Abstract
Daptomycin MICs for enterococci are typically 1- to 2-fold higher than those for Staphylococcus aureus, and there is an imminent need to establish the optimal dose for appropriate treatment of enterococcal infections. We investigated the bactericidal activity of daptomycin at various dose exposures compared to that of linezolid against vancomycin-resistant enterococcus (VRE) in an in vitro pharmacokinetic/pharmacodynamic model utilizing simulated endocardial vegetations over 96 h. Daptomycin at doses of 6, 8, 10, and 12 mg/kg of body weight/day and linezolid at a dose of 600 mg every 12 h were evaluated against two clinical vancomycin-resistant Enterococcus faecium strains (EFm11499 and 09-184D1051), one of which was linezolid resistant (09-184D1051), and one clinical vancomycin-resistant Enterococcus faecalis strain (EFs11496). Daptomycin MICs were 4, 2, and 0.5 μg/ml for EFm11499, 09-184D1051, and EFs11496, respectively. Bactericidal activity, defined as a ≥3 log10 CFU/g reduction from the initial colony count, was demonstrated against all three isolates with all doses of daptomycin; however, bactericidal activity was not sustained with the daptomycin 6- and 8-mg/kg/day regimens. Linezolid was bacteriostatic against EFm11499 and displayed no appreciable activity against 09-184D1051 or EFs11496. Concentration-dependent killing was displayed with more sustained reduction in colony count (3.58 to 6.46 and 5.89 to 6.56 log10 CFU/g) at 96 h for the simulated regimen of daptomycin at doses of 10 and 12 mg/kg/day, respectively (P ≤ 0.012). No E. faecium mutants with reduced susceptibility were recovered at any dosage regimen; however, the E. faecalis strain developed reduced daptomycin susceptibility with daptomycin at 6, 8, and 10 but not at 12 mg/kg/day. Daptomycin displayed a dose-dependent response against three VRE isolates, with high-dose daptomycin producing sustained bactericidal activity. Further research is warranted.
INTRODUCTION
Daptomycin (DAP) is a lipopeptide antibiotic with concentration-dependent activity against Gram-positive bacteria that is currently approved for the treatment of staphylococcus bacteremia and right-sided endocarditis at a dose of 6 mg/kg of body weight/day (7). Daptomycin also displays in vitro activity against almost all Enterococcus spp., including those resistant to other antibiotics, such as vancomycin, linezolid (LZD), and quinupristin-dalfopristin (3, 22, 38). Daptomycin exhibits a lower potency against enterococci than that against staphylococci, demonstrating higher Clinical Laboratory and Standards Institute (CLSI) breakpoints (≤4 μg/ml versus ≤1 μg/ml), MIC50 values (1 to 2 μg/ml versus 0.25 μg/ml), and MIC90 values (1 to 2 μg/ml versus 0.5 μg/ml) (11, 38). Based on in vivo neutropenic mice infection models, maximum concentration (Cmax)/MIC and area under the concentration-time curve (AUC)/MIC ratios are the best predictors for efficacy of daptomycin against infections caused by both Staphylococcus spp. and Enterococcus spp. (39) Additionally, in vitro pharmacokinetic/pharmacodynamic (PK/PD) models have demonstrated a clear dose-effect relationship of daptomycin with reduction of log10 CFU/ml (9). The simulated effective dose to achieve 80% maximal kill activity was 3 mg/kg for the two staphylococcal isolates (MICs of 0.125 and 0.25 μg/ml) and 6.8 mg/kg for the two Enterococcus faecium isolates (MICs of 2 and 4 μg/ml) (9). Based on the available data, the current Food and Drug Administration-approved dose of 6 mg/kg/day for Staphylococcus aureus bacteremia and right-sided infective endocarditis infections is likely suboptimal for infections caused by most enterococcus due to the higher MIC values and leads to the logical conclusion that higher daptomycin doses (i.e., >6 mg/kg/day) will be required to adequately treat these infections.
Clinical experience with daptomycin for the treatment of enterococcal infections is limited to several retrospective, observational studies of patients with enterococcal bacteremia (12, 14, 17, 18, 20, 21, 28, 29, 31, 32). Success rates in these series vary from 58.1% to 90% depending on the severity of illness of the included patients and the inclusion of clinical or microbiological results in the definition of success. Although these retrospective studies have several limitations, they provide some clinical support that standard doses of daptomycin (6 mg/kg) may be suboptimal for serious enterococcal infections, such as bacteremia and endocarditis. Additional data to support the use of high-dose daptomycin for the treatment of enterococcal infections are vital to ensuring its appropriate use and efficacy in treating enterococcal infections and establishing daptomycin as the preferred bactericidal regimen for the treatment of serious enterococcal infections. A retrospective analysis of high-dose daptomycin (≥8 mg/kg/day) in 250 patients with both S. aureus and enterococcal infections reported that daptomycin was safe and well-tolerated with no dose-response relationship to changes in creatine phosphokinase (CPK) levels (24). That and other studies suggest that the routine use of high-dose daptomycin, ranging from 8 to 14 mg/kg/day, to treat enterococcal infections is safe and clinically feasible (6, 13, 15, 16, 23, 24, 33). The purpose of the current study was to examine the effects of standard and various high-dose daptomycin regimens on both bactericidal killing and the emergence of nonsusceptibility in an in vitro model of enterococcal infection compared to the effects of linezolid.
(This study was presented in part at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2011, and at the 22nd European Congress of Clinical Microbiology and Infectious Diseases, London, United Kingdom, 2012.)
MATERIALS AND METHODS
Bacterial strains.
A total of three clinical vancomycin-resistant enterococcus (VRE) strains were evaluated. Two clinical E. faecium strains were evaluated (SF11499, daptomycin MIC of 4 μg/ml), one of which was linezolid resistant (09-184D1051, DAP MIC of 2 μg/ml), and one clinical Enterococcus faecalis strain (SF11496, DAP MIC of 0.5 μg/ml) was utilized. Isolates SF11499 (EFm11499) and SF11496 (EFs11496) were obtained from blood and urine sources, respectively, from a patient at Henry Ford Hospital in Detroit, MI. E. faecium isolate 09-184D1051 was recovered from a patient in Houston, TX.
Antimicrobials.
Daptomycin (DAP) analytical powder (Cubist Pharmaceuticals, Inc., Lexington, MA) was provided by the manufacturer. Linezolid (LZD) 2-μg/ml solution was commercially purchased (Detroit Receiving Hospital, Detroit, MI).
Media.
Mueller-Hinton broth II (MHB II; Difco, Detroit, MI) with 25 mg/liter of calcium and 12.5 μg/ml magnesium was used for susceptibility testing and in vitro pharmacodynamic simulated endocardial vegetation (SEV) models. Due to the dependency of daptomycin on calcium for antimicrobial activity, supplemented MHB II (SMHB II) containing 50 μg/ml of calcium was used for susceptibility testing, and that containing 75 μg/ml of calcium (50 and 75 SMHB, respectively) was used for in vitro SEV model experiments (due to binding of calcium by albumin) (25). Colony counts were determined using brain heart infusion agar (BHA; Difco, Detroit, MI). Nonsusceptibility was assessed with antibiotic-containing MHB II plus agar (Becton, Dickinson, Sparks, MD) supplemented to 50 mg/liter of calcium and BHA for daptomycin and linezolid, respectively.
Susceptibility testing.
MICs and minimum bactericidal concentrations (MBCs) of daptomycin and linezolid were determined in duplicate by broth microdilution at ∼5 × 105 CFU/ml in 50 SMHB II as specified above, according to the CLSI guidelines (11). Etest methodology, according to manufacturer recommendations, was used for any isolate observed to grow on DAP- or LZD-containing agar plates (Mueller-Hinton agar [MHA] for daptomycin, BHA for linezolid) used for screening changes in susceptibility during model experiments.
Simulated endocardial vegetations.
SEVs were prepared by mixing 0.05 ml of organism suspension (final inoculum, 108.5 CFU/g), 0.5 ml of human cryoprecipitate antihemophilic factor from volunteer donors (American Red Cross, Detroit, MI), and 0.025 ml of platelet suspension (platelets mixed with normal saline, 250,000 to 500,000 platelets per clot) in 1.5-ml siliconized Eppendorf tubes. Bovine thrombin (5,000 units/ml), 0.05 ml, was added to each tube after insertion of a sterile monofilament line into the mixture. The resultant simulated vegetations were then removed from the Eppendorf tubes with a sterile plastic needle (Becton, Dickinson, Sparks, MD) and introduced into the model. This methodology resulted in SEVs consisting of approximately 3 to 3.5 g/dl of albumin and 6.8 to 7.4 g/dl of total protein (1).
In vitro PK/PD model.
An in vitro PK/PD infection model consisting of a 250-ml glass apparatus with ports, where the SEVs were suspended, was utilized for all simulations. 75 SMHB II supplemented with 3.5 g/dl of human albumin (Baxter, Deerfield, IL) was used as the medium. The apparatus was prefilled with 250 ml of medium, and antibiotics were administered as boluses over a 96-h period into the central compartment via an injection port. The model apparatus was then placed into a 37°C incubator for the duration of the procedure, and a magnetic stir bar was placed in the medium for thorough mixing of the drug in the model. Fresh medium was continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex; Cole-Parmer Instrument Company, Chicago, IL) set to simulate the half-lives of the antibiotics. Simulated regimens included daptomycin at doses of 6 (DAP6), 8 (DAP8), 10 (DAP10), and 12 (DAP12) mg/kg daily (target peaks, 93.9, 123.3, 141.1, and 183.7 μg/ml, respectively; area under the concentration-time curve from 0 to 24 h [AUC0-24], 631.8 to 1,277.4 μg · h/ml; average half-life, 8 h) (6) and linezolid (LZD) at a dose of 600 mg every 12 h (target peak, 15.1 μg/ml; average half-life, 5 h) (19, 36). All models were run in duplicate to ensure reproducibility.
Pharmacodynamic analysis.
Two SEVs were removed from each model at 0, 4, 8, 24, 32, 48, 56, 72, and 96 h. The SEVs were homogenized and diluted in cold saline to be plated on BHI plates. For all samples, antimicrobial carryover was accounted for by serial dilution of the plated samples. If the anticipated dilution was near the MIC, samples were processed via vacuum filtration and washed through a 0.45-μm filter (Pall Corporation, Ann Arbor, MI) with normal saline to remove the antimicrobial agent. The limit of detection for determination of colony counts was 1 log10 CFU/g. Plates were then incubated at 37°C for 24 h, and the colony count was performed at the 24-h time point. The total reduction in log10 CFU/g over 96 h was determined by plotting time-kill curves based on the number of remaining organisms over the time period. Bactericidal activity (99.9% kill) and bacteriostatic activity were defined as a ≥3 log10 CFU/g or a <3 log10 CFU/g reduction in colony count from that of the initial inocula, respectively. Inactivity was defined as no observed reduction compared to results for the initial inocula. The time to achieve a 99.9% bacterial load reduction was determined by linear regression (if r2 ≥ 0.95) or visual inspection.
Pharmacokinetic analysis.
Pharmacokinetic samples were obtained, through the injection port of each model (duplicate samples) at 0, 0.5, 1, 2, 4, 8, 24, 32, 48, 56, 72, and 96 h for verification of target antibiotic concentrations. All samples were stored at −70°C until ready for analysis. Concentrations of DAP were determined by microbioassay utilizing Micrococcus luteus ATCC 9341. Briefly, blank quarter-inch disks were spotted with 10 μl of the standards or samples. Each standard was tested in duplicate by placing the disk on antibiotic medium 5 plates (Becton Dickinson, Sparks, MD) that were preswabbed with a 0.5 McFarland suspension of the test organism. Plates were incubated for 18 to 24 h at 37°C, and at 24 h, the zone sizes were measured using a protocol reader (Protocol; Microbiology International, Frederick, MD). Concentrations of 200, 100, 150, and 50 μg/ml were used as standards. This assay has a lower limit of detection of 5 μg/ml and demonstrates an interday coefficient of variation percentage (CV%) of ≤10.9% for daptomycin (2). Concentrations of LZD were determined using a validated high-performance liquid chromatography (HPLC) assay (30). Samples were measured using a system consisting of a ThermoFinnegan P4000 HPLC pump (San Jose, CA) with a model AS1000 fixed-volume autosampler, a model UV2000 UV detector, a Gateway Series e computer (Poway, CA), and the Chromquest HPLC data management system. The plasma standard curve for LZD ranged from 0.5 to 30 μg/ml and demonstrated a CV% of 1.04% to 4.39% for LZD (30). The half-life, area under the curve (AUC0-24 h), and peak concentration were determined by the trapezoidal method utilizing PK Analyst software (version 1.10; MicroMath Scientific Software, Salt Lake City, UT).
Nonsusceptibility.
Development of nonsusceptibility was evaluated at multiple time points throughout the simulation, at 24, 48, 72, and 96 h for days 1 to 4. Samples (100 μl) from each time point were plated on agar plates (BHI for linezolid, MHA for daptomycin) containing three times the MIC of the respective antibiotic to assess the development of resistance. Plates were then examined for growth after 24 to 48 h of incubation at 37°C. Any observed growth was tested for changes in susceptibility by both Etest and broth microdilution.
Statistical analysis.
Changes in CFU/g at 24, 48, 72, and 96 h were compared by one-way analysis of variance with Tukey's post hoc test. A P value of ≤0.05 was considered significant. All statistical analyses were performed using SPSS statistical software (release 20.0; SPSS, Inc., Chicago, IL).
RESULTS
Organism susceptibilities to DAP are displayed in Table 1. The two E. faecium isolates (EFm11499, 09-184D1051) and the E. faecalis isolate (EFs11496) were susceptible to DAP, displaying MICs of 4, 2, and 0.5 μg/ml, respectively. One E. faecium isolate (09-184D1051) was LZD resistant, with an MIC of 16 μg/ml. EFm11499 and EFs11496 were susceptible to LZD, with MICs of 2 and 1 μg/ml, respectively. MBCs of DAP were 4, 8, and 4 μg/ml for 09-184D1051, EFm11499, and EFs11496, respectively. MBCs of LZD were >16 for all isolates. No change in DAP or LZD susceptibility was found for either of the E. faecium isolates during the study. In vitro changes in susceptibility at 96 h are displayed in Table 1. Decreased susceptibility to DAP developed in EFs11496 when exposed to DAP6 and DAP8, producing a 32-fold increase in MIC (which increased from 0.5 to 16 μg/ml). In DAP10, one SEV sample from a single model developed an increased MIC to DAP (MIC of 8 μg/ml). This organism was found stable to three overnight passes onto antibiotic-free medium. No resistance was seen with DAP12 or LZD.
Table 1.
In vitro activity of daptomycin or linezolid in the pharmacokinetic/pharmacodynamic model
| Strain | DAP MIC (μg/ml) | Regimen | T99b | T99Sc | Reduction in log10 CFU/g from baseline (T0)d |
T96 mutant MIC (μg/ml)e | |
|---|---|---|---|---|---|---|---|
| 24 h | 96 h | ||||||
| 09-184D1051a | 2 | DAP6 | 32 h | NA | 1.72 ± 0.10† | 2.32 ± 0.05† | |
| DAP8 | 4 h | NA | 6 ± 0.32*† | 2.87 ± 0.37† | |||
| DAP10 | 4 h | 4 h | 5.3 ± 0.39*† | 4.71 ± 0.71*† | |||
| DAP12 | 4 h | 4 h | 6.4 ± 0.21*† | 6.04 ± 1.46*† | |||
| LZD | NA | NA | −1.81 ± 0.09 | −2.33 ± 0.03 | |||
| EFm11499a | 4 | DAP6 | 4 h | NA | 2.31 ± 0.85† | −0.01 ± 0.19 | |
| DAP8 | 8 h | NA | 3.34 ± 0.95† | 1.17 ± 0.16 | |||
| DAP10 | 4 h | 4 h | 4.46 ± 0.26*† | 3.58 ± 1.45*† | |||
| DAP12 | 8 h | 8 h | 5.01 ± 0.18*† | 6.56 ± 0.43*† | |||
| LZD | NA | NA | 0.83 ± 0.27 | 1.08 ± 0.89 | |||
| EFs11496 | 0.5 | DAP6 | 24 h | NA | 4.18 ± 1.27† | 0.65 ± 0.37 | 8 |
| DAP8 | 24 h | NA | 4.5 ± 0.7† | 2.27 ± 1.07† | 16 | ||
| DAP10 | 4 h | 4 h | 5.61 ± 0.18† | 6.46 ± 1.19*† | 8 | ||
| DAP12 | 4 h | 4 h | 6.87 ± 0.12*† | 5.89 ± 0.11*† | |||
| LZD | NA | NA | 0.24 ± 0.07† | 0.28 ± 0.25 | |||
No resistant mutants recovered from DAP or LZD simulated regimens.
NA, not achieved; T99, time to achieve a 99.9% colony reduction.
NA, not achieved; T99S, time to achieve a 99.9% colony reduction that was sustained to 96 h.
T0, time zero; *, P value of <0.05 for improved killing compared with that for the DAP 6 regimen; †, P value of <0.05 for improved killing compared with that for the LZD regimen.
Recovered nonsusceptible mutants (performed via BMD).
PK parameters of simulated regimens are displayed in Table 2. Observed pharmacokinetic parameters for LZD were within 12% of the targeted range. The Cmax and half-life for LZD were 14.4 ± 0.3 μg/ml and 4.4 ± 0.28 h (targeted values, 15.1 μg/ml and 5 h). Observed PK parameters for DAP were all within 11% of the targeted values. The Cmax and half-life observed were 105.1 ± 10.5 μg/ml and 7.93 h, 123.1 ± 7.4 μg/ml and 8.54 h, 144.2 ± 4.0 μg/ml and 7.87 h, and 188.7 ± 4.9 μg/ml and 8.36 h (targeted Cmax, 93.9, 123.3, 141.1, and 183.7 μg/ml; average half-life, 8 h) for DAP6, DAP8, DAP10, and DAP12, respectively. DAP AUC24/MIC ratios ranged from 235 to 4,367 (Table 2) and varied depending on the organism MIC.
Table 2.
Pharmacokinetic parameters of daptomycin and linezolid achieved in the PK/PD modela
| Drug, dosage, and strain | Cmax (μg/ml) (target value) | Half-life (h) | AUC0-24 (μg · h/ml) | AUC24/MICc |
|---|---|---|---|---|
| Daptomycin, 6 mg/kg/day | 105.1 ± 10.5 (93.9) | 7.86 ± 0.8 | 941.5 ± 31.2 | |
| 09-184D1051 | 471 | |||
| EFm11499 | 235 | |||
| EFs11496 | 1,883 | |||
| Daptomycin, 8 mg/kg/day | 123.1 ± 7.4 (123.3) | 8.54 ± 0.2 | 1,356.9 ± 121 | |
| 09-184D1051 | 678 | |||
| EFm11499 | 339 | |||
| EFs11496 | 2,714 | |||
| Daptomycin, 10 mg/kg/day | 144.2 ± 4.0 (141.1) | 7.87 ± 2.6 | 1,540.3 ± 280.9 | |
| 09-184D1051 | 770 | |||
| EFm11499 | 385 | |||
| EFs11496 | 3,080 | |||
| Daptomycin, 12 mg/kg/day | 188.7 ± 4.9 (183.7) | 8.36 ± 0.33 | 2,183.6 ± 95.7 | |
| 09-184D1051 | 1,092 | |||
| EFm11499 | 546 | |||
| EFs11496 | 4,367 | |||
| Linezolid, 600 mg q12hb | 14.4 ± 0.3 (15.1) | 4.4 ± 0.28 | 158.3 ± 3.7 | |
| 09-184D1051 | 79 | |||
| EFm11499 | 40 | |||
| EFs11496 | 317 |
Cmax, maximum concentration; AUC0-24; area under the concentration-time curve from 0 to 24 h. Results are expressed as means ± standard deviations.
q12h, every 12 h.
Varied based on organism MIC.
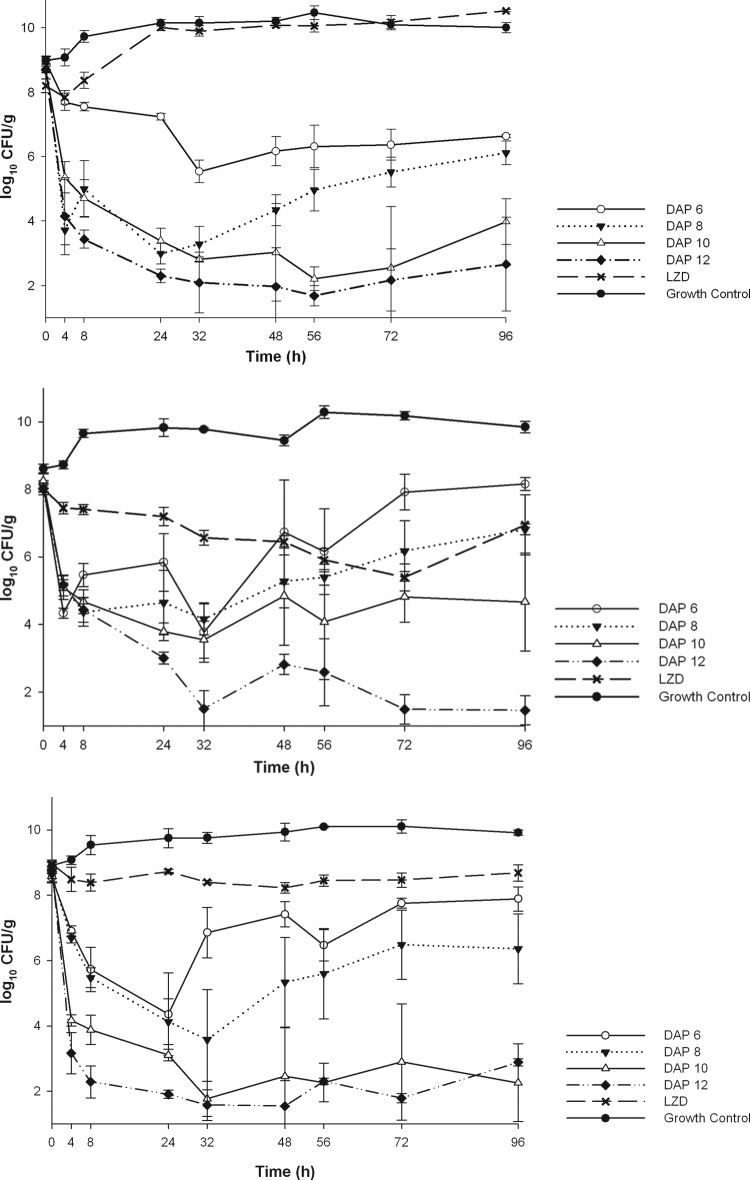
The in vitro activity of the simulated regimens is displayed in Table 1. LZD was bacteriostatic against EFm11499 and EFs11496 (Fig. 1) and displayed no appreciable activity against LZD-resistant 09-184D1051. All DAP regimens demonstrated bactericidal activity against LZD-resistant 09-184D1051. DAP6 and DAP8 displayed improved killing over that of LZD against 09-184D1051, EFm11499, and EFs11496, with times to a 99.9% kill (T99) of 32, 4, and 24 h for DAP6 and 4, 8, and 24 h for DAP8, respectively. However, both DAP6 and DAP8 failed to maintain bactericidal activity at 96 h in all three strains. DAP6 exhibited the least effect with the most regrowth, patterned closely by DAP8. In contrast, the rapid bactericidal activity of DAP10 and DAP12 was sustained to 96 h. These two regimens were similar except against EFm11499, which had the highest MIC for daptomycin. Against 09-184D1051, EFm11499, and EFs11496, DAP10 and DAP12 displayed rapid and sustained bactericidal activity (T99S) at 96 h, with a T99S of 4 h for all isolates for DAP10 and a T99S of 4, 8, and 4 h, respectively, for DAP12. These regimens were significantly more efficacious at decreasing the log10 CFU/g than DAP6, DAP8, and LZD at 72 and 96 h against 09-184D1051 (P = 0.008), at 96 h against EFm11499 (P = 0.012), and at 48 to 96 h against EFs11496 (P = 0.011). DAP10 had an overall kill count reduction of 4.46 to 5.61 log10 CFU/g at 24 h and 3.58 to 6.46 log10 CFU/g at 96 h. DAP12 had an overall kill count reduction of 5.01 to 6.87 log10 CFU/g at 24 h and 5.89 to 6.56 log10 CFU/g at 96 h. For EFm11499, DAP12 demonstrated significantly more killing than DAP10 at 72 and 96 h (P < 0.001), but activity was not significantly improved over that of DAP10 for 09-184D1051 or EFs11496 at 96 h.
Fig 1.
Activities of LZD, DAP6, DAP8, DAP10, and DAP12 against 09-184D1051 (A), EFm11499 (B), and EFs11496 (C).
DISCUSSION
Enterococcal infections are difficult to treat, especially in immunocompromised hosts and in those with deep-seated, high-inoculum infections, such as device-related infections and infective endocarditis (5, 26, 34, 37). Few therapeutic options are available, and bactericidal agents or combination therapy have been preferred for life-threatening infections (23). Linezolid demonstrates bacteriostatic activity, and prolonged therapy can result in thrombocytopenia. Quinupristin-dalfopristin is a last-line effort, being poorly tolerated with substantial toxicities. Daptomycin is a concentration-dependent cyclic lipopeptide with demonstrated in vitro bactericidal activity against enterococci. Daptomycin MIC values are higher for enterococci than for S. aureus (11, 38). Maximum effect (Emax) models suggest that increased doses (>7.9 mg/kg/day) may be needed to surmount this; however, a paucity of data exist evaluating escalating doses for activity against enterococci (9). Daptomycin resistance is still relatively rare; however, clinical cases are emerging for both E. faecium and E. faecalis, notably in patients with more complicated conditions (e.g., osteomyelitis, endocarditis, device-related infections, biofilm) (5, 26, 34). A recent review of daptomycin nonsusceptibility in enterococci from 23 studies from 2003 to 2010 reported an overall prevalence rate of 0.6% (23). The majority of the strains reported were vancomycin resistant (93.3%), with 88% being reported as E. faecium. Of interest, the most common dosage of daptomycin associated with resistance was 6 mg/kg/day. Although the optimal dosage of daptomycin for treatment of enterococcal infections is unknown, the authors suggested that dosages greater than what is currently recommended (4 to 6 mg/kg/day) may be required (23). The exact mechanism of enterococcal resistance to daptomycin is not fully elucidated. Similar to S. aureus resistance, enterococcal resistance is thought to result from several factors, including altered cell membrane composition and increased positive surface charge, altered ability of daptomycin to depolarize the cell, and cell wall thickening associated with genetic mutations; however, the affected genes appear to be different from those observed for S. aureus resistance (4, 23, 35, 40). Insights into the mechanism of daptomycin resistance in enterococci have recently been provided (4, 35). Whole-genome sequencing of a clinical strain pair of daptomycin-susceptible and -resistant E. faecalis obtained from the blood of a patient before and after daptomycin therapy, respectively, indicated that changes in two genes were necessary and sufficient for daptomycin resistance: (i) the liaF gene, which encodes a member of a three-component regulator (LiaFRS) that is likely to be involved in the stress-sensing response to cell envelope antibiotics and antimicrobial peptides, and (ii) the glycerophosphoryl diester phosphodiesterase gene, predicted to be involved in phospholipid metabolism. The genetic changes were associated with important ultrastructural alterations of the cell envelope and affected the ability of daptomycin to depolarize and permeabilize the cell membrane (4). In vitro selection of E. faecalis V583 in high concentrations of daptomycin resulted in changes in seven different genes. The predominant alteration was found in a gene encoding a putative cardiolipin synthase found in all resistant mutants observed. Cloning of the mutated allele of the cardiolipin synthase gene in a multicopy plasmid resulted in reduced susceptibility to daptomycin of V583, supporting the role of phospholipid enzymes in the resistance mechanism (35). Of note, changes in both the putative LiaFRS system and cardiolipin synthase have been observed with unrelated daptomycin-resistant clinical isolates of E. faecium and other E. faecalis isolates (4, 35).
The present study demonstrates a dose-response curve utilizing escalating doses of daptomycin compared with standard-dose linezolid against clinical vancomycin-resistant E. faecium and E. faecalis strains. In this study, we found that DAP6, as predicted, did not maintain bactericidal activity against E. faecium and E. faecalis, and regrowth was noted at 96 h. DAP10 and DAP12 displayed the most significant and sustained killing over the 96-h duration. We noted a concentration-dependent effect; as doses were escalated, bactericidal activity was prolonged. Overall, there was not a profound difference between results for the isolates. Although the overall doses and AUC requirements for effective and sustained activity were similar for E. faecalis and E. faecium, the AUC24/MIC ratios varied considerably based on the MIC (range, 0.5 to 4 μg/ml).
The PD parameter for daptomycin that best predicts outcome for S. aureus is the AUC24/MIC ratio (8, 27). Louie et al. reported an 80% maximal kill for S. aureus, and the AUC24/MIC ratio that correlated with bactericidal activity for the daptomycin dose of 6 mg/kg in animals was 245 to 516, depending on the organism MIC (27). Cha et al. reported an AUC24/MIC ratio of 502 and 705 for daptomycin doses of 6 and 8 mg/kg daily, respectively, for vancomycin-resistant E. faecium (10). In the current investigation, the AUC0-24 was proportional to the dose administered (6 to 12 mg/kg/day), and the corresponding AUC24/MIC ratio ranged from 235 to 4,367 and varied according to the organism MIC. The minimum AUC0-24 needed for sustained bactericidal activity was 1,540, and the corresponding AUC24/MIC ratio was 214 to 1,715, dependent upon the MIC (DAP MIC of 0.5 to 4 μg/ml).
Limitations of this study include the utilization of only three isolates for testing; therefore, the results may not be representative of those for all daptomycin and enterococcal interactions. A longer duration of exposure (e.g., >96 h) is needed to verify that killing is sustained and that there is suppression of resistance. In addition, a specific dose breakpoint should be pursued to determine the optimal AUC24/MIC exposure for each Enterococcus sp. best correlating with sustained bactericidal killing and suppression of emergence of resistance.
In conclusion, daptomycin doses of ≥10 mg/kg per day may be necessary to treat high-inoculum vancomycin-resistant E. faecium and E. faecalis infections, such as those found in patients with infective endocarditis.
ACKNOWLEDGMENTS
This investigator-initiated study was funded by a research grant from Cubist Pharmaceuticals.
We are grateful to Audrey Wanger for providing isolate 09-184D1051 and to Marcus Zervos for providing isolates SF11496 and SF11499.
M.J.R. has received grant support, has served as a consultant, or has participated as a speaker for Astellas, Cubist, Forest Laboratories, Pfizer, Rib-X, and Novartis and is supported in part by grant R21AI092055 from the NIAID. C.A.A. has received lecture fees, research support, and consulting fees from Pfizer, Inc., and Cubist and has served as a speaker for Novartis. C.A.A. is supported in part by NIH grants R00 AI72961 and R01 AI093749 from the NIAID. B.E.M. has received grant support from Johnson & Johnson, Astellas, Palumed, Intercell, and Cubist, has served as a consultant for Astellas, Cubist, The Medicines Company, Pfizer, Rib-X, AstraZeneca, and Duranta Therapeutics, and was supported in part by NIH grant R01 AI72961.
Footnotes
Published ahead of print 2 April 2012
REFERENCES
- 1. Akins RL, Rybak MJ. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akins RL, Rybak MJ. 2000. In vitro activities of daptomycin, arbekacin, vancomycin, and gentamicin alone and/or in combination against glycopeptide intermediate-resistant Staphylococcus aureus in an infection model. Antimicrob. Agents Chemother. 44:1925–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anastasiou DM, Thorne GM, Luperchio SA, Alder JD. 2006. In vitro activity of daptomycin against clinical isolates with reduced susceptibilities to linezolid and quinupristin/dalfopristin. Int. J. Antimicrob. Agents 28:385–388 [DOI] [PubMed] [Google Scholar]
- 4. Arias CA, et al. 2011. Genetic basis for in vivo daptomycin resistance in enterococci. N. Engl. J. Med. 365:892–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arias CA, et al. 2007. Failure of daptomycin monotherapy for endocarditis caused by an Enterococcus faecium strain with vancomycin-resistant and vancomycin-susceptible subpopulations and evidence of in vivo loss of the vanA gene cluster. Clin. Infect. Dis. 45:1343–1346 [DOI] [PubMed] [Google Scholar]
- 6. Benvenuto M, Benziger DP, Yankelev S, Vigliani G. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boucher HW, Sakoulas G. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601–608 [DOI] [PubMed] [Google Scholar]
- 8. Bowker KE, Noel AR, MacGowan AP. 2009. Comparative antibacterial effects of daptomycin, vancomycin and teicoplanin studied in an in vitro pharmacokinetic model of infection. J. Antimicrob. Chemother. 64:1044–1051 [DOI] [PubMed] [Google Scholar]
- 9. Cha R, Grucz RG, Jr, Rybak MJ. 2003. Daptomycin dose-effect relationship against resistant gram-positive organisms. Antimicrob. Agents Chemother. 47:1598–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cha R, Rybak MJ. 2003. Daptomycin against multiple drug-resistant staphylococcus and enterococcus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Diagn. Microbiol. Infect. Dis. 47:539–546 [DOI] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement, CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12. Crank CW, et al. 2010. Comparison of outcomes from daptomycin or linezolid treatment for vancomycin-resistant enterococcal bloodstream infection: a retrospective, multicenter, cohort study. Clin. Ther. 32:1713–1719 [DOI] [PubMed] [Google Scholar]
- 13. Cunha BA, Mickail N, Eisenstein L. 2007. E. faecalis vancomycin-sensitive enterococcal bacteremia unresponsive to a vancomycin tolerant strain successfully treated with high-dose daptomycin. Heart Lung 36:456–461 [DOI] [PubMed] [Google Scholar]
- 14. Dubrovskaya Y, Kubin C, Furuya E. 2008. Daptomycin compared to linezolid for primary treatment of vancomycin-resistant Enterococcus bacteremia (VREB). Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet American Society for Microbiology and Infectious Diseases Society of America, Washington, DC: http://www.icaac.org/ [Google Scholar]
- 15. Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Figueroa DA, et al. 2009. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin. Infect. Dis. 49:177–180 [DOI] [PubMed] [Google Scholar]
- 17. Gaffney M, McKinnon P, Mohr J, Zervos MJ. 2009. Clinical experience with daptomycin for the treatment of vancomycin-resistant enterococcal bacteremia. Abstr. 49th Annu. Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009 http://www.icaac.org/ [Google Scholar]
- 18. Gallagher JC, et al. 2009. Daptomycin therapy for vancomycin-resistant enterococcal bacteremia: a retrospective case series of 30 patients. Pharmacotherapy 29:792–799 [DOI] [PubMed] [Google Scholar]
- 19. Gee T, et al. 2001. Pharmacokinetics and tissue penetration of linezolid following multiple oral doses. Antimicrob. Agents Chemother. 45:1843–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grim SA, Hong I, Freeman J, Edwards C, Clark NM. 2009. Daptomycin for the treatment of vancomycin-resistant enterococcal infections. J. Antimicrob. Chemother. 63:414–416 [DOI] [PubMed] [Google Scholar]
- 21. Hjalmarson K, Craven D, Golan Y. 2008. The use of daptomycin in vancomycin-resistant Enterococcus (VRE) bacteremia: a single center experience. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet American Society for Microbiology and Infectious Diseases Society of America, Washington, DC: http://www.icaac.org/ [Google Scholar]
- 22. Johnson AP, Mushtaq S, Warner M, Livermore DM. 2004. Activity of daptomycin against multi-resistant Gram-positive bacteria including enterococci and Staphylococcus aureus resistant to linezolid. Int. J. Antimicrob. Agents 24:315–319 [DOI] [PubMed] [Google Scholar]
- 23. Kelesidis T, Humphries R, Uslan DZ, Pegues DA. 2011. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin. Infect. Dis. 52:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kullar R, et al. 2011. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy 31:527–536 [DOI] [PubMed] [Google Scholar]
- 25. Lamp KC, Rybak MJ. 1993. Teicoplanin and daptomycin bactericidal activities in the presence of albumin or serum under controlled conditions of pH and ionized calcium. Antimicrob. Agents Chemother. 37:605–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long JK, Choueiri TK, Hall GS, Avery RK, Sekeres MA. 2005. Daptomycin-resistant Enterococcus faecium in a patient with acute myeloid leukemia. Mayo Clin. Proc. 80:1215–1216 [DOI] [PubMed] [Google Scholar]
- 27. Louie A, et al. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marion C, Kennedy L, High K. 2008. Daptomycin or linezolid in the treatment of vancomycin-resistant enterococcal bacteremia in neutropenic cancer patients. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet American Society for Microbiology and Infectious Diseases Society of America, Washington, DC: http://www.icaac.org/ [Google Scholar]
- 29. Mave V, Garcia-Diaz J, Islam T, Hasbun R. 2009. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid? J. Antimicrob. Chemother. 64:175–180 [DOI] [PubMed] [Google Scholar]
- 30. McGee B, et al. 2009. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 53:3981–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKinnell JA, et al. 2011. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol. Infect. 139:1342–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohr JF, Friedrich LV, Yankelev S, Lamp KC. 2009. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE). Int. J. Antimicrob. Agents 33:543–548 [DOI] [PubMed] [Google Scholar]
- 33. Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. 2009. Safety and clinical outcomes when utilizing high-dose (≥8 mg/kg) daptomycin therapy. Ann. Pharmacother. 43:1211–1219 [DOI] [PubMed] [Google Scholar]
- 34. Munoz-Price LS, Lolans K, Quinn JP. 2005. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin. Infect. Dis. 41:565–566 [DOI] [PubMed] [Google Scholar]
- 35. Palmer KL, Daniel A, Hardy C, Silverman J, Gilmore MS. 2011. Genetic basis for daptomycin resistance in enterococci. Antimicrob. Agents Chemother. 55:3345–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pfizer, Inc 2010. Zyvox package insert. Pfizer, Inc., New York, NY [Google Scholar]
- 37. Poutsiaka DD, Skiffington S, Miller KB, Hadley S, Snydman DR. 2007. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. J. Infect. 54:567–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sader HS, Jones RN. 2009. Antimicrobial susceptibility of Gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007–2008). Diagn. Microbiol. Infect. Dis. 65:158–162 [DOI] [PubMed] [Google Scholar]
- 39. Safdar N, Andes D, Craig WA. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Steed ME, et al. 2011. Characterizing vancomycin-resistant enterococcus strains with various mechanisms of daptomycin resistance developed in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 55:4748–4754 [DOI] [PMC free article] [PubMed] [Google Scholar]