoline resistance, such as pfcrt, pfmdr1, pfmdr2, pfmrp, and pfnhe-1; furthermore, there was no significant association between Proveblue IC50s and the copy numbers of pfmdr1 and pfmdr2 (11).
In the present study, we tested the effects of Proveblue in combination with the standard antimalarial drugs chloroquine (CQ), monodesethylamodiaquine (MDAQ; the active metabolite of amodiaquine), quinine (QN), mefloquine (MQ), and dihydroartemisinin (DHA) and with atorvastatin (AVA), a potential antimalarial drug (9, 12).
The methodology of the in vitro potentiating test was previously described (7). We used nine well-established Plasmodium falciparum strains that had different phenotypic profiles: 3D7, W2, Palo Alto, FCR3, FCM29, ImtVol, ImtK2, ImtL1, and Imt10500 (3). Each strain was assessed once in triplicate for eight concentrations of standard drugs in combination with 10 concentrations of Proveblue ranging from 0.004 to 10 nM.
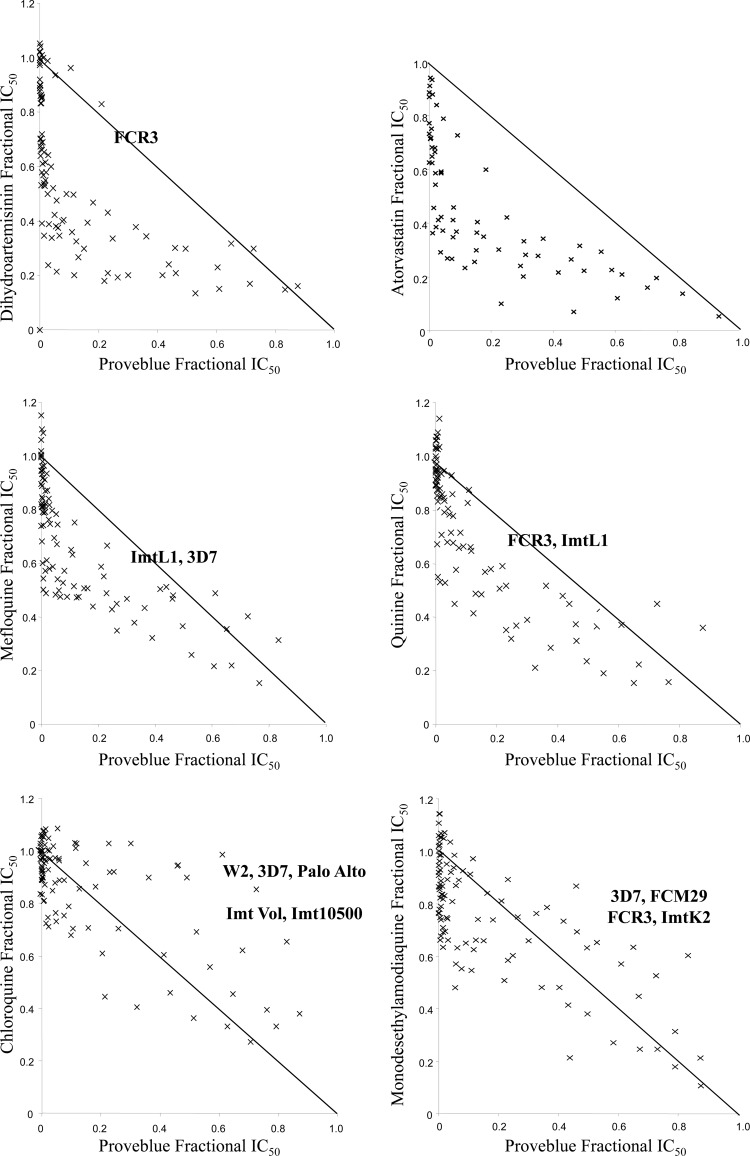
While Proveblue was shown to have antagonistic effects in combination with CQ and additive effects in combination with MDAQ against the nine P. falciparum strains (Fig. 1), Proveblue exhibited noticeable synergistic effects in combination with MQ and QN but high synergistic effects in combination with DHA and AVA. CQ IC50s were not significantly reduced in combination with Proveblue (Table 1). MQ and DHA IC50s were significantly reduced from 12.6% to 31.54% and from 18.9% to 48%, respectively, when Proveblue was added at concentrations ranging from 0.04 to 0.63 nM (9- to 140-fold less than the mean Proveblue IC50 for the nine strains and 0 to 2% of the growth inhibition obtained when used alone).
Fig 1.
In vitro effects of Proveblue in combination with DHA, AVA, MQ, QN, CQ, and MDAQ against nine strains of P. falciparum. Strains with antagonistic effects are above the additivity line, strains with synergistic effects are below the line, and strains with additive effects are on the line.
Table 1.
Reduction of the in vitro IC50s of CQ, MDAQ, QN, MQ, DHA, and AVA in combination with Provebluea
| Antimalarial | Avg % IC50 reduction [95% CI] (P value) with Proveblue at: |
||||
|---|---|---|---|---|---|
| 0.04 nMb | 0.08 nMc | 0.16 nMd | 0.31 nMe | 0.63 nMf | |
| CQ | 4.3 [0.9–7.7] (0.250) | 4.1 [0.6–7.6] (0.441) | 8.8 [2.9–14.7] (0.130) | 9.2 [1.1–17.4] (0.054) | 11.8 [2.2–21.3] (0.054) |
| MDAQ | 6.2 [0.–12.6] (0.859) | 15.1 [6.1–24.0] (0.075) | 15.4 [7.4–23.2] (0.044) | 19.3 [8.3–30.3] (0.039) | 17.4 [4.3–30.6] (0.008) |
| QN | 3.0 [0.–6.3] (0.820) | 7.5 [0.–17.4] (0.383) | 8.3 [1.8.–15.7] (0.074) | 15.3 [5.6–24.9] (0.004) | 20.6 [12.1–29.0] (0.009) |
| MQ | 12.6 [5.0–20.1] (0.027) | 15.1 [5.3–25.0] (0.020) | 20.9 [8.4–33.5] (0.004) | 25.6 [14.0–37.3] (0.004) | 31.5 [22.7–40.3] (0.004) |
| DHA | 18.9 [8.3–29.4] (0.012) | 23.7 [11.8–35.5] (0.008) | 33.0 [19.3–46.7] (0.008) | 41.2 [27.9–54.5] (0.004) | 48.0 [32.6–63.3] (0.009) |
| AVA | 24.6 [13.8–35.4] (0.020) | 37.0 [18.1–56.0] (0.020) | 43.6 [28.9–58.2] (0.020) | 56.3 [40.8–71.8] (0.020) | 63.1 [51.7–74.4] (0.020) |
CI, confidence interval. P values (for antimalarial plus Proveblue versus antimalarial alone) were determined by the Wilcoxon signed rank test. Significant P values (<0.05) are in bold.
Mean IC50/140.
Mean IC50/70.
Mean IC50/35.
Mean IC50/18.
Mean IC50/9.
These results were in agreement with the previous data on methylene blue noncompliant with the European Pharmacopoeia (Neph MB) that presented an antagonistic effect of Neph MB in combination with CQ against a CQ-resistant K1 strain but additive effects in combination with MQ and QN (2). More interestingly, the combination of Neph MB with artemisinin, artesunate, or artemether was found to act synergistically on the K1 strain (2). Garavito et al. found antagonism of Neph MB in combination with amodiaquine; additive effects in combination with CQ, MQ, and artemether; and synergy in combination with QN (5). Artemisinin induces a synergistic interaction with methylene blue; i.e., artemisinin reoxidizes leucomethylene blue, which is produced by reduction of methylene blue in parasites by the NADPH-flavin reductase system, in methylene blue, which both together oxidize FADH2 (6). This oxidation is inhibited by CQ, which interferes with redox processes.
In a previous study, we demonstrated that there was no significant correlation between DHA and Proveblue IC50s (r2 = 0.056; P = 0.275) (11). All of these data suggest that Proveblue could be effective as a good partner with artemisinin derivatives. Recent trials using artesunate provided evidence that Neph MB (despite not complying with the European Pharmacopoeia) is safe and relatively effective in uncomplicated falciparum malaria (4, 15). In addition, Neph MB has a gametocytocidal effect both in vitro and in vivo (1, 4). As suggested by in vitro combination data, the combination of Neph MB and CQ is not sufficiently effective against malaria in vivo (8).
Proveblue demonstrated synergistic effects in combination with AVA, a synthetic inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A. AVA IC50s were significantly reduced from 24.6% to 63.1% when Proveblue was added at concentrations ranging from 0.04 to 0.63 nM. Like Proveblue, AVA improved the in vitro activity of MQ (14), QN (10), or DHA (13) and the IC50s of AVA were unrelated to the mutations that occurred in the transport protein genes that are involved in quinoline resistance (9). The synergistic effect of AVA on MQ was significantly associated with the pfmdr1 copy number (14). However, there was no association between Proveblue activity and the pfmdr1 copy number (11). Even if we cannot explain the synergy between Proveblue and AVA, this observation supports the calls for in vivo evaluations in the murine malaria model.
These results confirm the therapeutic potential of Proveblue, which is a new methylene blue that contains limited organic impurities and heavy metals of recognized toxicity and could be integrated into new, low-cost antimalarial combination therapies.
ACKNOWLEDGMENT
This work was supported by the Délégation Générale pour l'Armement (grant 10ca405).
The conclusions of this article were in no way financially influenced.
Footnotes
Published ahead of print 5 March 2012
Contributor Information
Eric Baret, Unité de Parasitologie—UMR 6236 Institut de Recherche Biomédicale des Armées Marseille, France.
Michel Feraud, Provepharm SAS Marseille, France.
Bruno Pradines, Unité de Parasitologie—UMR 6236 Institut de Recherche Biomédicale des Armées Marseille, France.
REFERENCES
- 1. Adjalley SH, et al. 2011. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl. Acad. Sci. U. S. A. 108:E1214–E1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akoachere M, et al. 2005. In vitro assessment of methylene blue on chloroquine-sensitive and -resistant Plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob. Agents Chemother. 49:4592–4597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Briolant S, et al. 2010. Absence of association between piperaquine in vitro responses and polymorphisms in the pfcrt, pfmdr1, pfmrp, and pfnhe genes in Plasmodium falciparum. Antimicrob. Agents Chemother. 54:3537–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coulibaly B, et al. 2009. Strong gametocytocidal effect of methylene blue-based combination therapy against falciparum malaria: a randomized controlled trial. PLoS One 4:e5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garavito G, et al. 2007. Blood schizontocidal activity of methylene blue in combination with antimalarials against Plasmodium falciparum. Parasite 14:135–140 [DOI] [PubMed] [Google Scholar]
- 6. Haynes RK, et al. 2011. A partial convergence in action of methylene blue and artemisinins: antagonism with chloroquine, a reversal with verapamil, and an insight into the antimalarial activity of chloroquine. ChemMedChem 6:1603–1615 [DOI] [PubMed] [Google Scholar]
- 7. Henry M, et al. 2008. Dihydroethanoanthracene derivatives reverse in vitro quinoline resistance in Plasmodium falciparum malaria. Med. Chem. 4:426–437 [DOI] [PubMed] [Google Scholar]
- 8. Meissner PE, et al. 2006. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar. J. 5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parquet V, et al. 2009. Atorvastatin is a promising partner for antimalarial drugs in treatment of Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 53:2248–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parquet V, et al. 2010. Atorvastatin as a potential anti-malarial drug: in vitro synergy in combinational therapy with quinine against Plasmodium falciparum. Malar. J. 9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pascual A, et al. 2011. In vitro activity of Proveblue (methylene blue) on Plasmodium falciparum strains resistant to standard antimalarial drugs. Antimicrob. Agents Chemother. 55:2472–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pradines B, et al. 2007. Atorvastatin is 10-fold more active in vitro than other statins against Plasmodium falciparum. Antimicrob. Agents Chemother. 51:2654–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savini H, et al. 2010. Atorvastatin as a potential antimalarial drug: in vitro synergy in combinational therapy with dihydroartemisinin. Antimicrob. Agents Chemother. 54:966–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wurtz N, et al. 2010. Synergy of mefloquine activity with atorvastatin, but not chloroquine and monodesethylamodiaquine, and association with the pfmdr1 gene. J. Antimicrob. Chemother. 65:1387–1394 [DOI] [PubMed] [Google Scholar]
- 15. Zoungrana A, et al. 2008. Safety and efficacy of methylene blue combined with artesunate or amodiaquine for uncomplicated falciparum malaria: a randomized controlled trial from Burkina Faso. PLoS One 3:e1630. [DOI] [PMC free article] [PubMed] [Google Scholar]