Abstract
This analysis was conducted to determine whether the hepatitis C virus (HCV) viral kinetics (VK) model can predict viral load (VL) decreases for nonnucleoside polymerase inhibitors (NNPolIs) and protease inhibitors (PIs) after 3-day monotherapy studies of patients infected with genotype 1 chronic HCV. This analysis includes data for 8 NNPolIs and 14 PIs, including VL decreases from 3-day monotherapy, total plasma trough concentrations on day 3 (Cmin), replicon data (50% effective concentration [EC50] and protein-shifted EC50 [EC50,PS]), and for PIs, liver-to-plasma ratios (LPRs) measured in vivo in preclinical species. VK model simulations suggested that achieving additional log10 VL decreases greater than one required 10-fold increases in the Cmin. NNPolI and PI data further supported this result. The VK model was successfully used to predict VL decreases in 3-day monotherapy for NNPolIs based on the EC50,PS and the day 3 Cmin. For PIs, however, predicting VL decreases using the same model and the EC50,PS and day 3 Cmin was not successful; a model including LPR values and the EC50 instead of the EC50,PS provided a better prediction of VL decrease. These results are useful for designing phase 1 monotherapy studies for NNPolIs and PIs by clarifying factors driving VL decreases, such as the day 3 Cmin and the EC50,PS for NNPolIs or the EC50 and LPR for PIs. This work provides a framework for understanding the pharmacokinetic/pharmacodynamic relationship for other HCV drug classes. The availability of mechanistic data on processes driving the target concentration, such as liver uptake transporters, should help to improve the predictive power of the approach.
INTRODUCTION
The introduction of direct-acting antiviral (DAA) drugs with activity against the hepatitis C virus (HCV) is expected to significantly reduce the growing impact of this global epidemic, providing better treatment options to many patients and reducing the economic burden of disease. To realize the full potential of these emerging therapies, it is important to have an understanding of those factors which directly impact the clinical efficacy of these drugs. One important factor is the relationship between pharmacokinetics and pharmacodynamics (PK/PD), which aims to provide the link between a dosage regimen and a clinical outcome. For antiviral activity, this link requires knowledge of the concentration of drug at the site of action and the susceptibility of the virus to the particular drug of interest. Understanding the PK/PD of a particular drug or drug class then allows for the optimization of dosing regimens in clinical practice, enhances the ability to select the most appropriate doses during the drug development process, and helps guide the design and selection of future drug candidates.
The FDA recommends that in phase 1b studies in HCV patients, doses be selected so that the total plasma trough concentration (Cmin) is higher than the protein-shifted 50% effective concentration (EC50,PS) by severalfold (12). Although longer monotherapy studies are permitted for inhibitors with high barriers to resistance (e.g., the ELECTRON trial was amended to include one arm exploring 12 weeks of PSI-7977 monotherapy), for compounds with a low barrier to resistance, monotherapy longer than 3 days is not recommended due to the potential emergence of resistant viral strains. Three days should be sufficient for an initial exploration of the potential antiviral activity of a range of doses (12). The work presented here is aimed at understanding how to predict the PK/PD response after 3-day monotherapy to assist in the design of this proof-of-concept study for HCV nonnucleoside polymerase inhibitors (NNPolIs) and protease inhibitors (PIs) in early development.
A viral kinetics (VK) model describing the viral titers in HCV patients and their decline under therapy (e.g., with interferon alpha-2b) has been developed (50). The value of this approach has been demonstrated by many, e.g., in optimizing the design of HCV VK studies (18), in predicting the time required to reach undetectable viral load (VL) based on the observed 14-day VL (22), and in exploring potential mechanisms related to success of treatment and viral breakthrough during therapy (66), to name only a few demonstrated applications. Recently the VK model has been refined using the population approach with extensive clinical data in chronic HCV patients receiving peginterferon alpha-2a with or without ribavirin coadministration, with the added feature of a viral eradication boundary so that the percentage of patients achieving a cure can be simulated (66). The analysis described here was conducted to determine whether this refined HCV VK model could be used to predict VL decreases for NNPolIs and PIs in a 3-day monotherapy study with chronic HCV patients infected with genotype 1 (GT-1) based on in vitro replicon potency data and other preclinical data. Monotherapy and potency data for 8 NNPolIs and for 14 PIs were used in the analysis. As the FDA recommends guiding phase 2 study design using a mechanism-based VK model developed from phase 1 data (12), this analysis leverages that approach with even earlier application.
MATERIALS AND METHODS
NNPolI and PI GT-1 monotherapy study data.
Data for VL decreases in chronic HCV patients infected with GT-1 and treated in monotherapy for at least 3 days were obtained for 8 NNPolIs (Table 1) and for 14 PIs (Table 2). Here, monotherapy refers to DAA administered without interferon or ribavirin; two of the PIs (i.e., ABT-450 and narlaprevir) were administered with ritonavir as a PK enhancer. In vitro potency in the HCV replicon system has been reported for each compound. When available, potency (with 5 to 10% fetal bovine serum) for both GT 1a and GT 1b replicons, as well as protein-shifted potency (with 40 to 50% human serum added), were used in this analysis (Table 3). For PIs, the liver-to-plasma ratio (LPR) measured in vivo in a variety of preclinical species was also used (Table 4).
Table 1.
Summary of NNPolI 3-day monotherapy studies in HCV GT-1 patients
| Compound | Dose (mg) | Regimeni | N/N1a/N1ba | Day 3 Cmin (μg/ml)b | Day 3 VL decrease (log10 IU/mlc) |
Reference(s) | |
|---|---|---|---|---|---|---|---|
| Observed | Predictedd | ||||||
| ABT-072 | 100 | QD | 8/5/3 | 0.050 | −1.14 | −1.1 | 11, 15 |
| 300 | QD | 8h/5/2 | 0.15 | −1.07 | −1.5 | ||
| 600 | QD | 7/6/1 | 0.30 | −1.57 | −1.7 | ||
| ABT-333 | 400 | BID | 8/5/3 | 0.51 | −1.08 | −1.3 | 15, 26, 45 |
| 800 | BID | 8/6/2 | 0.51 | −0.95 | −1.3 | ||
| Filibuvir, PF-00868554 | 100 | BID | 6 | 0.551 | −0.70 | −1.0 | 19 |
| 300 | BID | 6 | 0.994 | —e | —e | ||
| 450 | BID | 6 | 0.868 | —e | —e | ||
| 300 | TID | 6 | 1.13 | −1.8 | −1.3 | ||
| IDX375 | 100 | BID | Not reported | 3.4 | −1.3 | −2.2 | 9, 21 |
| 200 | BID | Not reported | 6.8 | −2.3 | −2.5 | ||
| 400 | BID | Not reported | 14 | −2.7 | −2.8 | ||
| MK-3281 | 800 | BID | 11/7g/4 | 1.63 | −0.80/−2.9f | −1.6/−1.6f | 2 |
| Setrobuvir, ANA598 | 200 | BID | 11/5/6 | 35 | −1.43/−2.55f | −1.9/−3.1f | 35 |
| 400 | BID | 8/3/5 | 75 | −1.79/−2.50f | −2.2/−3.4f | ||
| 800 | BID | 8/4/4 | 190 | −2.50/−3.23f | −2.6/−3.8f | ||
| Tegobuvir, GS-9190 | 40 | BID | 11/8/3 | 0.62 | −1.0 | −1.2 | 1 |
| 120 | BID | 12/10/2 | 2.5 | −1.5 | −1.8 | ||
| VCH-222 | 750 | BID | 5/4/1 | 2.1 | −3.7 | −1.5 | 7 |
N, total number of patients; N1a, and N1b, number of GT-1a and GT-1b patients in a dose group. When only one number was reported, N1a and N1b were not available.
When not reported, Cmin values were calculated as described in Materials and Methods and the Appendix.
Units for tegobuvir were copies/ml.
Predicted using the VK model using the Cmin/EC50,PS ratio to estimate the PD effect.
Because emergence of resistant strains appeared to be affecting the two middle-dose groups, these values were left out of the table and the results plot since they are easy to misinterpret.
VL decreases (observed and predicted) for patients with GT-1a and GT-1b are listed separately (GT-1a/GT-1b) if the information was reported and there were >3 subjects for each.
This group included GT-1a and GT-NT (nontypeable).
One subject was GT-NT.
QD, once daily; BID, twice daily; TID, three times a day.
Table 2.
Summary of PI 3-day monotherapy studies in HCV GT-1 patients
| Compound (notes on study) | Dose (mg) | Regimena | N/N1a/N1bb | Day 3 Cmin (μg/ml)c | Day 3 VL decrease (log10 IU/ml) |
Reference(s) | |
|---|---|---|---|---|---|---|---|
| Observed | Predictedd | ||||||
| ABT-450 with 100 mg ritonavir | 50 | QD | 8/7/1 | 0.005 | −4.07 | −1.9 | 32 |
| 100 | QD | 8/5/3 | 0.017 | −3.89 | −2.5 | ||
| 200 | QD | 8/7/1 | 0.082 | −4.11 | −3.1 | ||
| ACH-1625 (2nd 600-mg group was fed) | 400 | QD | 6 | 0.0759 | −3.2 | −3.8 | 10 |
| 600 | QD | 6 | 0.0311 | −3.3 | −3.4 | ||
| 600 | QD | 6 | 0.0766 | −3.6 | −3.8 | ||
| Asunaprevir, BMS-650032 | 200 | BID | 4/3/1 | 0.027 | −3.2 | −2.8 | 41, 51 |
| 400 | BID | 4/4/0 | 0.054 | −3.4 | −3.0 | ||
| 600 | BID | 4/3/1 | 0.081 | −2.6 | −3.3 | ||
| BI 201335 | 20 | QD | 6/3/3 | 0.063 | −2.9 | −3.0 | 42 |
| 48 | QD | 7/2/5 | 0.19 | −3.5 | −3.5 | ||
| 120 | QD | 7/2/3 | 0.98 | −3.6 | −4.2 | ||
| 240 | QD | 6/3/2 | 3.5 | −4.0 | −4.6 | ||
| Boceprevir, SCH 503034 | 100 | BID | 12 | 0.011 | −0.3 | −0.26 | 65, 74 |
| 200 | BID | 12 | 0.022 | −0.5 | −0.43 | ||
| 400 | BID | 11 | 0.048 | −0.6 | −0.68 | ||
| 400 | TID | 10 | 0.19 | −1.7 | −1.2 | ||
| Danoprevir (1st 4 groups were TN, but 300-mg BID group were NRsf) | 100 | BID | 8/4/4 | 0.00003 | −1.1e | −0.73 | 13 |
| 100 | TID | 8/2/6 | 0.00024 | −2.1e | −1.6 | ||
| 200 | BID | 5/1/4 | 0.00020 | −2.6e | −1.5 | ||
| 200 | TID | 8/4/4 | 0.00101 | −3.9e | −2.2 | ||
| 300 | BID | 8/3/5 | 0.00043 | −2.3e | −1.8 | ||
| GS-9256 (1st 3 groups received solution formulation, last 2 got capsules) | 75 | BID | 12/6/6 | 1.9 | −2.6 | 34 | |
| 200 | BID | 8/6/2 | 21.6 | −2.9 | |||
| 300 | QD | 7/6/1 | 3.2 | −2.6 | |||
| 25 | BID | 9/8/1 | 0.2 | −1.1 | |||
| 75 | BID | 9/8/1 | 1.3 | −2.7 | |||
| GS-9451 | 60 | QD | 8/8/0 | 0.050 | −0.88 | 33 | |
| 200 | QD | 8/8/0 | 0.65 | −3.2 | |||
| 400 | QD | 9/9/0 | 1.86 | −3.6 | |||
| 200 | QD | 8/1/7 | 0.53 | −3.5 | |||
| IDX320 | 50 | QD | 5/0/5 | 0.065 | −2.5 | −3.1 | 59 |
| 100 | QD | 6/0/6 | 0.118 | −3.1 | −3.3 | ||
| 200 | QD | 5/0/5 | 0.174 | −3.1 | −3.5 | ||
| 400 | QD | 6/0/6 | 0.274 | −3.3 | −3.7 | ||
| 200 | BID | 6/0/6 | 0.781 | −3.8 | −4.1 | ||
| MK-5172 | 400 | QD | 5 | 0.0928g | −3.8 | 55 | |
| Narlaprevir, SCH 900518 (400 mg BID dose coadministered with 200 mg ritonavir) | 800 | TID | TN, 10/4/5 | 0.31 | −4.05 | 58 | |
| 400 | BID | TN, 10/4/4 | 1.7 | −4.0 | |||
| 800 | TID | TE, 10/1/5 | 0.31 | −3.95 | |||
| 400 | BID | TE, 10/3/6 | 1.7 | −3.55 | |||
| Telaprevir, VX-950 | 450 | TID | 10 | 0.78 | −3.11e | −1.9 | 61 |
| 750 | TID | 8 | 1.1 | −3.35e | −2.0 | ||
| 1,250 | BID | 8 | 0.68 | −2.89e | −1.8 | ||
| TMC435350 | 200 | QD | 6/4/2 | 4.61 | −3.6 | −4.9 | 43, 60 |
| 25 | QD | 9 | 0.071 | −2.7e | −3.3 | ||
| 75 | QD | 10 | 0.27 | −3.1e | −3.8 | ||
| Vaniprevir, MK-7009 | 25 | BID | 3/1/1 | 0.00056 | −2.1 | −2.3 | 36, 73 |
| 75 | BID | 6/5/0 | 0.0035 | −3.2 | −3.5 | ||
| 250 | BID | 6/5/1 | 0.014 | −3.4 | −3.9 | ||
| 500 | BID | 5/4/0 | 0.025 | −3.6 | −4.3 | ||
| 125 | QD | 5/2/2 | 0.00039 | −3.1 | −2.2 | ||
| 600 | QD | 4/4/0 | 0.0035 | −3.4 | −3.5 | ||
| 700 | BID | 6/5/1 | 0.044 | −4.05 | −4.4 | ||
QD, once daily; BID, twice daily; TID, three times a day.
N, total number of patients in each group being administered drug; N1a and N1b, the number of patients was broken down into the number of GT-1a and GT-1b patients when possible—when N1a and N1b do not add up to the total number, there were subjects where GT-1a or GT-1b could not be determined; when only one number was reported, N1a and N1b were not available; TN, treatment naïve; TE, treatment experienced.
Cmin values were sometimes reported but sometimes calculated as described in Materials and Methods and the Appendix.
Calculated using the most predictive PK/PD index, Cmin × LPR/EC50, as input for the model.
These data were from day 2.
NR, nonresponder.
Table 3.
Summary of selected properties and potency for NNPolIs and PIs
| Target and compound (site for NNPolIs) | MWa | EC50 (nM)b |
EC50,PS (nM)b |
Reference(s) | ||
|---|---|---|---|---|---|---|
| GT-1a | GT-1b | GT-1a | GT-1b | |||
| NNPolIs | ||||||
| ABT-072 (palm 1) | 1.1 | 0.3 | 20 | 2.5 | 71 | |
| ABT-333 (palm 1) | 8 | 2 | 100 | 20 | 71 | |
| Filibuvir (thumb 2) | 503.6 | 52 | 33 | 190 | 120 | 71 |
| IDX375 (palm 1) | 2.3 | 60 | 71 | |||
| MK-3281 (thumb 1) | 475.6 | 40 | 40 | 110 | 110 | 71 |
| Setrobuvir (palm 1) | 52 | 3 | 1,400c | 78c | 71 | |
| Tegobuvir (thumb) | 517.4 | 3.6 | 1.2 | 66d | 22d | 71 |
| 13.8 | 0.8 | 250d | 15d | 64 | ||
| 3.6 | 0.6 | 66d | 11 | 1a | ||
| VCH-222 (thumb 2) | 22 | 11 | 210 | 105 | 71 | |
| PIs | ||||||
| ABT-450 | 0.9 | 0.3 | 19 | 7 | 24 | |
| ACH-1625 | 15 | 11 | 50d | 37 | 20 | |
| Asunaprevir | 748.3 | 4 | 1 | 44 | ||
| BI 201335 | 869.8 | 6.5 | 3.1 | 72 | ||
| Boceprevir | 519.7 | 480 | 1,180 | 40, 72 | ||
| 550 | 520 | 1,350d | 1,280d | |||
| Danoprevir | 731.8 | 1.8e | 24, 63 | |||
| 2.2e | 1.3e | 40e | 18e | |||
| GS-9256 | 957.5 | 74 | 20 | 46 | ||
| GS-9451 | 910.5 | 22 | 7.5 | 8 | ||
| IDX320 | 0.5 | 3.4 | 30 | |||
| MK-5172 | 766.0f | 2 | 9.5 | 54 | ||
| Narlaprevir | 708.0 | 20 | 69 | |||
| Telaprevir | 679.8 | 1,100 | 8,600 | 20, 40, 72 | ||
| 700 | 540 | 4,460d | 3,440d | |||
| 213 | 408 | 1,360d | 3,600 | |||
| TMC435350 | 749.9 | 28.4 | 8.1 | 49d | 14.1 | 38 |
| Vaniprevir | 757.9 | 0.75 | 0.9 | 29 | 21 | 24, 40 |
| 5 | 13 | |||||
For compounds with an unknown molecular weight (MW), the average value of all the others for that target was used in calculations, i.e., 500 g/mol for NNPolIs and 760 g/mol for PIs.
EC50 is from a replicon assay with 5 to 10% fetal bovine serum, and EC50,PS is from a replicon assay with 40 to 50% human serum added, i.e., a protein-shifted assay.
Not measured experimentally. Calculated based on the observed protein-shift with 40% human serum of 26 (68).
Not measured experimentally. Calculated assuming the protein-shift observed for GT-1b.
Data from reference 24 were used because more data were available and GT-1b EC50s were consistent.
The MW of the potassium salt is 805.0.
Table 4.
Liver-to-plasma ratio (LPR) data for PIs in various preclinical species
| PI | LPR(s), species | Reference |
|---|---|---|
| ABT-450 | 6.7, dog | 24 |
| ABT-450 with ritonavir | 7.0, dog | 24 |
| ACH-1625 | >100 i.v. and 505 oral, rata; >55, dog | 67 |
| Asunaprevir | ≥40 across species | 44 |
| BI 201335 | 42, rat | 72 |
| Boceprevir | 30, rat | 5 |
| Danoprevir | 10, rat; 127, monkey | 14 |
| IDX320 | 32, mouse | 17 |
| Telaprevir | 5.7-16, mouse; 35, rat | 53 |
| TMC435350 | 39, rat | 38 |
| Vaniprevir | 330, chimpanzeeb | 40 |
For this analysis, an LPR value of 505 was used. i.v., intravenous.
Estimated using 12-h plasma and liver concentrations read off plots.
The monotherapy data presented here are from a variety of study designs in terms of doses, durations, patient populations, and HCV genotypes. Some of the studies were 3-day monotherapy studies, but more often the studies were longer monotherapy studies or included a period of monotherapy prior to a period of coadministration with pegylated interferon alpha-2a and ribavirin. From each study, only data up to day 3 of monotherapy in patients with GT-1a and GT-1b were included.
HCV viral titers can be measured with several different assays, and there can be differences in the reported VL depending on the assay used (27, 52). But regardless of the assay or whether a VL is reported in IU/ml or copies/ml, a 1-log10 decrease from baseline is a 10-fold decrease in VL. Therefore, the model prediction of VL drop could be compared to the experimental data regardless of the assay used to measure HCV viral titers.
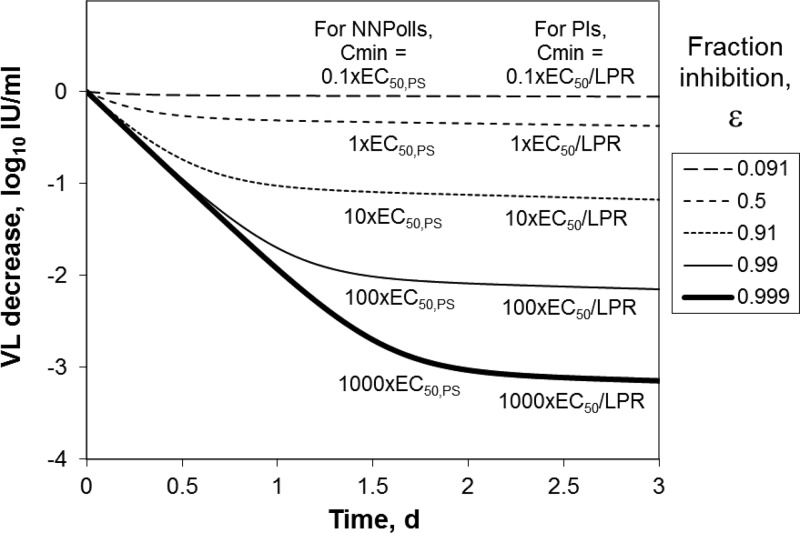
This analysis used VL decrease data on day 3 of monotherapy, with some exceptions. For telaprevir, danoprevir, and the two lower TMC435350 doses, the data were on day 2 of monotherapy because day 3 values were not reported. However, for two of those compounds (i.e., telaprevir and danoprevir), the model underestimated the log10 VL decreases. The prediction would have looked more accurate without including these compounds. Also, the VL decrease is most rapid at times less than 2 days and then slows down, as illustrated here with VK model simulations (Fig. 1) and also demonstrated in monotherapy studies, e.g., for danoprevir (13). Therefore, the 3-day time point would probably not have been that much lower, and it was appropriate to include these compounds in the analysis.
Fig 1.
Simulated VL decrease from baseline as a function of time in monotherapy of patients infected with HCV GT-1 for different levels of inhibition.
In monotherapy study reports, mean VL decrease and PK data were most often reported together. When mean and median VL data were both provided, median data were used in this analysis due to the high variability often observed in VK data. For setrobuvir, danoprevir, GS-9256, and telaprevir, as well as the high dose of TMC435350, median VL decrease data were reported. For VCH-222 and MK-5172, individual values were reported and the median was computed. For filibuvir, tegobuvir, MK-3281, and VCH-222, day 3 VL decrease values were read from graphed data. The procedure for reading data off plots was to capture the image using Snag It version 9.1.3 (TechSmith, Tokyo, Japan) and then extract the values from the images. For all PIs except ABT-450 and MK-5172, VL decreases were read off plots. For GS-9451, median maximum VL decreases were provided from a 3-day monotherapy study, and those values were used instead of reading the day 3 values from the plot because they occurred at or near day 3. In the case of ABT-072 and ABT-333, mean maximum VL decreases were provided from a 3-day monotherapy study, and those values were used instead of reading the day 3 values from plots because the maximum VL decrease appeared to take place on day 3.
PK data were read off plots for tegobuvir, setrobuvir, ABT-072, and VCH-222. For ABT-072 and vaniprevir, PK data in HCV patients were limited or not reported, and the Cmin values used here were from studies in healthy volunteers. For boceprevir, Cmin data were not reported in the monotherapy studies with GT-1; Cmin data from a study on monotherapy in patients with HCV genotypes 2 and 3 (65) were used instead. Additional details on calculations required for specific compounds (e.g., how repeat-dose Cmin was estimated from single-dose PK data when necessary) are located in the Appendix.
For PIs, LPR data were reported for a variety of species (Table 4). The LPR is typically determined in PK studies as either the measured total liver concentration divided by the total plasma concentration at a time point or as the ratio of area under the concentration-time curve (AUC) values for the total concentrations in the two tissues. In this analysis, it was assumed that the LPR was a constant value over time, independent of plasma concentrations (i.e., that uptake by liver transporters was not saturated), and that the human LPR could be approximated using the values determined in preclinical species (i.e., that the species difference in LPRs would be minimal). Although large species differences could exist, the measured LPR values in preclinical species were the only data available for incorporating this important consideration in the model. For danoprevir, calculations including LPR used the value in monkeys (127) instead of the value in rats (10) because the plasma concentrations were about 1 to 2 orders of magnitude lower in the monkey study and so, in the rat study, liver uptake transporters might have been saturated. For telaprevir, the average LPR between mice and rats (23) was used. For ACH-1625, the LPR in rats (505) was used because for dogs, it was reported as >55. For asunaprevir, a value of 40 was used since it was reported as ≥40 across species.
Replicon data.
When VL decreases were reported for GT-1 but not for GT-1a and GT-1b separately, an EC50 reflective of the proportion of each GT in a given dose group was used in the calculations, as follows:
| (1) |
where N1a and N1b are the number of GT-1a and GT-1b patients in a dose group, respectively, and EC50,1a and EC50,1b are the replicon EC50s for GT-1a and GT-1b, respectively. A similar method was used to estimate appropriate values for the EC50,PS. When N1a and N1b were not reported (e.g., as was the case for telaprevir), the average of the GT-1a and GT-1b EC50 (or EC50,PS) was used. If replicon data for only one GT were available, then that EC50 was used in calculations. If multiple values were available, a mean value was used unless otherwise noted. It should be noted that the replicon data were generated in different laboratories and may reflect slight variations in each assay (e.g., duration of assay).
Viral kinetics model.
The VK model of Snoeck et al. (66) was used for this analysis. Briefly, the model describes the infection of target cells (T, hepatocytes/ml) by HCV virions (V, virions/ml) to form infected cells (I, hepatocytes/ml), as follows:
| (2) |
| (3) |
| (4) |
where t is time, s is the hepatocyte production rate, r is the hepatocyte proliferation rate constant in both target and infected cells, Tmax is the total number of hepatocytes per ml, d is the hepatocyte death rate constant, β is the second-order rate constant for target cell infection, δ is the infected hepatocyte death rate constant, p is the virion production rate constant, c is the virion elimination rate constant, and ε is the inhibition caused by the NNPolI or PI described using a maximum effect (Emax) model assuming a maximum inhibition of 100%. The original model contained both infectious and noninfectious virions due to the effect of ribavirin, but equations 2 and 4 were written to describe VK during monotherapy, and therefore, only infectious virions were included. In this analysis, it was assumed that the inhibition from NNPolIs and PIs reduced viral production, i.e., both were incorporated in the model the same way. For NNPolIs and for PIs, the following method of parameterizing the Emax model was explored to determine whether it could predict VL decreases based on the inhibition at the day 3 total plasma trough concentration, Cmin:
| (5) |
Since the EC50,PS is from an assay with protein present, it was reasonable to compare total Cmin to this value for the antiviral activity prediction. For PIs, three additional ways to parameterize the Emax model were explored:
| (6) |
| (7) |
| (8) |
Regardless of whether EC50 or EC50,PS was used as the model input, Cmin in these equations is the total trough concentration on day 3. Equations 7 and 8 are based on the assumption that the effect of liver uptake transporters, approximated using the LPR measured in preclinical species, increases the free-drug concentration in the liver and thereby increases target inhibition. Additionally, equations 5 through 8 were written assuming that Cmin was the PK parameter responsible for antiviral activity, which was assumed for reasons presented in Discussion. Initially, a Hill coefficient (i.e., a sigmoidal Emax model) was explored as one possible way to improve the prediction of the day 3 VL decrease. However, it added another model parameter and did not improve the predictions. Therefore, no Hill coefficient was included in this analysis.
This analysis did not consider interindividual variability since individual data were unavailable. Instead simulations used the typical parameter values reported by Snoeck et al. (66), which were derived using a population modeling approach with an extensive data set from one phase 2 study and four phase 3 studies with chronic HCV patients who received treatment with peginterferon alpha-2a with or without ribavirin. The model development included patients with GT-1 and other genotypes, but we used model parameters for GT-1. The model was implemented in Berkeley Madonna version 8.3.11 (University of California at Berkeley, Berkeley, CA), a software package for solving systems of ordinary differential equations. Plots of the VL decrease from baseline on day 3 as a function of PK/PD indices were simulated in Berkeley Madonna using a parameter plot, which is a convenient way to tabulate the final value of multiple simulations for a parameter value changing over a specified range.
RESULTS
VK model simulations.
Simulated log10 VL decreases from baseline as a function of time from 3 days of monotherapy in chronic HCV GT-1-infected patients are shown in Fig. 1. These results indicate that for a compound to achieve a 1-log10 decrease in VL over 3 days, the inhibition of replication, ε, must be maintained at more than 90%. Figure 1 also lists the Cmin relative to the potency required for NNPolIs and PIs, as discussed further below. For both NNPolIs and protease inhibitors, each 10-fold increase in Cmin is expected to result in an additional VL decrease of about 1 log10.
In reports of monotherapy results, the authors have sometimes concluded that saturation of response was observed, e.g., for PI IDX320 (59). Therefore, this result, that additional 10-fold increases in Cmin will result in additional log10 VL decreases, requires more investigation. Next, observed clinical data were used to assess this prediction.
PK/PD relationship for NNPolIs.
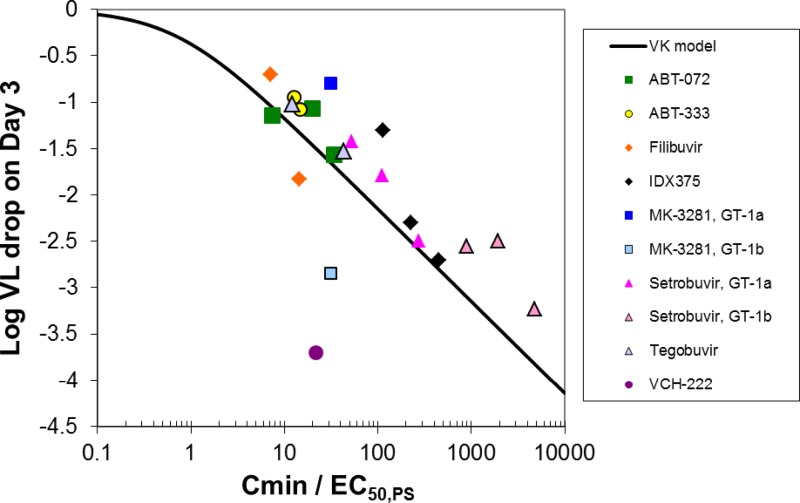
The NNPolIs included in this analysis, for which selected clinical data are summarized in Tables 1 and 5, achieved decreases of up to about 3.7-log10 IU/ml in VL from baseline in 3 days of monotherapy (i.e., for VCH-222), but for half the NNPolIs included here, the VL decreases were in the range of 1 to 2 log10 IU/ml. At the high dose in monotherapy studies, the day 3 total Cmin/EC50,PS ranged from 14 for filibuvir (VL decrease from baseline of 1.8 log10 IU/ml) to 4,800 for setrobuvir in GT-1b patients (VL decrease from baseline of 3.2 log10 IU/ml) (Table 5). Predicted log10 VL decreases from 3 days of NNPolI monotherapy in patients with GT-1 as a function of day 3 Cmin/EC50,PS are compared to observed data in Fig. 2. Plotting the data in this normalized way allows the comparison of simulation results with data for multiple NNPolIs. Although most individual studies did not cover a wide enough range of Cmin to fully characterize the dose-response relationship, by combining data for multiple compounds, the relationship is clarified.
Table 5.
Summary of selected NNPolI and PI results
| Compound | HD (mg) and regimena | HD day 3 log10 VL decrease | HD day 3 Cmin/EC50 | HD day 3 Cmin/EC50,PS | HD day 3 Cmin × LPR/EC50 | HD day 3 Cmin × LPR/EC50,PS | Cmin rangeb |
|---|---|---|---|---|---|---|---|
| NNPolIs | |||||||
| ABT-072 | 600 QD | −1.6 | 610 | 34 | 6.0-fold | ||
| ABT-333c | 400–800 BIDc | −1.1 | 180 | 15 | NAc | ||
| Filibuvir | 300 TID | −1.8 | 53 | 14 | 2.1-fold | ||
| IDX375 | 400 BID | −2.7 | 12,000 | 450 | 4.0-fold | ||
| MK-3281 | 800 BID | −0.80/−2.9d | 86/86d | 31/31d | NA | ||
| Setrobuvir | 800 BID | −2.5/−3.2d | 7,100/120,000d | 270/4,800d | 5.3-fold | ||
| Tegobuvir | 120 BID | −1.5 | 800 | 44 | 4.0-fold | ||
| VCH-222 | 750 BID | −3.7 | 210 | 22 | NA | ||
| PIs | |||||||
| ABT-450 with ritonavire | 50–200/100 QD | −3.9 to −4.1 | 7.9–130 | 0.37–6.1 | 55–910 | 2.6–43 | 16-fold |
| ACH-1625 | 600 QD fed | −3.6 | 7.7 | 2.3 | 3,900 | 1,200 | 2.5-fold |
| Asunaprevir | 600 BID | −3.4f | 33 | 1,300 | 1.5-fold | ||
| BI 201335 | 240 QD | −4.0 | 770 | 32,000 | 55-fold | ||
| Boceprevir | 400 TID | −1.7 | 0.35 | 0.071 | 10 | 2.1 | 17-fold |
| Danoprevirg | 200 TID | −3.9 | 0.79 | 0.048 | 100 | 6.0 | 34-fold |
| GS-9256 | 200 BID solution | −2.9 | 370 | 110-fold | |||
| GS-9451 | 400/200 QDd | −3.6/−3.5d | 93/78d | 11-fold | |||
| IDX320 | 200 BID | −3.8 | 300 | 9,700 | 12-fold | ||
| MK-5172 | 400 QD | −3.8 | 61 | 13 | NA | ||
| Narlaprevirh | 800 TID | −4.1 | 22 | 86 | NA | ||
| Narlaprevir with ritonavirh | 400/200 BID | −4.0 | 120 | 480 | NA | ||
| Telaprevirg | 750 TID | −3.4 | 2.7 | 0.33 | 63 | 7.6 | 1.6-fold |
| TMC435350 | 200 QD | −3.6 | 280 | 160 | 11,000 | 6,400 | 65-fold |
| Vaniprevir | 700 BID | −4.1 | 52 | 2.1 | 17,000 | 710 | >33-fold |
HD, high dose; QD, once daily; BID, twice daily; TID, three times a day. The high dose is considered to be the dose resulting in the highest Cmin; e.g., 200 mg TID would be considered a higher dose than 300 mg BID if it resulted in a higher Cmin.
Cmin range, reported as fold difference, is the HD Cmin divided by the Cmin at the lowest dose. NA, not applicable, is reported for studies that only included one dose level.
The Cmin and log10 VL decreases were similar for the 400 and 800 mg BID dose groups. Dose-limited absorption led to similar Cmin values at both doses. Here, the data are for the 400-mg BID dose group, which had a slightly higher VL drop.
Data or calculation for GT-1a/GT-1b, which were treated separately.
The VL decrease was independent of dose; the range reported is for the low to the high dose.
VL decrease is for second highest dose; HD had the lowest VL decrease, which seems spurious.
Most data are from day 3; for telaprevir and danoprevir, data are from day 2.
Data are for treatment-naïve instead of treatment-experienced groups because Cmin values and VL decreases for treatment-naïve patients were higher. The group with ritonavir was reported separately since it might affect the PK/PD relationship.
Fig 2.
Predicted (line) and observed (symbols) VL decrease from baseline on day 3 of NNPolI monotherapy (log10 IU/ml) as a function of Cmin/EC50,PS.
In general, the simulation results are consistent with the observed data for NNPolIs, with a tendency to overestimate VL decreases. The overestimation could be due to the emergence of resistant virus, which can be selected very readily upon treatment due to their presence at very low levels in the patient's viral population prior to the initiation of treatment or to de novo generation due to the error-prone nature of the HCV polymerase (28, 37). This issue was observed in the filibuvir monotherapy study, where emergence of resistant strains seemed apparent before 3 days in both the 300- and 450-mg twice-daily (BID) dose groups (19). Although there is a slight tendency to overestimate VL decreases, the model predicts VL decreases within about 0.5-log10 units with good confidence.
The NNPolIs in this analysis included four inhibitors of the palm 1 site and four for various thumb sites (Table 3). The model may predict VL decreases better for the palm 1 site than the thumb sites. Two of the less-good predictions are for the VCH-222 (thumb 2) and MK-3281 (thumb 1). But with only 8 compounds included in the analysis, the accuracy of the prediction for each site cannot be assessed with confidence.
For VCH-222, the log10 VL decrease was underestimated by about 2 log10 intervals. One possible explanation for this poor prediction is that VCH-222 could be a substrate for liver uptake transporters, as suggested by accumulation in human hepatocytes as observed with B-CLEAR data (4). The only LPR value for VCH-222 reported to date was low (a value of 5 observed in rats), which could indicate that the rat is not a good model for human hepatic uptake of VCH-222.
To see a wide range of PK/PD response in terms of VL decreases ranging from 1 to >3, the Cmin range in a study should cover orders of magnitude (Fig. 1 and 2). For one NNPolI, BI 207127, such a Cmin range was covered in a monotherapy study. BI 207127 could not be used in this analysis because EC50,PS data were not available. But in a BI 207127 monotherapy study in which doses ranged from 100 mg three times a day (TID) to 1,200 mg TID, the difference in Cmin of the high dose relative to the low dose was about 30-fold, and the median VL decrease was 0.2 log10 IU/ml for the low dose and 3.6 log10 IU/ml for the high dose (31). In compounds included in the current study, the largest Cmin range covered was for setrobuvir, which had a 3-day monotherapy study including doses of 200, 400, and 800 mg BID, with a difference in Cmin for the high dose relative to the low dose of about 5.3-fold. However, the range of Cmin/EC50,PS ratios included in this study was larger than might be expected based on the 5.3-fold difference in Cmin, due to the ability to assess GT-1a and GT-1b VL decrease data separately. Most of the monotherapy studies for NNPolIs did not cover a wide enough range of exposures to fully characterize the dose-response relationship.
PK/PD relationship for PIs.
A summary of monotherapy studies on PIs in chronic HCV GT-1 patients, including the average log10 VL decrease for each dose group, is listed in Table 2. Log10 VL decreases of about 4 log10 IU/ml on day 3 of monotherapy were achieved by ABT-450 coadministered with ritonavir, BI 201335, danoprevir, narlaprevir, and vaniprevir. The ratios of Cmin relative to the EC50 or the EC50,PS for the high-dose group, even for compounds with a similar VL decrease, varied widely (Table 5). Unlike NNPolIs, wide ranges of Cmin values were included in the PI monotherapy studies (e.g., up to a 110-fold range of Cmin from the low dose relative to the high dose for GS-9256).
LPR values were reported for various PIs in the mouse, rat, dog, monkey, and/or chimpanzee. The lowest LPR reported was about 7 for ABT-450 with and without ritonavir coadministration in dogs (Table 4). This low value of 7 suggests that for ABT-450 in dogs, liver uptake transporters may not have a large effect on liver concentrations, although LPR data should be interpreted with caution, as discussed further in Discussion. Given the good VL decreases achieved by this compound, it is possible that the dog model was not representative of humans in this instance. The highest LPR was for ACH-1625, with a value of 505 determined in rats.
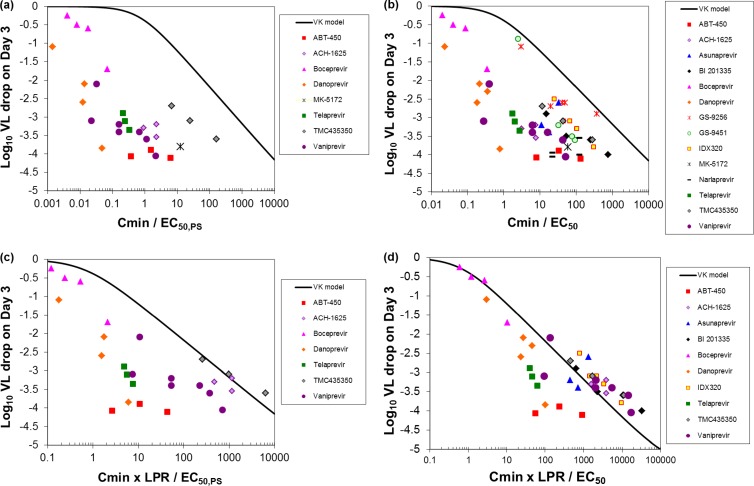
The purpose of this analysis was to determine whether an in vitro measure of potency incorporated in the VK model could be used to predict VL decreases. Four PK/PD indices were examined in this context. First, in an approach similar to that used for NNPolIs, Cmin/EC50,PS was used as model input and the VK model prediction was compared to observed data (Fig. 3a). However, this PK/PD index significantly underestimated VL decreases for PIs. The use of the Cmin/EC50 ratio also resulted in underestimation of VL decreases (Fig. 3b).
Fig 3.
Predicted (curve) and observed (symbols) VL decrease from baseline on day 3 of PI monotherapy (log10 IU/ml) as a function of (a) Cmin/EC50,PS, (b) Cmin/EC50, (c) Cmin × LPR/EC50,PS, and (d) Cmin × LPR/EC50. ABT-450 and two of the four narlaprevir doses were coadministered with ritonavir.
PIs often have high LPRs, suggesting that they are often good substrates for liver uptake transporters. These transporters can cause increased liver concentrations. Compounds that are good organic anion-transporting polypeptide (OATP) substrates with low to moderate permeability can accumulate in the liver, as reflected by a high LPR, thereby increasing the free concentration of PI at the target and improving antiviral activity (47). Therefore, PK/PD indices that included the LPR were also examined. The PK/PD index Cmin × LPR/EC50,PS still underestimated the log10 VL decrease (Fig. 3c). However, the PK/PD index Cmin × LPR/EC50 resulted in reasonably accurate predictions of VL decrease (Fig. 3d), predicting VL decreases on day 3 of monotherapy within about 1 log10 in most cases.
In general, increasing Cmin values led to increasing log10 VL decreases (Fig. 3). To achieve one additional log10 VL decrease, an additional 10-fold increase in Cmin was generally required, assuming that the LPR was independent of plasma concentrations. The exception was ABT-450 coadministered with ritonavir, for which Cmin covered a 16-fold range but which had VL decreases independent of Cmin.
The LPR was found to be important to improve the prediction of VL decreases in this analysis. For the two PK/PD indices that did not include the LPR, Cmin/EC50,PS and Cmin/EC50, the values of each index related to high VL decreases spanned about four orders of magnitude (Fig. 3). For the PK/PD index Cmin × LPR/EC50, there is still a lot of scatter at the highest VL decreases. However, the data are more consistent with the model. Although the EC50,PS relative to the total Cmin was initially expected to be a more reasonable predictor for PK/PD due to the high protein concentration in the assay, the EC50 was the better predictor in the case of the PIs. The LPR data are from a variety of species and study designs. The inconsistent LPR data and the uncertainty as to how the LPR in preclinical species translates to humans could be important reasons for discrepancies remaining between the model and observed data.
DISCUSSION
The current analysis was conducted with the goal of identifying the most important factors that determine the clinical pharmacodynamics of HCV PIs and NNPolIs. A clear relationship between Cmin, potency, and extent of VL decrease was characterized across drugs of a common class, and when these factors were incorporated into a model of HCV viral kinetics, the model provided an excellent prediction of clinically observed data across a wide range of studies. Interestingly, for HCV PIs and NNPolIs, different factors related to potency determined the pharmacodynamics in this analysis.
In HIV, the PK/PD of both protease inhibitors and nonnucleoside reverse transcriptase inhibitors are generally similar and, reportedly, often a function of both the plasma Cmin concentration (although the most appropriate measure of exposure for PD assessment is the subject of some debate) and the EC50 of the HIV (29, 49). Similarly, for both HCV PIs and NNPolIs, the combination of Cmin and potency appear to be important factors driving antiviral activity. However, the PK/PD of HCV compounds appears to be comparatively more complex and must additionally take into account the concentration of drug at the site of action in the liver. For the majority of HCV NNPolIs, active liver uptake does not appear to be an important determinant of liver concentrations, and EC50,PS was adequate to account for unbound drug getting to the site of action. However, for HCV PIs, active liver uptake appears to be common, and thus, the LPR for each compound must be considered a critical factor in predicting its antiviral activity.
Our results suggest that, in general, PIs achieve better clinical potency than the NNPolIs available to date, at least in part due to higher LPRs. As demonstrated by the VK model predictions, NNPolIs with greater potency and/or improved PK should be able to achieve reductions in VL after 3-day monotherapy similar to those observed with PIs. While the higher LPRs contribute to clinical potency differences, we cannot rule out the possibility of fundamental differences in the two targets which may also affect clinical potency. For example, ABT-072 (NNPolI) and ABT-450 (PI) have very similar EC50 and EC50,PS values for both HCV GT-1a and -1b (Table 3). ABT-450 reportedly has an LPR of approximately 7 in dogs with or without ritonavir coadministration. However, ABT-450 coadministered with ritonavir achieved a log10 VL decrease from baseline of about −4.1 with a plasma Cmin of 0.005 μg/ml at day 3, while for ABT-072, a 60-fold higher Cmin (0.3 μg/ml) resulted in a log10 VL reduction from baseline of about −1.6. As mentioned previously, it is possible that the dog model does not represent the human response in terms of LPR for ABT-450, but target differences also cannot be ruled out.
For PIs, clinical outcomes of patients undergoing treatment in combination with pegylated interferon-ribavirin can differ between HCV GT-1a and -1b, e.g., for boceprevir (3) and danoprevir (62). Differences observed with combination therapy outcomes have been primarily due to differences in the development of resistance between GT-1a and GT-1b. However, in the setting of 3-day monotherapy, there do not appear to be any relevant differences in potency by GT after accounting for the differences in EC50. Although expansion of preexisting mutants can take place early on, at least for some compounds, it seems unlikely that the number of resistant mutations is large enough to contribute such a difference to the measurement of total VL and account for the outlier compounds in the model. In other words, the same VL reduction in 3 days of monotherapy would be expected for a GT-1a or -1b virus with the same EC50. Therefore, the development of more potent PIs against GT-1a may be less important than the development of newer drugs or drug combinations with a higher barrier to resistance.
Limitations of the analysis.
One limitation of the current analysis is in understanding the target concentration for PIs. Here, the LPR was used to incorporate the effect of liver uptake transporters in the model. However, the LPR can also mislead. It is a gross estimate of relative amounts of total compound in the liver and plasma, and determination of liver concentrations does not differentiate between the concentration in the tissue versus the compound that has been excreted into the bile ducts. Some NNPolIs have high LPR values in preclinical species. For example, IDX375 has LPRs greater than about 100 in rats and mice (16) but did not seem to have increased antiviral activity, which suggests that liver uptake transporters are not responsible for the high LPR values. Also, species differences in LPR values can be difficult to interpret and to predict. Finally, the model assumes that the LPR remains relatively constant, but it is possible that the LPR could be a function of time that changes with drug concentration if hepatic transporters become saturated. A more rational, useful approach to using the LPR would be scaling in vitro hepatocyte uptake data for identified uptake transporter substrates, as illustrated in reference 56, and using the data for physiologically based pharmacokinetic (PBPK) model development to understand the free concentration in the liver.
The PK/PD approach for this analysis was relatively simple, focusing on Cmin as the concentration driving VL reduction via an Emax-type model. The Cmin concentration was utilized as it appears for both classes of compounds to be the pharmacodynamically linked variable and a primary determinant of clinical antiviral activity. However, a more sophisticated approach utilizing models that capture the full time course of both PK and PD may have the potential to further improve the observed relationships identified in this analysis, including the ability to predict the antiviral activity of new compounds. In several cases, the full PK profile was tested as an alternative to Cmin, but this did not significantly change the results for the compounds tested. However, there could be cases where inclusion of the entire PK profile would improve the model. In addition, there could be value in additional exploration of PK/PD indices or other modifications, such as the addition of a Hill coefficient, which could have the potential to further improve the model.
PK differences between HCV patients and healthy volunteers.
In HCV patients, as fibrosis progresses into cirrhosis, physiological changes occur that can affect PK. Factors affecting PK in patients with liver disease include differences in cardiac output, hepatic blood flow, number of functional hepatocytes, expression of P450s, albumin level, and renal function, among others (23). Decreased expression of several hepatic P450s and transporters is correlated with the progression of fibrosis (48). For several HCV compounds, significant differences in PK have been observed between healthy volunteers and HCV patients, e.g., for PI TMC435350 (60) and NNPolI IDX375 (9).
For ABT-072 and vaniprevir, PK data in HCV patients were either not available or insufficient to be used in this analysis and the Cmin values were based on PK in healthy volunteers. For ABT-072, the PK in HCV patients from 2 days of dosing appeared similar to the PK in healthy volunteers from 1 day of dosing, i.e., the half-life (T1/2); in healthy volunteers, it was 7.63 ± 2.32 (mean ± standard deviation; n = 8), and in HCV patients, it was 8.97 ± 0.20 (n = 4) (25). If vaniprevir exposures were higher in HCV patients than healthy volunteers, the prediction of VL decrease would have been less accurate, but the observed trend (an order of magnitude increase in Cmin resulting in an additional log10 IU/ml decrease in VL) should still be similar.
Considerations in designing a proof-of-concept monotherapy study.
The analysis presented here should be considered in the development of a DAA candidate. These results are useful for designing phase 1 monotherapy studies in HCV patients for NNPolIs and PIs and for understanding factors driving VL decreases. The FDA's draft guidance document on developing DAAs for treatment of HCV says that an initial proof-of-concept (phase 1b) monotherapy trial demonstrating initial antiviral activity of the DAA in HCV-infected patients should be conducted as part of an early clinical development program for a DAA candidate. The results from this trial would guide further development, including dose selection, in subsequent phase 2 dose and duration optimization trials in which patients are treated for longer durations (potentially up to 48 weeks) with the DAA as part of a combination regimen (12). Thus, the doses selected for the initial monotherapy trial are critical for informing future longer-term trials. The ability to predict clinical observations enables the identification of clinically meaningful doses for evaluation in the proof-of-concept monotherapy trial and provides a framework for the overall early development of the NNPolI or PI. Thus, the identification of factors that determine the clinical pharmacodynamics of HCV NNPolIs provides valuable information in guiding a 3-day proof-of-concept monotherapy study in HCV patients.
In planning the strategy for drug development, the value of a 1-day monotherapy study as an alternative to the 3-day or longer monotherapy study might be considered for the first study in HCV patients. The generic VK simulation is useful for understanding the utility of a 1-day monotherapy study. As shown in Fig. 1, if an NNPolI has Cmin > 100 × EC50,PS or a PI has Cmin > 100 × EC50/LPR, the VL decrease will be about 2 log10 independent of potency, as illustrated by the overlap of curves for times up to 1 day. If a compound has good potency, at least a 1-log10 VL reduction should be observed. A 1-day monotherapy study can be used to determine whether a compound will have any antiviral activity in HCV GT-1 patients. However, a 1-day study is not expected to differentiate between the activities of potent compounds or to determine whether a compound will have sufficient VL decreases compared to those for drugs on the market or in late-stage development.
Interestingly, although the 3-day proof-of-concept monotherapy study has important implications for the development of a DAA candidate and is used to guide further development of the DAA candidate, including larger phase 2 efficacy and safety trials, there is limited information within the FDA guidance document on this key initial trial, particularly with regard to dose selection. The guidance recommends only that the doses selected for the monotherapy trial should be predicted to provide plasma drug exposures expected to exceed, by severalfold, the protein binding-adjusted cell culture EC50 value of the agent and that subsequently these data be used to develop a mechanism-based VK model to guide phase 2 study design (12). For PIs, this strategy may often result in competitive VL decreases. However, for NNPolIs, higher exposures are probably needed to achieve high VL decreases in monotherapy. Although the value of the NNPolI might be mainly due to its antiviral activity on PI-resistant strains, a good VL decrease is still more desirable than a poor VL decrease. The results here suggest that for an NNPolI to achieve a 3-log10 VL decrease, Cmin > 700 × EC50,PS must be achieved. Therefore, good biopharmaceutical properties that allow high exposures in patients are desirable. Even for PIs, achieving high exposures helps to achieve high VL decreases, although there is more variability in how high the exposures have to be. For example, danoprevir resulted in a decrease of about 3.9 log10 IU/ml in VL within 3 days of monotherapy with a Cmin of 0.001 μg/ml, while TMC435350 achieved a decrease in VL of 3.6 log10 IU/ml with a Cmin of 4.8 μg/ml (Table 2).
The novel PK/PD approach presented here for the prediction of declines in VL incorporates the FDA-recommended consideration of the VK model but in an earlier application, and it leverages the immense available in vitro, preclinical, and clinical data for a range of NNPolIs and PIs in development to identify relationships between in vitro potency values, liver-to-plasma distributions (for PIs), and clinical PK/PD data to identify factors involved in clinical pharmacodynamics of NNPolIs and PIs and to characterize the VK-PK/PD relationship for two classes of DAAs. As practicality may limit the range of doses to be tested in the phase 1b trial, combining data from multiple compounds allows a broader range of exposures relative to potency to be evaluated and thus enables efficient identification of clinically meaningful doses for evaluation. Indeed, many individual studies did not cover a wide enough range of Cmin to fully characterize the dose-response relationship (Table 5); however, combining data for multiple competitor compounds elucidates the relationship.
For a given dose and the resulting Cmin, the predicted VL decrease can be expected to be within about 0.5 to 1 log10 of the observed value (Fig. 2, Fig. 3). This approach therefore enables the prediction of efficacious exposures and, through further physiologically based PK-modeling techniques, aids in the identification of appropriate doses and dose ranges to be evaluated in the clinical trial. Though this VK-PK/PD approach has demonstrated excellent prediction of clinically observed data across a wide range of studies, one must consider the uncertainty inherent in the predictions. It is noted that for PIs, there are several outliers that perform significantly better than the prediction, as the observed 4-log VL decrease values of Cmin × LPR/EC50 span three orders of magnitude (Fig. 3d). It appears that the PI model is particularly good for identifying less active compounds but could have the potential, if too rigorously applied, to improperly characterize some of the most potent compounds (e.g., danoprevir and ABT-450) as poor candidates. For these compounds, it is possible that the LPR is greater than the estimate, or there could be additional factors contributing to the antiviral activity that are not captured in the model.
The results here are specifically for NNPolIs and PIs but provide a framework that is generally applicable for anti-HCV drugs. Compounds that are not liver uptake transporter substrates may be expected to behave like NNPolIs, while compounds that are liver uptake transporter substrates may be expected to behave like PIs. Assessing model predictions against available clinical data from monotherapy studies for DAAs in clinical development for a given target should be done to gain confidence in this approach before applying it to new targets besides NNPolIs and PIs.
Conclusions.
The results presented here indicate a difference in PK/PD behavior for NNPolIs and PIs. For NNPolIs, using Cmin/EC50,PS as input to the VK model results in good prediction of VL decrease. For PIs, Cmin × LPR/EC50 is the PK/PD index that results in good prediction of VL decreases.
ACKNOWLEDGMENTS
We thank Pascal Chanu for the helpful discussions and for his effort in translating the VK model into a Berkeley Madonna model. We thank Irene Phillip for her help preparing the manuscript and Isa Najera, Xiao Tong, and Annabelle Lemenuel for their valuable input and for review of the manuscript. We thank Lisa Benincosa for her support and helpful suggestions. We also thank Jules Levin for making available online the HCV NNPolI and PI data presented at meetings.
All authors were employees at Hoffmann-La Roche Inc. at the time the work was performed.
APPENDIX
For one-compartment PK, the steady-state trough concentration, Cmin,SS, after n doses, Cmin,n, can be estimated as follows (70):
| (A1) |
where D is the dose, V is the volume of distribution, τ is the dosing interval (e.g., 12 h for BID administration), and T1/2 is the half-life. When the concentration at the end of the first dosing interval, Cmin,1, and T1/2 are available from a single-dose PK study, Cmin,n can be approximated using the following equation derived using equation A1:
| (A2) |
The following simplified equation can be used to estimate Cmin,SS if steady state was achieved by day 3:
| (A3) |
Assuming that it takes about 4 to 5 T1/2 intervals to achieve steady state, if T1/2 is less than about 14 to 18 h, equation A3 can be applied.
Monotherapy studies were often conducted for more than 3 days, and the Cmin value on the last day of the study was reported (e.g., for danoprevir). In such cases, if T1/2 was less than 18 h, it was assumed that steady state had been achieved by day 3 and that the Cmin on day 3 was the same value as the Cmin at the end of the study.
For some compounds, additional PK calculations were needed, as follows.
For ABT-072, the mean dose-normalized Cmin for once-daily administration for the 400- and 800-mg doses was used to estimate the day 3 Cmin for different doses, assuming dose-proportional PK, which had been observed for single doses up to 1,200 mg.
For ABT-333, the Cmin data were from a different study than the VL decrease data, which did not report PK. In the study that reported PK (26), ABT-333 was administered in 2 days of monotherapy followed by 26 days of coadministration with pegylated interferon and ribavirin. The Cmin values were similar on days 2, 3, 4, and 5 for both the 300- and 600-mg BID doses. It was assumed that due to dose-limited absorption, the Cmin for the 600-mg BID dose could be used to estimate the Cmin values for the 400- and 800-mg BID doses in the other study.
For IDX375, Cmin values were not reported (21); the PK for 200-mg BID dosing (9) were used to estimate Cmin after repeat dosing using equation A2 and assuming dose-proportional PK, which had been observed. This method was used because the T1/2 for IDX375 in HCV patients was 36.4 h.
For tegobuvir, single-dose PK data were reported (1). The Cmin on day 3 was calculated with equation A3.
For boceprevir, Cmin,SS values reported in a GT-2 and -3 study for doses of 200 mg BID, 400 mg BID, and 400 mg TID (65) were used to estimate Cmin values for the GT-1 studies, since the Cmin values were not reported in the GT-1 studies. The Cmin,SS was estimated as the average of the reported Cmin values on day 11, 12, 13, and 14. It was assumed that the day 3 Cmin would be about the same as the Cmin,SS because the T1/2 was about 8 h. The 200-mg TID Cmin was estimated from the 400-mg TID value, and the 100-mg BID Cmin was estimated from the 200-mg BID value, assuming dose-proportional PK.
For asunaprevir, only single-dose PK data were plotted in the monotherapy report (51). The Cmin data used in this analysis were steady-state values from a combination study with a 600-mg BID dose; the mean from two groups, asunaprevir with BMS-790052 or BMS-790052, pegylated interferon, and ribavirin, was used (41). To estimate Cmin for the other dose levels, linear PK were assumed. Although T1/2 was not reported, it was assumed that Cmin,SS could be used because of the claim that the compound PK supported BID administration, which suggests that T1/2 was less than 18 h.
For narlaprevir, the Cmin data used in this analysis were steady-state values from a combination study with peginterferon alpha-2b (58). It was assumed that Cmin,SS could be used because for the BID regimen (i.e., administered with ritonavir), the T1/2 was about 16 h, and for the TID regimen (i.e., no ritonavir), the T1/2 was about 5 h.
For BI 201335, the Cmin,SS on day 28 with coadministration with pegylated interferon alpha-ribavirin was reported. The day 3 Cmin was estimated by combining equations A3 and A2. The T1/2 of BI 201335 was estimated as 27.6 h, the average value for the four dose groups. This method might overestimate Cmin somewhat since the PK were greater than dose proportional.
For telaprevir, it was assumed that the Cmin on day 3 would be similar to the reported Cmin,SS since the T1/2 was about 4 h at doses of 450 mg and higher (6).
For TMC435350, the day 3 Cmin was reported for the study that included the 200-mg dose (60). Only Cmin,SS was reported for the study that included 25- and 75-mg doses (43). Nonlinear clearance apparently affected the 200-mg dose, which had about 20-fold higher AUC values than the 75-mg dose. Therefore, although the T1/2 for the 200-mg dose was about 41 h, it was assumed that the T1/2 intervals for the 25- and 75-mg doses were more like those of healthy volunteers (i.e., about 10 h) and that, for those two doses, the day 3 Cmin could be approximated as the Cmin,SS.
For vaniprevir, PK was reported to be nonlinear, with slightly greater than dose-proportional increases in PK (i.e., AUC, Cmax, and concentration in plasma at 12 h) with increasing doses. The exposures achieved in the monotherapy study were not reported. In the PK study with healthy volunteers, the doses and regimens were not the same as included in the monotherapy study. The day 3 Cmin was based on the Cmin of the closest dose, assuming dose-proportional kinetics, and then adjusted as necessary to reflect a Cmin,SS, which was assumed to represent the day 3 Cmin because the T1/2 was about 4 to 5 h following multiple oral doses (73). For example, the 25-mg BID day 3 Cmin was estimated based on the 40-mg PK, adjusting for the different dose by assuming dose-proportional PK between these similar doses and the effect of repeat exposure using equation A3. At the 40-mg dose, only the Cp at 6 h was reported, presumably from detection limit problems, and so the Cp at 12 h was adjusted for the expected decrease over 6 h.
Footnotes
Published ahead of print 2 April 2012
REFERENCES
- 1. Bavisotto L, et al. 2007. Antiviral, pharmacokinetic and safety data for GS-9190, a non-nucleoside HCV NS5B polymerase inhibitor, in a phase-1 trial in HCV genotype 1 infected patients. 58th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 2 to 6 November 2007, Boston, MA http://www.natap.org/2007/AASLD/AASLD_39.htm Accessed 14 March 2011 [Google Scholar]
- 1a. Bondy SS, Oare DA, Tse WC. June 2011. Pyridazine compound and use thereof. US patent 7,956,184
- 2. Brainard DM, et al. 2009. Safety and antiviral activity of NS5B polymerase inhibitor MK-3281 in genotype 1 and 3 HCV-infected patients. 60th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 30 October to 3 November 2009, Boston, MA http://www.natap.org/2009/AASLD/AASLD_23.htm Accessed 14 March 2011 [Google Scholar]
- 3. Brass CA, et al. 2011. Sustained virologic response and boceprevir resistance-associated variants observed in patients infected with HCV genotype 1a/1b when treated with boceprevir plus peginterferon alpha-2b/ribavirin: SVR rates among patients with G1b virus were consistently higher than G1a patients in both SPRINT-2 and RESPOND-2. The International Liver Congress 2011, 46th Annual Meeting of the European Association for the Study of the Liver (EASL), 30 March to 3 April 2011, Berlin, Germany http://www.natap.org/2011/EASL/EASL_96.htm Accessed 20 October 2011 [Google Scholar]
- 4. Chauret N, et al. 2009. Preclinical pharmacokinetic and ADME characterization of VCH-222, a novel non-nucleoside HCV NS5B polymerase inhibitor from Vertex/ViroChem. The International Liver Congress 2009, 44th Annual Meeting of the European Association for the Study of the Liver (EASL), 22 to 26 April 2009, Copenhagen, Denmark http://www.natap.org/2009/EASL/EASL_65.htm Accessed 8 June 2011 [Google Scholar]
- 5. Chen KX, Njoroge FG. 2010. The journey to the discovery of boceprevir: an NS3-NS4 HCV protease inhibitor for the treatment of chronic hepatitis C. Prog. Med. Chem. 49:1–36 [DOI] [PubMed] [Google Scholar]
- 6. Chu H-M, McNair L, Purdy S. 2004. Results of a phase I single-dose escalation study of the hepatitis C protease inhibitor VX-950 in healthy volunteers, abstr LB 20. Hepatology 40(Suppl 1):735A [Google Scholar]
- 7. Cooper C, Larouche R, Bourgault B, Chauret N, Proulx L. 2009. Safety, tolerability and pharmacokinetics of the HCV polymerase inhibitor VCH-222 following single dose administration in healthy volunteers and antiviral activity in HCV-infected individuals. The International Liver Congress 2009, 44th Annual Meeting of the European Association for the Study of the Liver (EASL) 22 to 26 April 2009, Copenhagen, Denmark: http://www.natap.org/2009/EASL/EASL_48.htm Accessed 8 June 2011 [Google Scholar]
- 8. Corsa A, et al. 2011. Preclinical properties of the novel HCV NS3 protease inhibitor GS-9451. The International Liver Congress 2011, 46th Annual Meeting of the European Association for the Study of the Liver (EASL), 30 March to 3 April 2011, Berlin, Germany: http://www.hivandhepatitis.com/2011_conference/easl2011/posters/corsa.pdf Accessed 23 August 2011 [Google Scholar]
- 9. de Bruijne J, et al. 2010. Phase I study in healthy volunteers and patients with IDX375, a novel non-nucleoside HCV polymerase inhibitor. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 30 October to 3 November 2010, Boston, MA http://www.natap.org/2010/AASLD/AASLD_93.htm Accessed 14 March 2011 [Google Scholar]
- 10. Detishin V, et al. 2011. Pharmacokinetics, pharmacodynamics, safety and tolerability of ACH-1625 (HCV NS3 protease inhibitor) in HCV genotype 1 infection. The International Liver Congress 2011, 46th Annual Meeting of the European Association for the Study of the Liver (EASL), 30 March to 3 April 2011, Berlin, Germany http://www.natap.org/2011/EASL/EASL_82.htm Accessed 23 August 2011 [Google Scholar]
- 11. Dumas E, et al. 2010. Pharmacokinetics of the HCV polymerase inhibitor ABT-072 following single and multiple dosing in healthy adult volunteers. The International Liver Congress 2010, 45th Annual Meeting of the European Association for the Study of the Liver (EASL), 14 to 18 April 2010, Vienna, Austria http://www.natap.org/2010/EASL/EASL_63.htm Accessed 9 March 2011 [Google Scholar]
- 12. Food and Drug Administration 2010. Guidance for industry. Chronic hepatitis C virus infection: developing direct-acting antiviral agents for treatment. Draft guidance, September 2010. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Silver Spring, Maryland: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/UCM225333.pdf Accessed 3 October 2011 [Google Scholar]
- 13. Forestier N, et al. 2011. Treatment of chronic hepatitis C patients with the NS3/4A protease inhibitor danoprevir (ITMN-191/RG7227) leads to robust reductions in viral RNA: a phase 1b multiple ascending dose study. J. Hepatol. 54:1130–1136 [DOI] [PubMed] [Google Scholar]
- 14. Gane EJ, et al. 2011. Antiviral activity, safety, and pharmacokinetics of danoprevir/ritonavir plus PEG-IFN α-2a/RBV in hepatitis C patients. J. Hepatol. 55:972–979 [DOI] [PubMed] [Google Scholar]
- 15. Gaultier IA, et al. 2011. 12-Week effiacy [sic] and safety of ABT-072 or ABT-333 with pegylated interferon + ribavirin, following 3-day monotherapy in genotype 1 HCV-infected treatment-naïve subjects. (APASL), 17 to 20 February 2011, Bangkok, Thailand http://www.natap.org/2011/APSL/APSL_02.htm Accessed 7 March 2011 [Google Scholar]
- 16. Good SS, et al. 2009. Preclinical pharmacokinetic and safety profile of IDX375, a novel and potent non-nucleoside HCV polymerase inhibitor. The International Liver Congress 2009, 44th Annual Meeting of the European Association for the Study of the Liver (EASL), 22 to 26 April 2009, Copenhagen, Denmark http://www.hivandhepatitis.com/2009icr/easl/pdf/IDX375_Poster_Handout_EASL09_FINAL.pdf Accessed 28 September 2011 [Google Scholar]
- 17. Good SS, et al. 2010. Pharmacokinetic and safety profile of IDX320, a novel and potent HCV protease inhibitor. The International Liver Congress 2010, 45th Annual Meeting of the European Association for the Study of the Liver (EASL), 14 to 18 April 2010, Vienna, Austria http://www.idenix.com/hcv/IDX320PK_EASL_2010_handout_FINAL.pdf Accessed 2 September 2011 [Google Scholar]
- 18. Guedj J, Bazzoli C, Neumann AU, Mentre F. 2011. Design evaluation and optimization for models of hepatitis C viral dynamics. Stat. Med. 30:1045–1056 [DOI] [PubMed] [Google Scholar]
- 19. Hammond JL, et al. 2008. Antiviral activity of the HCV polymerase inhibitor PF-00868554 administered as monotherapy in HCV genotype 1 infected subjects. 59th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 31 October to 1 November 2008, San Francisco, CA http://www.natap.org/2008/AASLD/AASLD_18.htm Accessed 24 March 2011 [Google Scholar]
- 20. Huang M, et al. 2010. Antiviral activity, combination and resistance of ACH-1625, a potent HCV NS3 protease inhibitor. The International Liver Congress 2010, 45th Annual Meeting of the European Association for the Study of the Liver (EASL), 14 to 18 April 2010, Vienna, Austria http://www.natap.org/2010/EASL/EASL_70.htm Accessed 2 September 2011 [Google Scholar]
- 21. Idenix press release 2011. IDX375, an HCV non-nucleoside polymerase inhibitor. Idenix, Cambridge, MA: http://www.dailymarkets.com/stock/2011/02/28/idenix-pharmaceuticals-reports-fourth-quarter-and-year-end-2010-financial-results/ Accessed 14 March 2011 [Google Scholar]
- 22. Itakura J, et al. 2011. Changes in hepatitis C viral load during first 14 days can predict the undetectable time point of serum viral load by pegylated interferon and ribavirin therapy. Hepatol. Res. 41:217–224 [DOI] [PubMed] [Google Scholar]
- 23. Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. 2010. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin. Pharmacokinet. 49:189–206 [DOI] [PubMed] [Google Scholar]
- 24. Kempf D, et al. 2010. Pharmacokinetic enhancement with ritonavir: more than it seems? 5th International Workshop on Clinical Pharmacology of Hepatitis Therapy, 23 to 24 June 2010, Boston, MA http://regist2.virology-education.com/5thHEP_PK/docs/14_Kempf.pdf Accessed 16 November 2011 [Google Scholar]
- 25. Klein CE, et al. 2009. Safety, tolerability, pharmacokinetics and antiviral activity of the HCV polymerase inhibitor ABT-072 following single and multiple dosing in healthy adult volunteers and two days of dosing in treatment-naïve HCV genotype 1-infected subjects. HEP DART 2009, 6 to 10 December 2009, Kohala Coast, HI: http://www.natap.org/2009/hepDART/hepDART_01.htm Accessed 9 November 2011 [Google Scholar]
- 26. Klein E, Menon RM, Cohen DE, Awni WM, Bernstein BM. 2009. Pharmacokinetics of a polymerase inhibitor, ABT-333, in treatment-naïve HCV genotype 1-infected subjects following treatment with 2 days of ABT-333 followed by 26 days of ABT-333 plus pegylated interferon and ribavirin. HEP DART 2009, 6 to 10 December 2009, Kohala Coast, HI: http://www.natap.org/2009/hepDART/hepDART_04.htm Accessed 10 November 2011 [Google Scholar]
- 27. Konnick EQ, Erali M, Ashwood ER, Hillyard DR. 2002. Performance characteristics of the COBAS Amplicor Hepatitis C Virus (HCV) Monitor, version 2.0, international unit assay and the National Genetics Institute HCV Superquant assay. J. Clin. Microbiol. 40:768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuntzen T, et al. 2008. Naturally occurring dominant resistance mutations to hepatitis C virus protease and polymerase inhibitors in treatment-naïve patients. Hepatology 48:1769–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. la Porte C. 2008. Inhibitory quotient in HIV pharmacology. Curr. Opin. HIV AIDS 3:283–287 [DOI] [PubMed] [Google Scholar]
- 30. Lallos LB, et al. 2010. In vitro antiviral activity of IDX320, a novel and potent macrocyclic HCV protease inhibitor. The International Liver Congress 2010, 45th Annual Meeting of the European Association for the Study of the Liver (EASL), 14 to 18 April 2010, Vienna, Austria http://www.natap.org/2010/EASL/EASL_21.htm Accessed 2 September 2011 [Google Scholar]
- 31. Larrey D, et al. 2009. BI 207127 is a potent HCV RNA polymerase inhibitor during 5 days monotherapy in patients with chronic hepatitis C. 60th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 30 October to 3 November 2009, Boston, MA http://www.natap.org/2009/AASLD/AASLD_10.htm Accessed 15 March 2011 [Google Scholar]
- 32. Lawitz E, et al. 2010. Initial antiviral activity of the HCV NS3 protease inhibitor ABT-450 when given with low-dose ritonavir as 3-day monotherapy: preliminary results of study M11-602 in genotype 1 (GT1) HCV-infected treatment-naïve subjects. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 29 October to 2 November 2010, Boston, MA http://www.hivandhepatitis.com/2010_conference/aasld/posters/lawitz2.pdf Accessed 10 June 2011 [Google Scholar]
- 33. Lawitz E, et al. 2010. Three-day, dose-ranging study of the HCV NS3 protease inhibitor GS-9451. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 29 October to 2 November 2010, Boston, MA http://www.natap.org/2010/AASLD/AASLD_44.htm Accessed 2 September 2011 [Google Scholar]
- 34. Lawitz E, et al. 2010. Dose-ranging, three-day monotherapy study of the HCV NS3 protease inhibitor GS-9256. The International Liver Congress 2010, 45th Annual Meeting of the European Association for the Study of the Liver (EASL), 14 to 18 April 2010, Vienna, Austria http://www.natap.org/2010/EASL/EASL_08.htm Accessed 2 September 2011 [Google Scholar]
- 35. Lawitz E, et al. 2009. Antiviral activity of ANA598, a potent non-nucleoside polymerase inhibitor, in chronic hepatitis C patients. The International Liver Congress 2009, 44th Annual Meeting of the European Association for the Study of the Liver (EASL), 22 to 26 April 2009, Copenhagen, Denmark http://www.anadyspharma.com/products_in_development/pdf/ANA598.EASL2009.pdf Accessed 18 October 2011 [Google Scholar]
- 36. Lawitz E, et al. 2008. Safety, tolerability and antiviral activity of MK-7009, a novel inhibitor of the hepatitis C virus NS3/4A protease, in patients with Chronic HCV genotype 1 infection. 59th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 31 October to 1 November 2008, San Francisco, CA http://www.natap.org/2008/AASLD/AASLD_09.htm Accessed 2 September 2011 [Google Scholar]
- 37. Le Pogam S, et al. 2008. Existence of hepatitis C virus NS5B variants naturally resistant to non-nucleoside, but not to nucleoside, polymerase inhibitors among untreated patients. J. Antimicrob. Chemother. 61:1205–1216 [DOI] [PubMed] [Google Scholar]
- 38. Lin T, et al. 2009. In vitro activity and preclinical profile of TMC435350, a potent hepatitis C virus protease inhibitor. Antimicrob. Agents Chemother. 53:1377–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reference deleted.
- 40. Liverton NJ, et al. 2010b. MK-7009, a potent and selective inhibitor of hepatitis C virus NS3/4A protease. Antimicrob. Agents Chemother. 54:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lok A, et al. 2010. Combination therapy with BMS-790052 and BMS-650032 alone or with pegylated interferon and ribavirin (pegIFN/RBV) results in undetectable HCV RNA through 12 weeks of therapy in HCV genotype 1 null responders. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 30 October to 3 November, Boston, MA http://www.natap.org/2010/AASLD/AASLD_16.htm Accessed 2 September 2011 [Google Scholar]
- 42. Manns MP, et al. 2011. Potency, safety, and pharmacokinetics of the NS3/4A protease inhibitor BI201335 in patients with chronic HCV genotype-1 infection. J. Hepatol. 54:1114–1122 [DOI] [PubMed] [Google Scholar]
- 43. Manns M, et al. 2008. Safety and antiviral activity of TMC435350 in treatment-naive genotype 1 HCV-infected patients. 59th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 31 October to 1 November 2008, San Francisco, CA http://www.natap.org/2008/AASLD/AASLD_10.htm Accessed 2 September 2011 [Google Scholar]
- 44. McPhee F. 2010. Identification and preclinical profile of the novel HCV NS3 protease inhibitor BMS-650032. The International Liver Congress 2010, 45th Annual Meeting of the European Association for the Study of the Liver (EASL), 30 March to 3 April 2010, Berlin, Germany http://www.kenes.com/easl2010/posters/Abstract649.htm Accessed 2 September 2011 [Google Scholar]
- 45. Menon R, et al. 2009. Pharmacokinetics, safety and tolerability of the HCV polymerase inhibitor ABT-333 following multiple ascending doses and effect of coadministration of ketoconazole in healthy subjects. The International Liver Congress 2009, 44th Annual Meeting of the European Association for the Study of the Liver (EASL), 22 to 26 April 2009, Copenhagen, Denmark http://www.natap.org/2009/EASL/EASL_53.htm Accessed 14 March 2011 [Google Scholar]
- 46. Mo H, et al. 2010. Enhanced in vitro antiviral activity and suppression of resistance by combining GS-9256, a novel protease inhibitor, with GS-9190, a non-nucleoside NS5B inhibitor. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 29 October to 2 November 2010, Boston, MA http://www.hivandhepatitis.com/2010_conference/aasld/posters/mo.pdf Accessed 25 August 2011 [Google Scholar]
- 47. Monteagudo E, Fonsil, et al. 2010. The metabolism and disposition of a potent inhibitor of hepatitis C virus NS3/4A protease. Xenobiotica 40:826–839 [DOI] [PubMed] [Google Scholar]
- 48. Nakai K, et al. 2008. Decreased expression of cytochromes p450 1A2, 2E1, and 3A4 and drug transporters Na(+)-taurocholate-cotransporting polypeptide, organic cation transporter 1, and organic anion-transporting peptide-C correlates with the progression of liver fibrosis in chronic hepatitis C patients. Drug Metab. Dispos. 36:1786–1793 [DOI] [PubMed] [Google Scholar]
- 49. Neely MN, Rakhmanina NY. 2011. Pharmacokinetic optimization of antiretroviral therapy in children and adolescents. Clin. Pharmacokinet. 50:143–189 [DOI] [PubMed] [Google Scholar]
- 50. Neumann AU, et al. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103–107 [DOI] [PubMed] [Google Scholar]
- 51. Pasquinelli C, et al. 2009. Safety, tolerability, pharmacokinetics and antiviral activity following single- and multiple-dose administration of BMS-650032, a novel HCV NS3 inhibitor, in subjects with chronic genotype 1 HCV infection. 60th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 30 October to 3 November 2009, Boston, MA http://www.natap.org/2009/AASLD/AASLD_13.htm Accessed 2 September 2011 [Google Scholar]
- 52. Pawlotsky JM, et al. 2000. Standardization of hepatitis C virus RNA quantification Hepatol. 32:654–659 [DOI] [PubMed] [Google Scholar]
- 53. Perni RB, et al. 2006. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob. Agents Chemother. 50:899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petry A, et al. 2010. Safety, tolerability, and pharmacokinetics after single and multiple doses of MK-5172, a novel HCV NS3/4a protease inhibitor with potent activity against known resistance mutants, in healthy subjects. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 29 October to 2 November 2010, Boston, MA http://www.hivandhepatitis.com/2010_conference/aasld/posters/petry2.pdf Accessed 2 September 2011 [Google Scholar]
- 55. Petry A, et al. 2010. Safety and antiviral activity of MK-5172, a novel HCV NS3/4a protease inhibitor with potent activity against known resistance mutants, in genotype 1 and 3 HCV-infected patients. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 29 October to 2 November 2010, Boston, MA http://www.natap.org/2010/AASLD/AASLD_34.htm Accessed 2 September 2011 [Google Scholar]
- 56. Poirier A, et al. 2008. Design, data analysis, and simulation of in vitro drug transport kinetic experiments using a mechanistic in vitro model. Drug Metab. Dispos. 36:2434–2444 [DOI] [PubMed] [Google Scholar]
- 57. Reference deleted.
- 58. Reesink H, et al. 2009. Safety and antiviral activity of SCH 900518 administered as monotherapy and in combination with peginterferon alfa-2b to naïve and treatment-experienced HCV-1 infected patients. The International Liver Congress 2009, 44th Annual Meeting of the European Association for the Study of the Liver (EASL), 22 to 26 April 2009, Copenhagen, Denmark http://www.natap.org/2009/EASL/EASL_39.htm Accessed 2 September 2011 [Google Scholar]
- 59. Reesink HW, et al. 2010. Antiviral activity, safety and pharmacokinetics of IDX320, a novel macrocyclic HCV protease inhibitor, in a 3-day proof-of-concept study in patients with chronic hepatitis C. 61st Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 29 October to 2 November 2010, Boston, MA http://www.natap.org/2010/AASLD/AASLD_92.htm Accessed 2 September 2011 [Google Scholar]
- 60. Reesink HW, et al. 2010. Rapid HCV-RNA decline with once daily TMC435: a phase I study in healthy volunteers and hepatitis C patients. Gastroenterology 138:913–921 [DOI] [PubMed] [Google Scholar]
- 61. Reesink HW, et al. 2006. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology 131:997–1002 [DOI] [PubMed] [Google Scholar]
- 62. Rouzier R, et al. 2011. Activity of danoprevir plus low-dose ritonavir in combination with peginterferon alfa-2a (40KD) plus ribavirin in previous null responders. The International Liver Congress 2011, 46th Annual Meeting of the European Association for the Study of the Liver (EASL), 30 March to 3 April 2011, Berlin, Germany http://www.natap.org/2011/EASL/EASL_21.htm Accessed 20 October 2011 [Google Scholar]
- 63. Seiwert SD, et al. 2008. Preclinical characteristics of the hepatitis C virus NS3/4A protease inhibitor ITMN-191 (R7227). Antimicrob. Agents Chemother. 52:4432–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shih IH, et al. 2011. Mechanistic characterization of GS-9190 (tegobuvir), a novel nonnucleoside inhibitor of hepatitis C virus NS5B polymerase. Antimicrob. Agents Chemother. 55:4196–4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Silva M, et al. 2011. Antiviral activity of boceprevir monotherapy in treatment-naive subjects with chronic hepatitis C genotype 2/3. 21st Conference of the Asian Pacific Association for the study of the Liver (APASL), 17 to 20 February, Bangkok, Thailand http://www.natap.org/2011/APSL/APSL_03.htm Accessed 2 September 2011 [Google Scholar]
- 66. Snoeck E, et al. 2010. A comprehensive hepatitis C viral kinetic model explaining cure. Clin. Pharmacol. Ther. 87:706–713 [DOI] [PubMed] [Google Scholar]
- 67. Stauber K, et al. 2010. Characterization of the hepatoselective distribution of ACH-1625: a potent, clinical stage HCV NS3 protease inhibitor. The International Liver Congress 2010, 45th Annual Meeting of the European Association for the Study of the Liver (EASL), 14 to 18 April 2010, Vienna, Austria http://www.natap.org/2010/EASL/EASL_69.htm Accessed 2 September 2011 [Google Scholar]
- 68. Thompson PA, et al. 2008. Pathway to selection of a potent, well tolerated development candidate in HCV. http://www.anadyspharma.com/products_in_development/pdf/ICAR-2008-Final.pdf Accessed 28 June 2011
- 69. Tong X, et al. 2010. Preclinical characterization of the antiviral activity of SCH 900518 (narlaprevir), a novel mechanism-based inhibitor of hepatitis C virus NS3 protease. Antimicrob. Agents Chemother. 54:2365–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. University of California, San Francisco 2007. Course notes from the 20th annual Pharmacokinetics for Pharmaceutical Scientists course, Department of Biopharmaceutical Sciences, School of Pharmacy, University of California, San Francisco, CA [Google Scholar]
- 71. Watkins WJ, Ray AS, Chong LS. 2010. HCV NS5B polymerase inhibitors. Curr. Opin. Drug Discov. Devel. 13:441–465 [PubMed] [Google Scholar]
- 72. White PW, et al. 2010. Preclinical characterization of BI 201335, a C-terminal carboxylic acid inhibitor of the hepatitis C virus NS3-NS4A protease. Antimicrob. Agents Chemother. 54:4611–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wright DH, et al. 2008. Safety, tolerability, and pharmacokinetic data following single- and multiple-dose administration of MK-7009, a hepatitis C virus non-structural 3/4a protease inhibitor, to healthy male subjects. 59th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 31 October to 1 November 2008, San Francisco, CA http://www.natap.org/2008/AASLD/AASLD_25.htm Accessed 2 September 2011 [Google Scholar]
- 74. Zeuzem S. 2005. SCH 503034 protease inhibitor monotherapy in hepatitis C virus genotype-1 IFN-a non-responders. 56th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD), 11 to 15 November 2005, San Francisco, CA http://www.natap.org/2006/HCV/031706_02.htm Accessed 2 September 2011 [Google Scholar]