Abstract
Interesting antischistosomal properties have been documented for the antimalarial mefloquine, a 4-quinolinemethanol. We evaluated the antischistosomal activities of nine mefloquine-related compounds belonging to the 4-pyridinemethanols, 9-phenanthrenmethanols, and 4-quinolinemethanols. Eight compounds revealed high activities against Schistosoma mansoni in vitro, with two drugs (the 4-quinolinemethanols WR7573 and WR7930) characterized by significantly lower half-maximal inhibitory concentrations (IC50s) (2.7 and 3.5 μM, respectively) compared to mefloquine (11.4 μM). Mefloquine and WR7930 showed significantly decreased IC50s when incubated in the presence of hemoglobin. High worm burden reductions (WBR) were obtained with enpiroline (WBR, 82.7%; dosage, 200 mg/kg of body weight) and its threo isomers (+)-threo (WBR, 100%) and (−)-threo (WBR, 89%) and with WR7930 (WBR, 87%; dosage, 100 mg/kg) against adult S. mansoni in mice. Furthermore, excellent in vitro and in vivo antischistosomal activity was observed for two WR7930-related structures (WR29252 and WR7524). In addition, mefloquine (WBR, 81%), enpiroline (WBR, 77%), and WR7930 (WBR, 100%) showed high activities against S. haematobium harbored in mice following single oral doses of 200 mg/kg. These results provide a deeper insight into the structural features of the arylmethanols that rule antischistosomal activity. Further studies should be launched with enpiroline and WR7930.
INTRODUCTION
Human schistosomiasis is a neglected tropical disease whose burden is mainly concentrated in sub-Saharan Africa and affects approximately 207 million people (30). The three main schistosome species parasitizing humans are Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum. Today, treatment with praziquantel is the core component of schistosomiasis control programs (32, 34, 35). There is no question that heavy reliance on a single drug bears a risk of drug resistance development. Indeed, in different regions of endemicity, lower cure rates have already been observed (9). An additional disadvantage of praziquantel is its stage-dependent susceptibility, showing only poor efficacy against immature schistosome stages (23). Therefore, drug discovery in the field of schistosomiasis remains an important task. Antischistosomal properties of the antimalarial drug mefloquine were first mentioned in 2008, when it was shown that a dosage of 150 mg/kg of body weight significantly reduced the egg burden in S. mansoni-infected mice (33). Further investigations revealed that mefloquine possesses good in vivo efficacy, with a single oral dosage of 200 mg/kg resulting in a total worm burden reduction of 72.3% in S. mansoni-infected mice. Another interesting characteristic of mefloquine is its efficacy against the juvenile immature stage (13). Finally, a randomized clinical trial that investigated the effect of mefloquine and mefloquine-artesunate in S. haematobium-infected patients was recently performed. It was shown that a combination therapy of mefloquine-artesunate resulted in moderate cure and high egg reduction rates (15).
Based on these promising findings, we were motivated to test mefloquine-related compounds belonging to the three major groups of arylmethanols well described in antimalarial research, namely, 4-quinolinemethanols, 9-phenanthrenmethanols, and 4-pyridinemethanols (4), in order to elucidate their potential as antischistosomal lead candidates. In addition, a study on mefloquine-related compounds might provide us with a deeper understanding of structural features needed for antischistosomal activity of arylmethanols. The selected nine arylmethanols were first tested against S. mansoni schistosomula and adults in vitro. Promising compounds were followed up in vivo. Candidates characterized by high in vivo activity against S. mansoni were tested against S. haematobium. In addition, promising candidates were incubated in the presence of hemoglobin, hemin, or red blood cells to compare drug activities in relation to the postulated mechanistic heme dependency of arylmethanols (6). Finally, isothermal microcalorimetry (IMC) was used to investigate the antischistosomal properties of lead candidates in greater detail and to compare their levels of activity with mefloquine.
MATERIALS AND METHODS
Drugs and media.
The following 11 arylmethanols were kindly provided by the Walter Reed Army Institute of Research (WRAIR): compounds 1 (WR171669; halofantrine), 2 (WR178460; n-desbutylhalofantrine), 3 [WR190420; 1-(1,3-dichloro-6-trifluoromethyl-9-phenanthryl)-2-(2-piperidyl)ethanol], 4 [WR148946; 1-(2,6-bis(4-(trifluoromethyl)phenyl)pyri-dine-4-yl)-2-(dibutylamino)ethanol], 5 [WR151312; 1-(2,6-bis(4-(trifluoromethyl)phenyl)pyri-dine-4-yl)-2-(butylamino)ethanol], 6 [WR154904; (2,6-bis(4-(trifluoromethyl)phenyl)pyridine-4-yl)(piperidin-2-yl)methanol], 7 (WR180409; enpiroline), 8 {WR7573; (2-(4-chlorophenyl)benzo[h]quinolin-4-yl)(piperidin-1-yl)methanol}, 9 [WR7930; (6,8-dichloro-2-phenylquinolin-4-yl)(piperidin-1-yl)methanol], 12 [WR29252; 6,8-dichloro-2-(4′-chlorophenyl)-alpha-(di-n-butylaminomethyl)-4-quinoline methanol], and 13 [WR7524; alpha-butylaminomethyl-2-(4′-chlorophenyl)-6,8-dichloro-4-quinoline methanol]. Additionally, we received the two threo enantiomers of enpiroline (compounds 10 [WR247733] and 11 [WR247734]) from the WRAIR. Mefloquine was kindly provided by Mepha AG (Aesch, Switzerland). The chemical structures of the arylmethanols investigated are shown in Fig. 1 to 3. For the in vitro studies, all drugs were dissolved in stock solutions of dimethyl-sulfoxide (DMSO) (Sigma-Aldrich Chemie GmbH) (10 mg/ml). For the in vivo studies, drugs were freshly prepared as water-based suspensions in 7% (vol/vol) Tween 80 and 3% (vol/vol) ethanol before per os administration to animals.
Fig 1.
Chemical structures of the arylmethanols investigated: halofantrine (compound 1); n-desbutylhalofantrine (compound 2); WR190420 (compound 3); WR148946 (compound 4); WR151312 (compound 5); WR154904 (compound 6); enpiroline/WR180409 (compound 7); WR7573 (compound 8); WR7930 (compound 9).
Fig 3.
Chemical structures of the WR7930-related compounds WR29252 (compound 12) and WR7524 (compound 13).
The hemin solution (1.5 mM) was prepared as follows: 50 mg of hemin-chloride (Fluka Analytical, Netherlands) was dissolved in 10 ml of 0.1 M NaOH and 39.5 ml of phosphate-buffered saline (PBS), and 0.5 ml of 1 M HCl was added to adjust the pH to ∼7.4. The hemoglobin solution (0.23 mM) was prepared using 750 mg of hemoglobin from bovine blood (Sigma-Aldrich) dissolved in identical amounts of NaOH, PBS, and HCl as described above. Supplemented RPMI media were achieved by adding 8% of freshly prepared hemin solution (final concentration of 120 μM), 10% of prepared hemoglobin solution (final concentration of 23 μM), or 2% red blood cells (RBC) from concentrate (group A; Rh positive).
Animals and parasites.
All animal studies were conducted at the Swiss Tropical and Public Health Institute (Basel, Switzerland) following Swiss national and cantonal regulations on animal welfare (permission no. 2070). Three-week-old (weight, ca. 14 g) female NMRI mice were purchased from Charles River (Sulzfeld, Germany) or Harlan Laboratories (Blackthorn, United Kingdom). Three-week-old Syrian golden hamsters were purchased from Charles River. All animals were allowed to adapt for 1 week under controlled conditions (temperature, ca. 22°C; humidity, ca. 50%; 12-h light and 12-h dark cycle; free access to rodent diet and water) before initiation of experiments. Mice were infected subcutaneously with 80 to 100 S. mansoni (Liberian strain) cercariae. Cercariae were harvested from infected intermediate Biomphalaria glabrata host snails by exposure to light for 3 h, following the standard procedures of our laboratory. The S. haematobium (Egyptian strain) infection was performed using Bulinus truncatus truncatus snails (kindly provided by the Biomedical Research Institute, Rockville, MD). Mice and hamsters were subcutaneously infected with 300 to 350 and with 120 S. haematobium cercariae, respectively.
In vitro studies with S. mansoni. (i) Preparation of NTS.
Cercariae of S. mansoni were mechanically transformed into newly transformed schistosomula (NTS) (12, 18). The concentration of the obtained schistosomulum suspension was adjusted to 100 NTS per 50 μl with Medium 199 (Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated fetal calf serum (iFCS), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Invitrogen, Carlsbad, CA). NTS suspensions were incubated at 37°C in an atmosphere of 5% CO2 in ambient air for a minimum of 12 to 24 h until usage, ensuring completed conversion from cercariae to NTS (8).
(ii) Preparation of adult schistosomes.
At 7 to 8 weeks postinfection with S. mansoni, NMRI mice were sacrificed by administration of CO2 and dissected, and adult worms were harvested from hepatic portal veins and mesenteric veins (36). Schistosomes were placed in RPMI 1640 culture medium supplemented with 5% heat-inactivated fetal calf serum (iFCS), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Invitrogen, Carlsbad, CA) at 37°C in an atmosphere of 5% CO2 until use. Likewise, adult flukes were collected from S. haematobium-infected NMRI mice; however, they were harvested 12 to 14 weeks postinfection.
(iii) Drug sensitivity assays with NTS.
Drugs were tested at a concentration range of 0.37 to 90 μg/ml (0.37, 1.1, 3.3, 10, 30, and 90 μg/ml). For that purpose, flat-bottom 96-well plates (BD Falcon) were prepared with Medium 199 supplemented with iFCS and antibiotics. Appropriate drug dilutions followed by the NTS suspension, containing 100 NTS per 50 μl, were added to each well to achieve a total volume of 250 μl per well. The highest DMSO concentration used, diluted in Medium 199, served as a control. Plates were incubated at 37°C in an atmosphere of 5% CO2. Based on microscope readouts (Carl Zeiss, Germany; magnification, ×80), NTS were evaluated with regard to death, changes in motility, viability, and morphological alterations at three different time points (24, 48, and 72 h post-drug exposure). Drug effects were evaluated using a viability scale, as described previously (12, 18). Briefly, parasite fitness, morphology, and motility were classified with scores ranging from 4 (hyperactivity, increased motility) and 3 (normal activity, no morphological changes) to 0 (all worms dead). Each concentration was tested in duplicate, and experiments were repeated at least three times. Motility scale values obtained at the 72-h time point were used to determine half-maximal inhibitory concentrations (IC50s) of the individual drugs using CompuSyn software (version 3.0.1 [2007]; ComboSyn, Inc.).
(iv) Drug sensitivity assay with adult schistosomes.
For the in vitro screening of adult flukes, RPMI 1640 medium, supplemented with iFCS and antibiotics, was placed into flat-bottom 24-well plates (BD Falcon). Dilution series were performed with DMSO stock solutions (concentration, 10 mg/ml) to obtain final concentrations of each test drug of 1, 3.3, 10, 30, and 90 μg/ml in a final volume of 1.4 ml per well. Three worms of both sexes were placed into each well. Experiments investigating the influence of iron-containing products were carried out with both schistosoma worms, which were incubated in supplemented RPMI media for 7 days without addition of RBC or any other additional iron source before usage, and freshly dissected worms. Wells with the highest concentration of DMSO in medium served as controls. Phenotypes were monitored after 24, 48, and 72 h using the motility scale described by Ramirez et al. (25) and an inverse microscope (Carl Zeiss, Germany; magnification, ×80). Each experiment was performed at least three times. IC50s were calculated with CompuSyn software as described above for NTS.
In vivo studies with S. mansoni and S. haematobium.
Groups of 3 to 5 infected NMRI mice or 4 Syrian golden hamsters characterized by a patent schistosome infection (49 days for S. mansoni and 90 days for S. haematobium) were treated orally with the test drug using single doses (100 or 200 mg/kg of body weight). Seven or eight (n = 12 for S. haematobium infection) untreated mice or four hamsters served as controls. At 14 days posttreatment, animals were sacrificed by the CO2 method and dissected. Worms were sexed and counted (36). Worm burdens of treated mice were compared to those of control animals and reductions of worm burden calculated.
IMC drug assay with adult S. mansoni.
Two lead candidates (enpiroline and WR7930), characterized by high WBR, were further characterized and compared to mefloquine using an isothermal microcalorimeter as described by Manneck et al. (17). Briefly, schistosome heat production and motility data (derived from noise amplitudes) were measured using a 48-channel isothermal microcalorimeter (model TAM 48; TA Instruments, New Castle, DE) over a time period of 120 h. Samples were prepared in glass ampoules with 2,900 μl of medium (supplemented RPMI 1640) containing 3 or 4 adult worms. Prewarmed (37°C) ampoules were placed in channels, and equilibration was performed for 5 h until a stable signal was observed. Drug suspensions (at a concentration of 30, 300, or 900 μg/ml) in supplemented medium (volume of 100 μl) were injected, using 1-ml syringes (BD Plastipak; Becton, Dickinson S.A., Madrid, Spain) to reach final concentrations (1, 10, and 30 μg/ml per ampoule). Ampoules with dead worms served as negative controls, and ampoules with worms treated with the highest concentration of DMSO served as positive controls. The heat flow was recorded as 1 data point per min over at least 120 h. Experiments using each concentration were repeated at least three times.
Statistics.
Parasite motility of treated and untreated NTS and adult flukes were calculated as means (± standard deviation) using Microsoft Excel software. IC50s were determined using the CompuSyn software. To compare the IC50s of lead candidates determined in various media, the Kruskal-Wallis test was performed (results were considered significant at P ≤ 0.05%) (StatsDirect statistical software, version 2.7.2; StatsDirect Ltd., United Kingdom). For in vivo studies, the Kruskal-Wallis test was also utilized, to compare the medians of the responses of the treatment and control groups. A difference in median values was considered to be statistically significant at a level of 5%. Noise amplitudes and heat flows observed in calorimetric in vitro assays with adult S. mansoni were analyzed using R software and Microsoft Excel. As described by Manneck et al. (16), noise amplitude values follow an exponential decay. Endpoints of worm motility were determined by the intersection of the sample amplitude curve with the background signal noise of dead worms. The statistical significance of the means of heat flows (calculated with Microsoft Excel) was assessed using the parametric paired t test at a 5% level of significance (StatsDirect statistical software, version 2.7.2; StatsDirect Ltd., United Kingdom).
RESULTS
In vitro activities of selected arylmethanols against NTS and adult S. mansoni.
IC50s of nine compounds tested against NTS and adult S. mansoni worms are summarized in Table 1. With exception of one drug (compound 4 [WR148946]; IC50 of 12.1 μM), all compounds tested showed higher activities (IC50s of 0.2 to 3.70 μM) than mefloquine (IC50 of 6.1 μM) against NTS. Compounds 1, 2, and 6 to 9 showed significantly higher activity than mefloquine. The highest activity against NTS was observed with compounds 2, 6, and 9 (IC50s of 0.2 μM). A similar finding was obtained for adult S. mansoni. Eight compounds revealed higher activities against the adult stage of the parasite than mefloquine (IC50 of 11.4 μM). Two compounds (compounds 8 and 9) revealed significantly lower IC50s (2.7 and 3.5 μM) than mefloquine. Compounds showing increased activity compared to mefloquine against adult S. mansoni were followed up in vivo.
Table 1.
In vitro IC50s of selected arylmethanols for NTS and adult S. mansoni 72 h posttreatmenta
| Methanol group | Compound | IC50 (SD) [μM] |
|
|---|---|---|---|
| NTS | Adult worms | ||
| 4-Quinoline | Mefloquine | 6.1 (1.3) | 11.4 (6.4) |
| 9-Phenanthrene | 1 (WR171669) | 1.9 (0.4) | 7.2 (1.6) |
| 2 (WR178460) | 0.2 (0.2) | 7.1 (6.4) | |
| 3 (WR190420) | 3.0 (2.6) | 5.4 (1.7) | |
| 4-Pyridine | 4 (WR148946) | 12.1 (8.2) | >52.2 |
| 5 (WR151312) | 2.7 (3.1) | 4.0 (3.4) | |
| 6 (WR154904) | 0.2 (0) | 5.3 (6.2) | |
| 7 (WR180409) | 0.9 (0.2) | 6.1 (4.7) | |
| 4-Quinoline | 8 (WR7573) | 1.4 (1.3) | 2.7 (0.2) |
| 9 (WR7930) | 0.2 (0.3) | 3.5 (1.6) | |
Experiments were carried out 3 times in duplicate for NTS (n = 6) and 3 times for adult schistosomes (n = 3) for each tested drug. SD, standard deviation.
In vivo activity of arylmethanols against adult S. mansoni.
In vivo activities of eight arylmethanols are summarized in Table 2. Previous studies have reported worm burden reductions of 45.8% and 64.0% when a single oral dose of mefloquine at 100 mg/kg of body weight was administered to S. mansoni-infected mice (13, 14). At 100 mg/kg, compound 6 completely lacked activity. A low total worm burden reduction of 18.9% was observed for compound 5. Moderate total worm burden reductions of 37.1%, 40.5%, and 52.0% were achieved with compounds 3, 8, and 2, respectively. Significant total worm burden reductions of 58.5% (P = 0.02) and 60.4% (P = 0.01) (and female worm burden reductions of 58.6% and 68.6%) were documented for halofantrine and enpiroline, respectively. The highest activity (total and female worm burden reductions of 87.0% and 91.9%, respectively [P = 0.01]) was documented for compound 9 (WR7930).
Table 2.
Effect of selected arylmethanols on worm burden in mice harboring adult S. mansoni (patent infection, 49 days), stratified by sex and worm distributiona
| Compound (100 mg/kg) | No. of mice investigated | No. of mice that died | No. of mice cured | Mean no. of worms (SD) |
TWR (%) | P value | FWBR (%) | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Mesenteric veins | Total | Females | ||||||||
| Control | 8 | 0.6 (1.0) | 13.0 (3.0) | 13.9 (2.5) | 6.4 (2.5) | ||||||
| 5 | 4 | 0 | 0 | 0.8 (0.4) | 10.5 (5.2) | 11.3 (5.1) | 4.8 (2.2) | 18.9 | 0.3 | 25.5 | 0.35 |
| 6 | 3 | 0 | 0 | 0 (0) | 21.3 (12.5) | 21.3 (12.5) | 10.3 (6.1) | 0 | 0.92 | 0 | 0.84 |
| 7 | 6 | 2 | 0 | 0.5 (0.9) | 5.0 (3.5) | 5.5 (3.2) | 2.0 (1.9) | 60.4 | 0.01 | 68.6 | 0.01 |
| 8 | 4 | 0 | 0 | 1.0 (1.0) | 7.3 (4.5) | 8.3 (4.5) | 4.0 (2.9) | 40.5 | 0.03 | 37.3 | 0.2 |
| Control | 7 | 0.3 (0.8) | 24.9 (7.6) | 25.0 (8.1) | 12.3 (3.9) | ||||||
| 2 | 4 | 1 | 0 | 0.5 (0.5) | 11.5 (3.9) | 12.0 (6.0) | 5.5 (2.5) | 52.0 | 0.11 | 55.4 | 0.09 |
| 9 | 4 | 0 | 0 | 2.3 (0.8) | 1.0 (1.7) | 2.3 (0.8) | 1.0 (0.7) | 87.0 | 0.01 | 91.9 | 0.01 |
| Control | 6 | 1.3 (1.0) | 30.0 (3.9) | 31.3 (3.7) | 14.5 (3.5) | ||||||
| 1 | 3 | 0 | 0 | 1.0 (2.3) | 11.7 (6.2) | 13.0 (5.8) | 5.0 (1.7) | 58.5 | 0.02 | 58.6 | 0.02 |
| Control | 9 | 2.0 (3.5) | 27.0 (27.7) | 29.0 (28.5) | 17.8 (24.1) | ||||||
| 3 | 4 | 0 | 0 | 0.8 (1.5) | 16.0 (11.5) | 18.3 (10.9) | 7.8 (4.5) | 37.1 | 0.76 | 67.9 | 0.76 |
| MQ (data from reference 13) | 45.8 | 56.3 | |||||||||
| MQ (data from reference 14) | 64.0 | 77.6 | |||||||||
SD, standard deviation; TWR, total worm burden reduction; FWBR, female worm burden reduction; MQ, mefloquine.
Halofantrine was not considered further, since, in a previous study, an increased dose (400 mg/kg) achieved only a moderate total worm burden reduction of 51.7% (13). Enpiroline, an antimalarial drug candidate that had already been tested in phase 2 trials (7), and its enantiomers (compounds 10 and 11) were selected for further studies along with WR7930 and two closely related molecules (compounds 12 and 13).
In vitro activity of WR7930, enpiroline, and mefloquine against adult S. mansoni determined using IMC.
The effects of the lead candidates enpiroline, WR7930, and mefloquine on the heat flow and motility of adult S. mansoni determined using three different concentrations (1, 10, and 30 μg/ml) were compared using IMC over a period of 5 days.
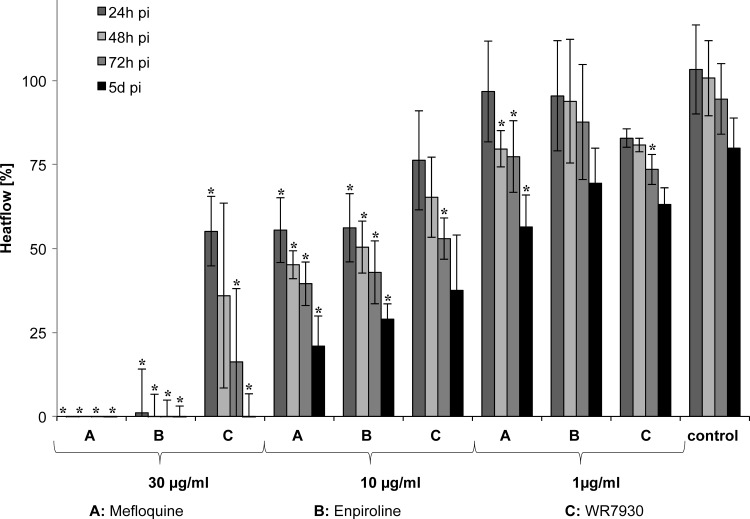
Enpiroline and mefloquine showed similar effects on the heat flow (Fig. 4) and motility of adult S. mansoni, which is consistent with the microscopically obtained results. Concentrations of 30 and 10 μg/ml of each drug resulted in immediate losses of motility and a significant decrease of heat flow. The lowest tested concentration (1 μg/ml) of both drugs had only a minor effect on the motility (all worms were active for >5 days). At this concentration, however, the heat flow was significantly decreased compared to that seen with the control at 48 h post-mefloquine treatment whereas enpiroline administration did not result in a significant decrease in heat flow. Heat flow and motility were less affected by WR7930. A significant heat flow reduction at 5 days posttreatment with WR7930 was observed only with the highest concentration (30 μg/ml). Motility loss was documented 19.3 h posttreatment at the highest concentration (30 μg/ml).
Fig 4.
Heat flow of schistosomes recorded over 5 days (d) posttreatment with (A) mefloquine, (B) enpiroline, or (C) WR7930 at concentrations of 30, 10, and 1 μg/ml. Bars indicate standard deviations. Experiments were conducted in quadruplicate with 4 worms each. Asterisks (*) indicate heat flow values which are significantly (P ≤ 0.05) lower than control values obtained with untreated worms. pi, postinjection.
In vitro and in vivo activity of enpiroline and its enantiomers against S. mansoni.
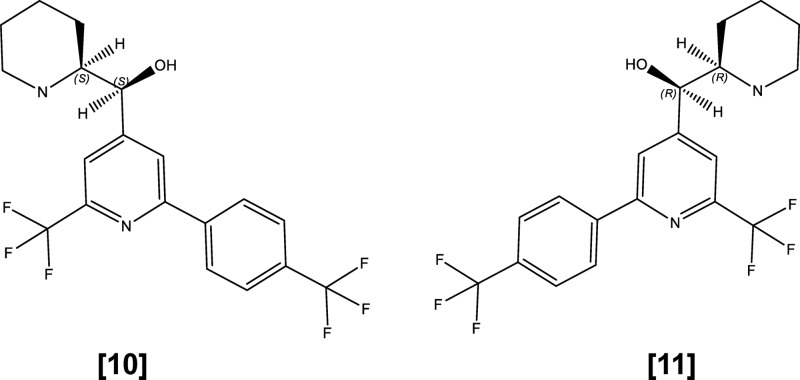
The two enantiomers of enpiroline (structures shown in Fig. 2) showed similar antischistosomal activities in vitro as determined using a microscopic readout (Table 3). The ratio of the microscopically evaluated IC50 of the (−) isomer (9.5 μM) to the IC50 of the (+) isomer (9.0 μM) against adult S. mansoni is 1.06. Slightly higher activities against NTS (0.9 μM) and adult S. mansoni worms (6.1 μM) were observed with enpiroline.
Fig 2.
Chemical structures of the enantiomers of enpiroline: (−)-threo enpiroline (compound 10); (+)-threo enpiroline (compound 11).
Table 3.
In vitro activity of enpiroline (threo racemate) and its (−)-threo/(+)-threo enantiomers against NTS and adult S. mansoni 72 h posttreatment as assessed microscopicallya
| Compound | IC50 (SD) [μM] |
|
|---|---|---|
| NTS | Adult worms | |
| Enpiroline | 0.9 (0.2) | 6.1 (4.7) |
| (−)-threo | 3.6 (4.7) | 9.5 (6.5) |
| (+)-threo | 2.8 (1.7) | 9.0 (5.9) |
Experiments were carried out 3 times in duplicate for NTS (n = 6) and 3 times for adult schistosomes (n = 3) for each tested drug. SD, standard deviation.
IMC revealed that, at a concentration of 20 μM, the (−)-threo isomer achieved a loss of movement of schistosomes 27 h posttreatment compared to 46.5 h for the (+)-threo enantiomer. On the other hand, at a concentration of 20 μM, enpiroline inhibited the motility of adult S. mansoni immediately after exposure.
The in vivo efficacies obtained with enpiroline and its threo enantiomers at 200 mg/kg are summarized in Table 4. No significant differences in activity between the two enantiomers and enpiroline could be observed. In vivo treatment of mice with the (+)-threo enantiomer resulted in complete elimination of worms. However, 4 out of 5 mice died following treatment with this drug. Highly significant total and female worm burden reductions were observed following treatment of S. mansoni-infected mice with the racemate enpiroline (82.7% and 83.8%, respectively) and the (−) isomer (89.0% and 91.9%, respectively) at 200 mg/kg. Based on these findings, the in vitro cytotoxicity was assessed using two different cell lines (HepG2 and L6 rat skeleton cells). The lead compounds (WR7930, enpiroline, and both enantiomers) showed patterns of cytotoxicity similar to that seen with the parent drug mefloquine. Hepatic HepG2-cells were strongly affected after treatment with the highest concentration (30 μg/ml), whereas the other tested concentrations (3 and 0.3 μg/ml) did not reduce the cell viability. After a 72-h treatment of L6 rat skeleton cells, IC50s ranging from 9.7 to 16.5 μM were observed. Detailed in vitro cytotoxicity data are summarized in Tables S1 and S2 in the supplemental material.
Table 4.
Effect of single oral doses of enpiroline and its (+)-threo and (−)-threo enantiomers at 200 mg/kg on worm burden in mice harboring adult S. mansoni (patent infection, 49 days), stratified by sex and worm distributiona
| Compound | No. of mice investigated | No. of mice that died | No. of mice cured | Mean no. of worms (SD) |
TWR (%) | P value | FWBR (%) | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver | Mesenteric veins | Total | Females | ||||||||
| Control | 7 | 0.3 (0.8) | 24.9 (7.6) | 25.0 (8.1) | 12.3 (3.9) | ||||||
| (−)-threo | 4 | 0 | 0 | 1.3 (0.4) | 1.3 (0.9) | 2.8 (1.1) | 1.0 (0.7) | 89.0 | 0.01 | 91.9 | 0.01 |
| (+)-threo | 5 | 4 | 1 | 0 | 0 | 0 | 0 | 100.0 | 100.0 | ||
| Enpiroline | 4 | 1 | 1 | 0.7 (0.9) | 3.7 (1.9) | 4.3 (4.2) | 2.0 (2.2) | 82.7 | 0.02 | 83.8 | 0.02 |
| MQ (data from reference 13) | 72.3 | 93.0 | |||||||||
SD, standard deviation; TWR, total worm burden reduction; FWBR, female worm burden reduction; MQ, mefloquine.
In vitro and in vivo activities of WR7930-related compounds.
Since WR7930 showed very good in vivo antischistosomal activity at an oral dosage of 100 mg/kg in mice, two related structures featuring desbutyl and dibutyl aminostructures (Fig. 3) were investigated in vitro and in vivo. As shown in Table 5, desbutyl compound 13 showed lower in vitro activity against NTS (IC50, 4.3 μM) and similar activity against adult schistosomes (IC50, 3.9 μM) compared to WR7930 and higher activity than mefloquine on both stages. On the other hand, compound 12, a dibutyl analogue, showed lower activity than WR7930 against NTS (IC50, 5.7 μM) and even less activity than mefloquine against adult S. mansoni (IC50, 27.9 μM) (Table 5). Both compounds showed high antischistosomal in vivo activity, achieving total worm burden reductions of 75.0% (compound 12) and 81.9% (compound 13).
Table 5.
In vitro activity of WR7930-related compounds against NTS and adult S. mansoni and its in vivo effect on S. mansoni in infected NMRI mice treated with an oral dosage of 100 mg/kga
| Treatment group |
In vitro IC50 (SD) [μM] |
In vivo |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NTS | Adult worms | No. of mice investigated | No. of mice cured | Mean no. of worms (SD) |
TWR (%) | P value | FWBR (%) | P value | |||
| Liver | Mesenteric veins | Total | |||||||||
| Control | 9 | 2.0 (3.5) | 27.0 (27.7) | 29.0 (28.5) | |||||||
| Compound 12 | 5.7 (3.3) | 27.9 (6.8) | 4 | 0 | 1.5 (1.0) | 5.8 (2.6) | 7.3 (2.8) | 75.0 | 0.31 | 83.1 | 0.18 |
| Compound 13 | 4.3 (2.2) | 3.9 (0.7) | 4 | 0 | 2.8 (2.8) | 3.3 (2.5) | 5.3 (1.5) | 81.9 | 0.12 | 93.0 | 0.02 |
In vitro experiments were carried out 3 times in duplicate for NTS (n = 6) and 3 times for adult schistosomes (n = 3) for each tested drug. SD, standard deviation; TWR, total worm burden reduction; FWBR, female worm burden reduction.
Influence of hemin, hemoglobin, or red blood cells on drug activity against adult schistosomes.
As shown in Fig. 5 IC50s of enpiroline did not differ significantly following supplementation of RPMI medium with hemin, hemoglobin, or RBC (RPMI, 6.1 μM; hemin, 8.0 μM; hemoglobin, 5.0 μM; RBC, 7.5 μM) (P > 0.05). Mefloquine revealed similar activities in RPMI medium, RPMI medium plus hemin, and RPMI medium plus RBC (11.4, 9.7, and 9.9 μM, respectively) (P > 0.05). A significantly 57-fold-lower IC50 (0.2 μM) was determined for mefloquine in the presence of hemoglobin compared to standard culture medium conditions (P < 0.03). Similarly, the highest antischistosomal activity of WR7930 was calculated in the presence of hemoglobin (0.2 μM). An 18-fold-higher IC50 (3.5 μM) was observed when adult schistosomes were exposed to WR7930 in RPMI medium followed by incubation in the presence of RBC (IC50, 6.0 μM) and hemin (IC50, 11.3 μM) (P < 0.05). All female worms died 4 h post-WR7930 exposure at the highest concentration (30 μg/ml), whereas males often stayed alive until 6 to 24 h postexposure. This effect could be observed in all investigated media.
Fig 5.
IC50s calculated following exposure of adult S. mansoni worms to mefloquine, enpiroline, or WR7930 using different culture media (RPMI medium or RPMI medium supplemented with 120 μM hemin, 23 μM hemoglobin, or 2% RBC). Error bars indicate standard deviations of experiments (n = 5). Asterisks (*) indicate IC50s which are significantly (P ≤ 0.05) lower than the control values determined using RPMI medium.
No differences in results were observed in all experiments using starved or freshly dissected S. mansoni.
In vivo activity of enpiroline, WR7930, and mefloquine against S. haematobium.
Enpiroline, mefloquine, and WR7930 were studied in S. haematobium-infected mice and hamsters. In vivo findings are summarized in Table 6.
Table 6.
Activity of enpiroline, mefloquine, and WR7930 at doses of 100 and 200 mg/kg against S. haematobium in mice and hamstersa
| Host | Drug [dose (mg/kg)] | No. of animals investigated | No. of animals cured | Mean no. of worms (SD) |
TWR (%) | P value | ||
|---|---|---|---|---|---|---|---|---|
| Liver | Mesenteric veins | Total | ||||||
| NMRI mice | Control | 12 | 2.3 (2.4) | 3.0 (4.0) | 5.3 (5) | |||
| Enpiroline [200] | 4 | 3 | 0 (0) | 1.3 (2.5) | 1.3 (2.5) | 76.6 | 0.05 | |
| Mefloquine [200] | 4 | 1 | 0.7 (1.2) | 0.3 (0.6) | 1.0 (1.0) | 81.3 | 0.03 | |
| WR7930 [200] | 4 | 4 | 0 (0) | 0 (0) | 0(0) | 100.0 | 0.01 | |
| Syrian golden hamsters | Control | 4 | 5.5 (4.2) | 13.7 (6.7) | 15.8 (4.6) | |||
| Mefloquine [100] | 4 | 0 | 1.5 (1.9) | 4.5 (2.6) | 6.0 (2.9) | 61.9 | 0.02 | |
| Mefloquine [200] | 4 | 3 | 0.3 (0.5) | 0.8 (1.5) | 1.0 (2.0) | 93.7 | 0.02 | |
SD, standard deviation; TWR, total worm burden reduction; FWBR, female worm burden reduction.
Mefloquine showed good activity against S. haematobium in vivo. Worm burden reductions of 61.9% and 93.7% were observed at dosages of 100 and 200 mg/kg, respectively, in hamsters. Similarly, a worm burden reduction of 81.3% was observed in S. haematobium-infected mice treated with a single dose at 200 mg/kg. Enpiroline showed comparable activity, with a worm burden reduction of 76.6% at a dose of 200 mg/kg in S. haematobium-infected mice. Complete elimination of worms was achieved by treating S. haematobium-infected mice with WR7930 at 200 mg/kg.
DISCUSSION
Mefloquine possesses a compelling antischistosomal prototype and might therefore serve as a starting point to identify one or more related lead compounds with high antischistosomal efficacy.
Nine compounds, representatives of 3 chemical classes, the 4-quinolinemethanols, 9-phenanthrenmethanols, and 4-pyridinemethanols, served as initial sources in our small structure-activity-relationship study. To our knowledge, compounds belonging to the 4-pyridinemethanols had not been tested against schistosomes to date. We selected four representatives of this group, including enpiroline, since the drug is characterized by good pharmacokinetic features and has already undergone clinical testing against malaria in humans (3, 7). Three compounds from the 9-phenanthrenmethanols, halofantrine, n-desbutylhalofantrine (the main human metabolite of halofantrine), and a derivative characterized by a piperidyl (compound 3) similar to mefloquine, were chosen. Finally, two 4-quinolinemethanol analogs of WR7930 complemented our study.
Our findings confirm the potential of arylmethanols as lead structure candidates. Eleven compounds revealed high activities in vitro. In addition, 5 of 12 selected compounds showed in vivo activity in S. mansoni-infected NMRI mice that, relative to the activity of mefloquine, was comparable [enpiroline and its (−)-threo enantiomer] or even increased (WR7930 and its related structures WR29252 and WR7524). Activity was observed among all chemical subclasses, and no final conclusion on the structural needs for an ideal antischistosomal arylmethanol can be drawn.
The similarity of the susceptibilities seen with arylmethanols tested against Plasmodium and Schistosoma species is worth highlighting. When the tested 4-pyridinemethanols (compounds 4 to 7) were ranked in the order of their activities, a pattern was observed in our study that was similar to that observed in studies carried out in Plasmodium falciparum-infected owl monkeys (28). In more detail, enpiroline was found to be the most active representative followed by compound 5 showing moderate activity and compound 6 showing only minimal activity against P. falciparum. A similar trend was also observed with activity against S. mansoni. In addition, in our study, halofantrine and its n-desbutyl derivative showed IC50s in similar ranges, a result that is in line with data on various Plasmodium strains (2).
Furthermore, the above-mentioned study from Schmidt and colleagues (28) demonstrated higher activity of desbutyl drugs against two Plasmodium species. A similar result was observed in the present study against schistosomes. This similarity between the two parasites with respect to structural needs might point to similar modes of action of arylmethanols against schistosomes and Plasmodium species.
Regarding the 4-quinolinemethanols, we observed high in vitro activity for three of four representatives (compounds 8, 9, and 13) and moderate (compound 8) to very good (compound 9, 12, and 13) in vivo activity for all four compounds. WR7930 (compound 9), which possesses a piperidyl substituent on the methanol side chain, showed the highest worm burden reduction, followed by its desbutyl and dibutyl relatives. A correlation between the side-chain modification (dibutyl, desbutyl, and piperidyl substituent) and antischistosomal activity was observed in vitro for all compounds examined on NTS. All compounds tested with dibutyl substituents showed greatly reduced activities, in contrast to the piperidyl derivatives, which revealed high activities. A similar trend was observed for activity against the adult worms. Interestingly, these structure-activity correlations were also described for 4-quinolinemethanols tested against Plasmodium (29). Despite the exceptionally good activity of WR7930, it has to be mentioned that the drug possesses mild to moderate phototoxic properties (27). This fact in particular has to be kept in mind, as the drug would be used in tropical surroundings.
Mefloquine and enpiroline demonstrated similar activities in vivo and in vitro, according to the results obtained with both IMC and microscopy. Both compounds immediately affected the motility of adult S. mansoni. In contrast, a delayed loss of motility and a reduction of heat flow after exposure to WR7930 were observed using IMC. On the other hand, the highest worm burden reductions in vivo were observed with WR7930. The high in vivo activity of WR7930 might be explained by excellent pharmacokinetic properties. A long half-life of up to 25 to 30 days for the drug in humans was previously described (24).
In our work, we also studied the threo diastereomers of enpiroline. Earlier studies related to malaria research did not detect significant differences in the activities of erythro and threo diastereomers. Based on these data, the erythro isomers were not tested in the present work. An additional advantage of the threo isomers is that it is easier to prepare these compounds in larger quantities (28). Our finding that both isomers and the racemate show similar antischistosomal activities is again in accordance with data on P. falciparum (11). A recent study by Manneck et al. showed that there was no significant difference between the activities of the diastereomers and its enantiomers of mefloquine in S. mansoni-infected mice (16). Interestingly, the (+)-threo isomer showed high toxicity at a dosage of 200 mg/kg in S. mansoni-infected mice (4 out of 5 treated mice died), while the (−)-threo isomer was well tolerated. Toxicity studies performed by the WRAIR in connection with preclinical trials for antimalarial candidates documented low levels of toxicity, with similar patterns determined for the two enantiomers (unpublished findings). Details on in vitro cytotoxicity assessments performed using two different cell lines are provided in Tables S1 and S2 in the supplemental material. Previous studies have shown that threo enpiroline has acceptable levels of acute and subacute toxicity in mice (28). In addition, the drug was well tolerated in clinical trials using total dosages of 1,500 mg (7). Nonetheless, our observations should be kept in mind, as the (−)-threo isomer might offer a therapeutic benefit in terms of minimized adverse events.
Though the activity of mefloquine against S. mansoni and S. japonicum in rodents has been well described (13, 37), we have for the first time elucidated the efficacy of mefloquine against S. haematobium in mice and hamsters. S. haematobium represents one of the most important human-pathogenic Schistosoma species (26). It is therefore of high importance to find a potential drug candidate which shows activity against all major Schistosoma species. Promisingly, no species-specific sensitivity was observed with all three lead candidates (mefloquine, enpiroline, and WR7930), revealing comparable activities against S. mansoni and S. haematobium. However, since the mouse is a poor host for drug testing against S. haematobium (as evidenced by the very low worm recoveries in mice), the results obtained for enpiroline and WR7930 should be confirmed in hamsters (as was done for mefloquine), since it is the better host for rodent S. haematobium infections (1, 5).
The degradation of hemoglobin provides adult schistosomes with essential nutrients such as amino acids for growth, development, and reproduction (31). Aggregation into hemozoin represents a heme (the resulting end product of hemoglobin digestion) detoxification pathway in S. mansoni (22). Mungthin and colleagues showed that hemoglobin degradation plays a central role in the mechanism of 4-aminoquinolines on Plasmodium species (20), and drug hematin binding was suggested as a possible mechanism (10). Another study postulated that exposure of S. mansoni worms to chloroquine inhibits heme aggregation (21). Our in vitro studies have demonstrated that the addition of hemoglobin to the incubation medium strongly impacts the activity of quinolinemethanols. Interestingly, pronounced activities in the presence of hemoglobin were detected only for representatives of the quinolinemethanols tested, WR 7930 and mefloquine. Enpiroline, a pyridinemethanol, did not show any differences in activity under different culture conditions. Surprisingly, the addition of red blood cells, which contain lots of hemoglobin, had no influence on the activity of the test drugs. One reason might be that an impact of red blood cells on activity would be visible only over a longer duration of incubation, since red blood cells have to be lysed in order to liberate intracellular hemoglobin. Overall, all the drugs (mefloquine, enpiroline, and WR7930) revealed good antischistosomal activity without addition of hemoglobin, hemin, or RBC, suggesting the presence of a second, non-heme-dependent mode of action for their antischistosomal activity. Similarly, an interaction with hematin polymerization may not be sufficient to explain the activity of such drugs as mefloquine against Plasmodium (20). We have recently demonstrated that mefloquine interferes with glycolysis in S. mansoni schistosomula (19).
In conclusion, our study has confirmed the high antischistosomal activity of compounds with an mefloquine scaffold. We identified four candidates, WR7930, its two derivatives, and enpiroline, that are characterized by high antischistosomal properties in vivo. Interestingly, two of these compounds have already undergone extensive testing as antimalarial drugs.
WR7930 showed the highest activity in vivo; however, due to its phototoxic potential, as observed in a previous study performed with humans (24), it does not represent a lead candidate. Nonetheless, both derivatives of WR7930 tested in the present work showed remarkably high antischistosomal activities; hence, these compounds should be studied in preclinical studies in greater detail. In addition, this chemical structure might serve as a starting point for further structure-activity studies. Finally, enpiroline offers the advantages that it has already been used in clinical trials and is characterized by a good pharmacokinetic and safety profile. Hence, the drug would be available for clinical testing in proof-of-concept studies. However, whether the drug offers a therapeutic benefit superior to that of mefloquine remains to be confirmed.
Supplementary Material
ACKNOWLEDGMENTS
The following reagent was obtained through BEI Resources, NIAID, NIH: Schistosoma haematobium Exposed Bulinus truncatus subsp. truncatus (NR-21965). We thank Helene Kettiger and Lorenz Hofer very much for their helping hands with parts of the in vitro experiments.
J. Keiser is grateful to the Swiss National Science Foundation for financial support (grants PPOOA-114941 and PPOOP3_135170).
Footnotes
Published ahead of print 2 April 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Agnew AM, Lucas SB, Doenhoff MJ. 1988. The host-parasite relationship of Schistosoma haematobium in CBA mice. Parasitology 97(Pt. 3):403–424 [DOI] [PubMed] [Google Scholar]
- 2. Basco LK, Gillotin C, Gimenez F, Farinotti R, Le Bras J. 1992. Antimalarial activity in vitro of the N-desbutyl derivative of halofantrine. Trans. R. Soc. Trop. Med. Hyg. 86:12–13 [DOI] [PubMed] [Google Scholar]
- 3. Basco LK, Gillotin C, Gimenez F, Farinotti R, Le Bras J. 1992. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br. J. Clin. Pharmacol. 33:517–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canfield CJ. 1980. Antimalarial aminoalcohol alternatives to mefloquine. Acta Trop. 37:232–237 [PubMed] [Google Scholar]
- 5. Cheever AW. 1985. Schistosoma haematobium: the pathology of experimental infection. Exp. Parasitol. 59:131–138 [DOI] [PubMed] [Google Scholar]
- 6. Corrêa Soares JB, et al. 2009. Interference with hemozoin formation represents an important mechanism of schistosomicidal action of antimalarial quinoline methanols. PLoS Negl. Trop. Dis. 3:e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosgriff TM, et al. 1984. Evaluation of the 4-pyridinemethanol WR 180,409 (enpiroline) in the treatment of induced Plasmodium falciparum infections in healthy, non-immune subjects. Am. J. Trop. Med. Hyg. 33:767–771 [DOI] [PubMed] [Google Scholar]
- 8. Cousin CE, Stirewalt MA, Dorsey CH, Watson LP. 1986. Schistosoma mansoni: comparative development of schistosomules produced by artificial techniques. J. Parasitol. 72:606–609 [PubMed] [Google Scholar]
- 9. Doenhoff MJ, Cioli D, Utzinger J. 2008. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 21:659–667 [DOI] [PubMed] [Google Scholar]
- 10. Dorn A, et al. 1998. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 55:727–736 [DOI] [PubMed] [Google Scholar]
- 11. Karle JM, Olmeda R, Gerena L, Milhous WK. 1993. Plasmodium falciparum: role of absolute stereochemistry in the antimalarial activity of synthetic amino alcohol antimalarial agents. Exp. Parasitol. 76:345–351 [DOI] [PubMed] [Google Scholar]
- 12. Keiser J. 2010. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology 137:589–603 [DOI] [PubMed] [Google Scholar]
- 13. Keiser J, et al. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keiser J, Manneck T, Vargas M. 2011. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J. Antimicrob. Chemother. 66:1791–1797 [DOI] [PubMed] [Google Scholar]
- 15. Keiser J, et al. 2010. Efficacy and safety of mefloquine, artesunate, mefloquine-artesunate, and praziquantel against Schistosoma haematobium: randomized, exploratory open-label trial. Clin. Infect. Dis. 50:1205–1213 [DOI] [PubMed] [Google Scholar]
- 16. Manneck T, Braissant O, Ellis W, Keiser J. 2011. Schistosoma mansoni: antischistosomal activity of the four optical isomers and the two racemates of mefloquine on schistosomula and adult worms in vitro and in vivo. Exp. Parasitol. 127:260–269 [DOI] [PubMed] [Google Scholar]
- 17. Manneck T, Braissant O, Haggenmuller Y, Keiser J. 2011. Isothermal microcalorimetry to study drugs against Schistosoma mansoni. J. Clin. Microbiol. 49:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manneck T, Haggenmuller Y, Keiser J. 2010. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology 137:85–98 [DOI] [PubMed] [Google Scholar]
- 19. Manneck T, Keiser J, Müller J. 2012. Mefloquine interferes with glycolysis in schistosomula of Schistosoma mansoni via inhibition of enolase. Parasitology 139:497–505 [DOI] [PubMed] [Google Scholar]
- 20. Mungthin M, Bray PG, Ridley RG, Ward SA. 1998. Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob. Agents Chemother. 42:2973–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliveira MF, et al. 2004. Inhibition of heme aggregation by chloroquine reduces Schistosoma mansoni infection. J. Infect. Dis. 190:843–852 [DOI] [PubMed] [Google Scholar]
- 22. Oliveira MF, et al. 2000. Haemozoin in Schistosoma mansoni. Mol. Biochem. Parasitol. 111:217–221 [DOI] [PubMed] [Google Scholar]
- 23. Pica-Mattoccia L, Cioli D. 2004. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int. J. Parasitol. 34:527–533 [DOI] [PubMed] [Google Scholar]
- 24. Pullman TN, et al. 1948. The use of SN-10,275 in the prophylaxis and treatment of sporozoite-induced vivax malaria, Chesson strain. J. Clin. Invest. 27:12–16 [PubMed] [Google Scholar]
- 25. Ramirez B, et al. 2007. Schistosomes: challenges in compound screening. Expert Opin. Drug Discov. 2(Suppl 1):S53–S61 [DOI] [PubMed] [Google Scholar]
- 26. Rollinson D. 2009. A wake up call for urinary schistosomiasis: reconciling research effort with public health importance. Parasitology 136:1593–1610 [DOI] [PubMed] [Google Scholar]
- 27. Rothe WE, Jacobus DP. 1968. Laboratory evaluation of the phototoxic potency of quinolinemethanols. J. Med. Chem. 11:366–368 [DOI] [PubMed] [Google Scholar]
- 28. Schmidt LH, Crosby R, Rasco J, Vaughan D. 1978. Antimalarial activities of various 4-pyridinemethanols with special attention to WR-172,435 and WR-180,409. Antimicrob. Agents Chemother. 14:420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt LH, Crosby R, Rasco J, Vaughan D. 1978. Antimalarial activities of various 4-quinolonemethanols with special attention to WR-142,490 (mefloquine). Antimicrob. Agents Chemother. 13:1011–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. 2006. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6:411–425 [DOI] [PubMed] [Google Scholar]
- 31. Timms AR, Bueding E. 1959. Studies of a proteolytic enzyme from Schistosoma mansoni. Br. J. Pharmacol. Chemother. 14:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Utzinger J, Keiser J. 2004. Schistosomiasis and soil-transmitted helminthiasis: common drugs for treatment and control. Expert Opin. Pharmacother. 5:263–285 [DOI] [PubMed] [Google Scholar]
- 33. Van Nassauw L, Toovey S, Van Op den Bosch J, Timmermans JP, Vercruysse J. 2008. Schistosomicidal activity of the antimalarial drug, mefloquine, in Schistosoma mansoni-infected mice. Travel Med. Infect. Dis. 6:253–258 [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization 2011. Schistosomiasis: number of people treated in 2009. Wkly. Epidemiol. Rec. 86:73–80 [PubMed] [Google Scholar]
- 35. World Health Organization 2006. Preventive chemotherapy in human helminthiasis. World Health Organization, Geneva, Switzerland [Google Scholar]
- 36. Xiao SH, et al. 2007. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 51:1440–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiao SH, Mei JY, Jiao PY. 2011. Effect of mefloquine administered orally at single, multiple, or combined with artemether, artesunate, or praziquantel in treatment of mice infected with Schistosoma japonicum. Parasitol. Res. 108:399–406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.