Abstract
Malaria remains a significant risk in many areas of the world, with resistance to the current antimalarial pharmacopeia an ever-increasing problem. The M1 alanine aminopeptidase (PfM1AAP) and M17 leucine aminopeptidase (PfM17LAP) are believed to play a role in the terminal stages of digestion of host hemoglobin and thereby generate a pool of free amino acids that are essential for parasite growth and development. Here, we show that an orally bioavailable aminopeptidase inhibitor, CHR-2863, is efficacious against murine malaria.
INTRODUCTION
Malaria is a major cause of morbidity and mortality worldwide, with as many as 1 million deaths each year (3). While four Plasmodium species commonly infect humans, malaria caused by Plasmodium falciparum is responsible for most deaths, particularly in children under the age of 5 and pregnant women (2, 24). Both prevention and treatment of P. falciparum malaria are under threat because of the spread of drug-resistant parasites. Resistance to chloroquine is now considered almost universal (36), while the efficacy of affordable antimalarial drugs, such as sulfadoxine-pyrimethamine, the major drug used for intermittent preventative therapy, is declining (19, 22). Artemisinin and its derivatives may be our last line of drug defense, although recent reports have indicated signs of reduced effectiveness of artemisinin combination therapies (ACTs) (23, 28). For the development of the next generation of antimalarial agents, new targets and pathways susceptible to interruption by chemotherapy need to be identified.
The asexual intraerythrocytic stages of Plasmodium development are responsible for the clinical symptoms attributable to malaria, and most antimalarial drugs target these stages of the parasite's life cycle (15, 30). Many laboratories have focused on the hydrolytic process of hemoglobin (Hb) digestion within the parasite's specialized digestive vacuole as a target for drug discovery (4, 7, 16, 18, 32). During the asexual development phase, malaria parasites degrade 65 to 75% of their host cell's Hb, a process that ultimately results in the release of amino acids that are used by the parasite for protein anabolism (29) and the maintenance of osmotic pressure within the infected erythrocyte (14). Two metalloaminopeptidase enzymes, the P. falciparum M1 alanine aminopeptidase (PfM1AAP) and the P. falciparum M17 leucine aminopeptidase (PfM17LAP), may be critical in the terminal stages of Hb degradation and the release of free amino acids (6, 21). We have shown that inhibitors of these aminopeptidases, such as the natural Phe-Leu dipeptide analog bestatin, derived from the fungus Streptomyces olivoretticuli, and several synthetic phosphinate dipeptide analogs, can kill P. falciparum parasites in culture. These compounds are also effective against the rodent malaria parasite Plasmodium chabaudi chabaudi in vivo when administered intraperitoneally (i.p.) (32, 33). We have also recently reported the production and characterization of functionally active recombinant P. falciparum M1 alanine aminopeptidase (rPfM1AAP) and M17 leucine aminopeptidase (rPfM17LAP) and their crystal structures complexed to the aminopeptidase inhibitors bestatin and the phosphinate hPheP[CH2]Phe (20, 21, 34).
The aminopeptidase inhibitor CHR-2863 {(S)-[(R)-2-((S)-hydroxycarbamoyl-methoxy-methyl)-4-methyl-pentanoylamino]-phenyl-acetic acid cyclopentyl ester} (Fig. 1A) is a hydroxamate-containing ester compound closely related to CHR-2797 (tosedostat), a potent inhibitor of a number of intracellular mammalian aminopeptidases. While some hydroxamate-containing compounds are known to be promiscuous and inhibit various families of metalloenzymes, such as histone deacetylases and matrix metalloproteinases, this is not the case for these aminopeptidase inhibitors (13). Tosedostat has been shown to exert antiproliferative effects against a range of human tumor cell lines in vitro and in vivo and has completed phase I/II trials for the treatment of acute myeloid leukemia (AML) and myelodysplasia (13). Both CHR-2863 and CHR-2797 are converted into pharmacologically active charged acid products inside cells by the action of cytoplasmic carboxyesterases (23). In the case of CHR-2863, the ester is converted into (S)-[(R)-2-((S)-hydroxycarbamoyl-methoxy-methyl)-4-methyl-pentanoylamino]-phenyl-acetic-acid (CHR-6768) (Fig. 1A). Here, we show that CHR-2863 and CHR-6768 kill P. falciparum malaria parasites in culture and that oral administration of the former is efficacious against rodent malaria in vivo.
Fig 1.
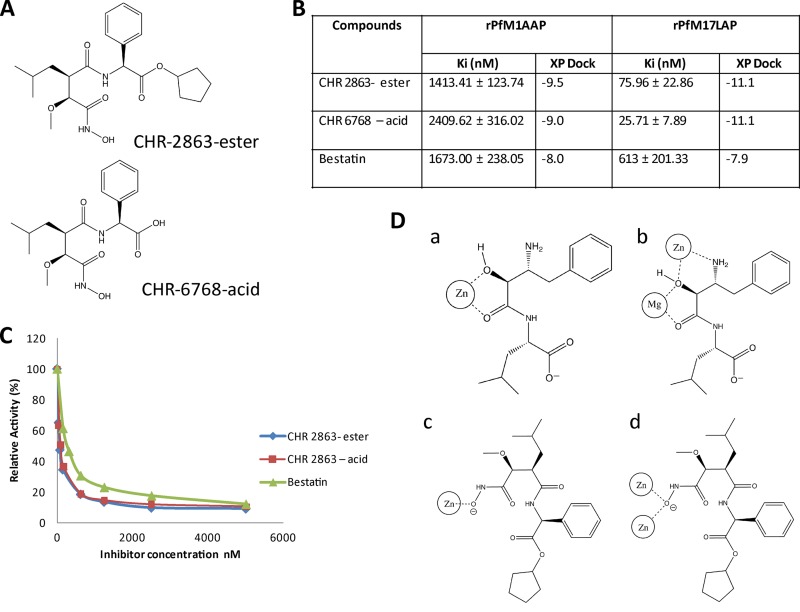
Structure, enzyme kinetics, and binding mode of the novel aminopeptidase inhibitor CHR-2863. (A) CHR-2863 and CHR-6768. (B) Ki values and XP dock scores of CHR-2863 and CHR-6768 compared to those of bestatin when assessed against rPfM1AAP and rPfM17LAP. (C) Inhibition curves of aminopeptidase activity in malaria extracts. The substrate (H-Leu-NHMec) was maintained at 25 μM, while the inhibitor compounds were varied. Soluble malaria extract was prepared as previously reported (21), and 5 μg total proteins was used in each assay. (D) Schematic representations of metal-ligand interaction diagrams for rPfM1AAP and rPfM17LAP illustrating the different cavity binding orientations observed in the solid state for bestatin and the proposed structures for CHR 2863 based on docking analysis. (a) rPfM1AAP and bestatin (derived from X ray data; PDB code 3EBH); (b) rPfM17LAP and bestatin X-ray (derived from PDB code 3KR4); (c) rPfM1AAP docked with CHR-2863; (d) rPfM17LAP docked with CHR-2863.
MATERIALS AND METHODS
Compounds.
The compounds CHR-2863 and CHR-6769 {(S)-[(R)-2-((S)-hydroxycarbamoyl-methoxy-methyl)-4-methyl-pentanoylamino]-phenyl-acetic-acid} were supplied by Chroma Therapeutics Ltd., Abingdon, United Kingdom, and synthesized as previously described (13). Bestatin was purchased from Sigma.
Parasites and parasite extracts.
The P. falciparum chloroquine-sensitive clone 3D7, derived from P. falciparum isolate NF54, and the multidrug-resistant clone K1 were used in all in vitro drug assays. The parasites were cultured as previously described (35). Soluble malaria extracts were prepared as previously reported (34), and 5 μg total protein was used in each assay. The murine malaria species used in in vivo assays was the nonlethal Plasmodium chabaudi chabaudi obtained from frozen stocks and passaged once in female C57BL/6 mice.
Recombinant PfM1AAP and PfM17LAP.
rPfM1AAP and rPfM17LAP were prepared and purified in our laboratory as previously described (21, 34).
Enzyme inhibitor constants.
Enzyme assays employed the fluorogenic peptide substrate H-leucine-7-amido-4-methylcoumarin (H-Leu-NHMec), a substrate that is cleaved efficiently by both PfM1AAP and PfM17LAP. Initial rates were obtained at 37°C using a range of substrate (H-Leu-NHMec) concentrations spanning the enzyme's Km (0.2 to 1,000 μM) and at fixed enzyme concentrations in 50 mM Tris-HCl, pH 7.5. Progress curves were monitored until a final steady-state velocity, Vs, was reached. Ki values were determined from Dixon plots of 1/Vs versus the inhibitor concentration when [S] is much less than Km, as described previously (20, 21, 34).
In silico docking of inhibitors.
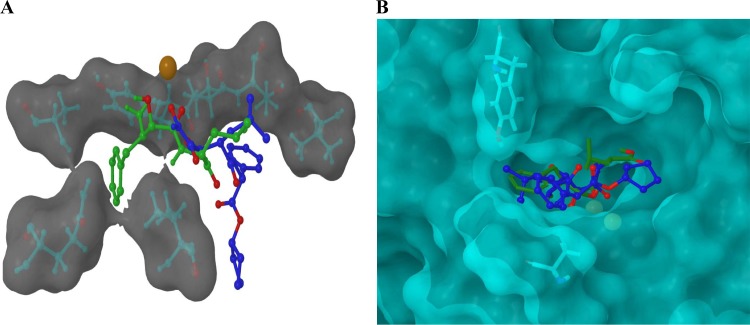
Molecular analysis and docking studies were carried out using the Schrodinger 2011 suite of modeling tools. Docking grids were prepared, using Glide (Fig. 2A) (5, 10), from solid-state structures of unliganded proteins deposited in the RCSB Protein Data Bank (PDB) (http://www.pdb.org) (rPfM1AAP from PDB code 3EBG; rPfM17LAP from PDB code 3KQZ) after using the Schrodinger Protein Preparation Tool (Schrodinger Suite 2011 Protein Preparation Wizard) and an Impref minimization to a converged heavy-atom root mean square (RMS) of 0.3 Å. Bestatin and CHR2863 ligands were prepared using LigPrep (version 2.5; Schrodinger) incorporating Epik metal states (8, 26, 31) at pH 7. Docking runs were carried out using the XP mode of the Glide module.
Fig 2.
(A) The calculated locations of CHR-2863 and bestatin within the binding site of rPfM1AAP obtained by molecular docking studies using Glide. CHR-2863 (blue) is predicted to occupy a region of the substrate-binding cavity different from that observed in both the X-ray-derived solid-state structure and our docking analysis for bestatin (green). Oxygen atoms are highlighted in red, the zinc ion is highlighted in orange/brown and the proximal hydrophobic residues are shown inset into a semitransparent Van der Waals (VDW) molecular surface. (B) The calculated locations of CHR-2863 and bestatin within the binding site of rPfM17LAP obtained by molecular docking studies using Glide. Analysis revealed that the phenyl group of CHR-2863 and the isopropyl group of bestatin occupy the same hydrophobic region of the rPfM17LAP protein, while the phenyl group of CHR- 2863 projects into a region proximal to Tyr493 and Ala388. Oxygen atoms are highlighted in red, the zinc ions are highlighted in yellow, and the proximal residues are shown inset into a semitransparent VDW molecular surface.
Parasite growth and sensitivity to inhibitors.
Serial dilutions of each inhibitor were prepared in culture media and added, with [3H]hypoxanthine (0.5 μCi/well), to asynchronous cultures at a 0.5% parasitemia and 2% hematocrit. The amount of [3H]hypoxanthine incorporated into parasites was measured after an incubation of 48 h. The concentrations of inhibitor required to prevent incorporation by 50% (IC50s) were determined by nonlinear regression analysis (SigmaPlot). Each assay was performed in triplicate on two separate occasions, and the data were pooled and are presented as mean ± standard deviation (SD).
Morphological assessment of inhibitors.
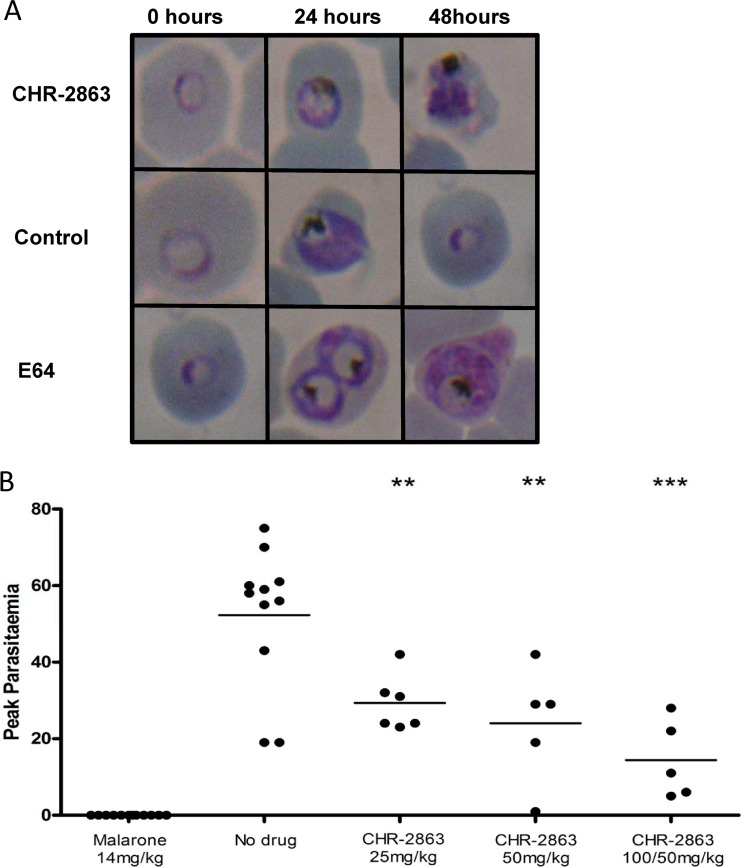
Asynchronous cultures of P. falciparum 3D7 were exposed to CHR-2863 (5 μM). Morphological changes were assessed over 24 and 48 h and compared to those of no-drug controls, together with the well-characterized inhibitor E64 (Fig. 3A). Giemsa-stained blood smears were examined by light microscopy (×1,000 magnification).
Fig 3.
(A) Morphological changes in P. falciparum parasites seen over a 48-h period when treated with 5 μM CHR-2863 compared to an untreated control and a known cysteine protease inhibitor, E64. (B) Mice infected with P. c. chabaudi parasites were dosed by oral gavage with a range of concentrations of CHR-2863. Five groups of mice were used: (i) a no-drug vehicle control group, (ii) a group given one daily dose of 100 mg/kg of CHR-2863 on days 1 and 2 followed by one daily dose of 50 mg/kg on days 3 and 4, (iii) a group given one daily dose of 50 mg/kg of CHR-2863 on days 1 to 4, (iv) a group given one daily dose of 25 mg/kg of CHR-2863 on days 1 to 4, and (v) a group given a daily dose of 14 mg/kg malarone (atovaquone at 10 mg/kg plus proguanil at 4 mg/kg) on days 1 to 3. Each mouse received 1 × 105 P. c. chabaudi parasites intravenously on day 0, and all compounds, including the vehicle control, were administered by oral gavage in a total volume of 200 μl. Dosing of the animals commenced 24 h after inoculation of parasites and was repeated at 24-h intervals. Peak parasitemia corresponds to the percentage of parasitized red blood cells quantified from Giemsa-stained thin films. The doses on the x axis correspond to the amount of CHR-2863 given per day over 4 days in mg/kg. A black horizontal line indicates the mean parasitemia of each of the test and control groups. **, P < 0.01; ***, P < 0.001.
In vivo suppression test.
In vivo activity was assessed using a modified 4-day suppression test (27) employing the P. c. chabaudi murine malaria model in C57/BL6J mice (Fig. 3B). Five groups of mice were used in these experiments, including (i) a no-drug vehicle control group; (ii) a group given one daily dose of 100 mg/kg of body weight of CHR-2863 on days 1 and 2, followed by one daily dose of 50 mg/kg on days 3 and 4; (iii) a group given one daily dose of 50 mg/kg of CHR-2863 on days 1 to 4; (iv) a group given one daily dose of 25 mg/kg of CHR-2863 on days 1 to 4; and (v) a group given a daily dose of 14 mg/kg malarone (atovaquone at 10 mg/kg plus proguanil at 4 mg/kg) on days 1 to 3. Each mouse received 1 × 105 P. c. chabaudi parasites intravenously on day 0, and all compounds, including the vehicle control, were administered by oral gavage in a total volume of 200 μl. Dosing of the animals commenced 24 h after inoculation of parasites and was repeated at 24-h intervals. All procedures used in the assay were ratified by the Queensland Institute of Medical Research Animal Ethics Committee.
RESULTS
CHR-2863 and CHR-6768 are potent inhibitors of malaria aminopeptidases.
Data describing the activities of CHR-2863 (Fig. 1A, ester), CHR-6768 (Fig. 1B, acid), and bestatin against recombinant PfM1AAP and PfM17LAP demonstrate that CHR-2863 is a potent inhibitor of both aminopeptidases (Fig. 1B); it was almost twice as potent (Ki = 1.4 μM) as the acid derivative CHR-6768 (Ki = 2.4 μM) and about equally as potent as bestatin (Ki = 1.6 μM) against the rPfM1AAP enzyme. While both CHR-2863 and CHR-6768 showed far greater potency (20- to 100-fold) against rPfM17LAP than rPfM1AAP, the acid CHR-6768 (Ki = 0.025 μM) exhibited a 3-fold greater inhibition constant than CHR-2863 (Ki = 0.075 μM). CHR-2863 and CHR-6768 were 8- and 24-fold more potent, respectively, against rPfM17LAP than bestatin (Fig. 1B).
Using the peptide H-Leu-NHMec as a substrate, we assessed the relative potencies of CHR-2863, CHR-6768, and bestatin against the aminopeptidase activity in freeze-thaw soluble extracts of P. falciparum parasites (34). The inhibition curves presented (Fig. 1C) were performed with 25 μM substrate. Both CH-2863 and CHR-6768 are more potent inhibitors of aminopeptidase activity in malaria extracts than bestatin. These results are consistent with data generated with purified recombinant enzymes (Fig. 1B). Since the precise concentrations of native PfM1AAP and PfM17LAP in these extracts are not known, we determined the concentrations of inhibitor that inhibited 50% of the total enzyme activity in these samples: 75 nM, 78 nM, and 300 nM for the CHR-2863, CHR-6768, and bestatin, respectively.
In silico modeling identifies a different binding modality of CHR-2863 than of bestatin.
Analysis of solid-state data within the protein structural data bank (http://www.rcsb.org) on several hydroxamic acid-based structures similar to CHR-2863 that bind to zinc metalloproteins suggested that compounds of this type chelate the metal ion via the terminal hydroxamic acid regions of the molecules. In conjunction with the PDB analysis outlined above, we used computer simulation and docking techniques to formulate a molecular interpretation of how CHR-2863 inhibits rPfM1AAP and rPfM17LAP. This analysis was implemented by modeling the ligand structure into the unliganded active site of the structures of the relevant malaria enzymes previously determined by us (20, 21). These analyses predicted that compounds such as CHR-2863 bind strongly to the active sites of these enzymes and also that they may adopt orientations within the binding cavities of rPfM1AAP and rPfM17LAP different from that reported by us for bestatin (20, 21). In both the rPfM1AAP and rPfM17LAP proteins, CHR-2863 interacts via a monoanionic binding mode that exists between the terminal hydroxamic region of the chelator and the metal ion(s) of the protein. This is in contrast to the bestatin mode of binding observed in the solid state of both rPfM1AAP and rPfM17LAP, in which the metal ion(s) of the protein interacts with the center region of the bestatin proximal to the hydroxyl methylene group (Fig. 1D). Changes are also observed in the overall orientation of the molecules within the binding cavity distal to the metal center. For example, in rPfM1AAP, both the docking simulation and solid-state X-ray data (obtained from PDB code 3EBH) reveal that the phenyl ring of bestatin is situated within a hydrophobic cavity formed by amino acid residues Tyr575, Ala320, Gln317, and Val459. Other bestatin-protein noncovalent contacts correlate well with that observed in the solid-state structure, although in the simulation, the bestatin amide NH-Cα bond is rotated by 180° to that observed in the X-ray data. In contrast, the CHR-2863 simulation locates the bulk of the molecule in a region of the protein different from that occupied by bestatin, so that the hydrophobic phenyl group of CHR-2863 is located in a hydrophobic region described by Val550, Thr576, and Thr577. This could be attributable to the increased molecular volume of CHR-2863 over bestatin (∼370 Å3 versus ∼310 Å3) (Fig. 2A).
Differences were also noted between CHR-2863 and bestatin in the rPfM17LAP system. In this data set, the docked bestatin molecule once again occupied a position within the protein cavity similar to that observed in the bestatin-liganded solid-state structure (obtained from PDB code 3KR4). For example, the phenyl region of bestatin is located in a hydrophobic area bounded by Leu492, Ala577, Phe398, and Met396 in both the simulation and the X-ray solid-state structure. Interestingly, the simulation again rotated the bestatin amide NH-Cα bond 180° to that observed in the X-ray data. While the larger CHR-2863 molecule was slightly removed to the region occupied by bestatin, it was much less pronounced than in the rPfM1AAP system. Further analysis revealed that the hydrophobic phenyl group of CHR-2863 and the isopropyl group of bestatin occupy the same hydrophobic region of the rPfM17LAP protein while the phenyl group of CHR-2863 projects into an region adjacent to that normally occupied by bestatin, proximal to Tyr493 and Ala388 (Fig. 2B).
CHR-2863 is a more potent in vitro inhibitor of P. falciparum than bestatin.
We investigated the potential antimalarial activity of CHR-2863 and the corresponding acid CHR-6768. This was first examined in vitro against P. falciparum 3D7 using [3H]hypoxathanine incorporation as a measure of parasite growth, In these assays, bestatin exhibited an IC50 of 5 μM, within the range we have previously reported (32). The in vitro antimalarial potency of the CHR-2863 ester (IC50, 370 nM) was 5-fold greater than that of the acid CHR-6768 (IC50, 2 μM). A similar antimalarial potency (IC50, 376 nM) was obtained when the CHR-2863 ester was tested against the P. falciparum multidrug-resistant clone K1.
CHR-2863 induces vacuolization in P. falciparum parasites.
Analysis of parasite morphology after treatment with CHR-2863 demonstrated that, similar to bestatin, CHR-2863 induces a slow-growth phenotype with some vacuolization. In contrast, the well-characterized cysteine protease inhibitor E64, which is believed to specifically target cysteine proteases (falcipains) within the P. falciparum digestive vacuole, caused significant swelling of this organelle (Fig. 2A). Whether the effect of CHR-2863 was due to inhibition of both PfM17LAP and PfM1AAP or of one specific enzyme cannot be realistically determined from these observations, since the compound inhibits both enzymes.
CHR-2863 shows antimalarial activity and is orally bioavailable.
The clinical significance of the antimalarial activity demonstrated by CHR-2863 was examined in vivo. Using a modified 4-day suppression test, we assessed the treatment of mice infected with the murine malaria species P. c. chabaudi. Treatment with CHR-2863 significantly reduced the peak parasitemia in all groups compared to the vehicle control group, with higher doses showing an increased effect on parasite growth (Fig. 2B).
DISCUSSION
To support its rapid growth in vivo, P. falciparum must acquire nutrients from the host cell and the extracellular environment. Digestion of host cell hemoglobin is essential for the parasite to acquire sufficient amino acids to facilitate parasite anabolism. We and others have previously shown that the two P. falciparum aminopeptidases PfM1AAP and PfM17LAP are essential to this process, and while there is some overlapping substrate specificity between the enzymes, they both appear to be essential for parasite viability, indicating that they may play different roles in the life cycle of the parasite (1, 11).
CHR-2863 is a hydroxamate-containing ester compound closely related to CHR-2797 (tosedostat), itself a potent inhibitor of a number of intracellular mammalian aminopeptidases. In order to assess whether CHR-2863 could inhibit P. falciparum aminopeptidases, we determined the inhibitor constants of the compound and its acid derivative against recombinant PfM1AAP and PfM17LAP. In both cases, these compounds showed significantly increased activity against PfM17LAP compared to bestatin, with Kis in the low nanomolar range with an almost 10-fold increase in inhibitory activity. The figures for the Glide dock scores suggested that CHR-2863 would be an inhibitor comparable to, if not better than, bestatin, and the values correlated well with the observed inhibitory data (Fig. 1B). This increase in inhibitory activity, however, was not repeated when the two compounds were assessed against rPfM1AAP. In this case, both inhibitors were in the range observed for bestatin, the low μM range. While the substrate specificity of PfM1AAP is much broader than that of PfM17LAP, most inhibitors of these enzymes appear to target PfM17LAP preferentially to PfM1AAP, except for a series recently described by Harbut et al. (11), where they extensively modified the bestatin scaffold in an attempt to create specific inhibitors for both enzymes.
We previously reported, using the murine malaria model P. c. chabaudi, that the aminopeptidase inhibitors bestatin and hPheP[CH2]Phe exhibited in vivo antimalarial activity and thus provided proof of concept for aminopeptidases being considered targets for antimalaria drug development (32). Given the apparent higher activity of the ester derivative (CHR-2863) than of the acid (CHR-6768) in an in vitro P. falciparum assay (IC50, 370 nM vs 2 μM), we assessed only the antimalarial activity of CHR-2863 in vivo using the rodent P. c. chabaudi model. A treatment dose of 25 mg/kg given only once a day reduced the parasite burdens in treated mice significantly compared to controls, and further dose increments showed a dose-dependent reduction in the parasite burden in the treated groups (Fig. 2). These data provide further evidence of the successful use of aminopeptidase inhibitors as antimalarial agents by demonstrating for the first time that oral administration of CHR-2863 reduces parasite burdens in vivo (Fig. 2).
Drug resistance is such a major problem for the control and treatment of malaria that novel targets and lead compounds for the development of new drugs are in continual demand (25). This study establishes the possibility that PfM17LAP and PfM1AAP could become antimalarial drug targets by demonstrating a clear relationship between the ability of inhibitors to inactivate the enzymes and to kill malaria parasites in vitro and in vivo. The compounds CHR-2863 and CHR-6768 are some of the most potent inhibitors of M17 LAPs reported to date (9, 11). Their potency makes them attractive lead compounds for the development of antimalaria therapeutic agents. The present studies show that CHR-2863 has exhibited in vivo antimalarial activity when dosed orally at 25 mg/kg once daily. These data demonstrate that it is now possible to target malaria aminopeptidases by oral administration of inhibitors, an important consideration, since bioavailability is a prerequisite for the future progression of antimalaria drugs into the development pipeline. Previous phase I/II studies with a similar compound, tosedostat (60 mg to 180 mg for 28 days), have shown that it is one of only a few orally delivered monotherapy agents that have shown promising activity and good tolerability in elderly and/or refractory or relapsed AML and myelodysplasia patients (17). Bestatin is currently used as a maintenance treatment for patients with acute nonlymphocytic leukemia who are in complete remission after chemotherapy as a result of randomized trials for such patients. An oral treatment of 30 mg daily for 2 years has been shown to be safe in over 2,000 patients, with only infrequent and mild adverse reactions (hepatic dysfunction, skin reaction, or gastrointestinal toxicity, including nausea, vomiting, and anorexia). Thus, there is a precedent for using aminopeptidase inhibitors for disease prevention in humans and evidence that they have few side effects (12). Nonetheless, the toxicity of any new antimalarial drug needs to be investigated fully prior to human experimentation.
ACKNOWLEDGMENTS
C.L.P. was supported by an Australian Postgraduate award. D.L.G. is the recipient of an NHMRC Career Development Award. The work outlined was supported in part by NHMRC grant no. 571917 to D.L.G. and J.P.D. T.S.A. and K.R.T. acknowledge the support of the Australian NHMRC. J.P.D. holds the Canada Research Chair in Infectious Diseases, and this work was supported in part by a Discovery Project funded by the Canada Institute of Health Research (CIHR).
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Dalal S, Klemba M. 2007. Roles for two aminopeptidases in vacuolar hemoglobin catabolism in Plasmodium falciparum. J. Biol. Chem. 282:35978–35987 [DOI] [PubMed] [Google Scholar]
- 2. Dev V, Phookan S, Sharma VP, Dash AP, Anand SP. 2006. Malaria parasite burden and treatment seeking behavior in ethnic communities of Assam, Northeastern India. J. Infect. 52:131–139 [DOI] [PubMed] [Google Scholar]
- 3. Enserink M. 2008. Epidemiology. Lower malaria numbers reflect better estimates and a glimmer of hope. Science 321:1620. [DOI] [PubMed] [Google Scholar]
- 4. Ersmark K, et al. 2004. Potent inhibitors of the Plasmodium falciparum enzymes plasmepsin I and II devoid of cathepsin D inhibitory activity. J. Med. Chem. 47:110–122 [DOI] [PubMed] [Google Scholar]
- 5. Friesner RA, et al. 2004. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 47:1739–1749 [DOI] [PubMed] [Google Scholar]
- 6. Gardiner DL, Trenholme KR, Skinner-Adams TS, Stack CM, Dalton JP. 2006. Overexpression of leucyl aminopeptidase in Plasmodium falciparum parasites. Target for the antimalarial activity of bestatin. J. Biol. Chem. 281:1741–1745 [DOI] [PubMed] [Google Scholar]
- 7. Gavigan CS, Machado SG, Dalton JP, Bell A. 2001. Analysis of antimalarial synergy between bestatin and endoprotease inhibitors using statistical response-surface modelling. Antimicrob. Agents Chemother. 45:3175–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenwood JR, Calkins D, Sullivan AP, Shelley JC. 2010. Towards the comprehensive, rapid, and accurate prediction of the favorable tautomeric states of drug-like molecules in aqueous solution. J. Comput. Aided Mol. Des. 24:591–604 [DOI] [PubMed] [Google Scholar]
- 9. Grembecka J, Mucha A, Cierpicki T, Kafarski P. 2003. The most potent organophosphorus inhibitors of leucine aminopeptidase. Structure-based design, chemistry, and activity. J. Med. Chem. 46:2641–2655 [DOI] [PubMed] [Google Scholar]
- 10. Halgren TA, et al. 2004. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 47:1750–1759 [DOI] [PubMed] [Google Scholar]
- 11. Harbut MB, et al. 2011. Bestatin-based chemical biology strategy reveals distinct roles for malaria M1- and M17-family aminopeptidases. Proc. Natl. Acad. Sci. U. S. A. 108:E526–E534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ichinose Y, et al. 2003. Randomized double-blind placebo-controlled trial of bestatin in patients with resected stage I squamous-cell lung carcinoma. J. Natl. Cancer Inst. 95:605–610 [DOI] [PubMed] [Google Scholar]
- 13. Krige D, et al. 2008. CHR-2797: an antiproliferative aminopeptidase inhibitor that leads to amino acid deprivation in human leukemic cells. Cancer Res. 68:6669–6679 [DOI] [PubMed] [Google Scholar]
- 14. Lew VL, Macdonald L, Ginsburg H, Krugliak M, Tiffert T. 2004. Excess haemoglobin digestion by malaria parasites: a strategy to prevent premature host cell lysis. Blood Cells Mol. Dis. 32:353–359 [DOI] [PubMed] [Google Scholar]
- 15. Li X, et al. 2009. Plasmodium falciparum signal peptide peptidase is a promising drug target against blood stage malaria. Biochem. Biophys. Res. Commun. 380:454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li X, Chen H, Jeong JJ, Chishti AH. 2007. BDA-410: a novel synthetic calpain inhibitor active against blood stage malaria. Mol. Biochem. Parasitol. 155:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lowenberg B, et al. 2010. Phase I/II clinical study of Tosedostat, an inhibitor of aminopeptidases, in patients with acute myeloid leukemia and myelodysplasia. J. Clin. Oncol. 28:4333–4338 [DOI] [PubMed] [Google Scholar]
- 18. Luksch T, et al. 2008. Computer-aided design and synthesis of nonpeptidic plasmepsin II and IV inhibitors. ChemMedChem 3:1323–1336 [DOI] [PubMed] [Google Scholar]
- 19. Marks F, et al. 2005. Parasitological rebound effect and emergence of pyrimethamine resistance in Plasmodium falciparum after single-dose sulfadoxine-pyrimethamine. J. Infect. Dis. 192:1962–1965 [DOI] [PubMed] [Google Scholar]
- 20. McGowan S, et al. 2010. Structure of the Plasmodium falciparum M17 aminopeptidase and significance for the design of drugs targeting the neutral exopeptidases. Proc. Natl. Acad. Sci. U. S. A. 107:2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGowan S, et al. 2009. Structural basis for the inhibition of the essential Plasmodium falciparum M1 neutral aminopeptidase. Proc. Natl. Acad. Sci. U. S. A. 106:2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mugittu K, et al. 2004. Therapeutic efficacy of sulfadoxine-pyrimethamine and prevalence of resistance markers in Tanzania prior to revision of malaria treatment policy: Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase mutations in monitoring in vivo resistance. Am. J. Trop. Med. Hyg. 71:696–702 [PubMed] [Google Scholar]
- 23. Noedl H, et al. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620 [DOI] [PubMed] [Google Scholar]
- 24. Nosten F, et al. 2004. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends Parasitol. 20:425–432 [DOI] [PubMed] [Google Scholar]
- 25. Nwaka S, Hudson A. 2006. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 5:941–955 [DOI] [PubMed] [Google Scholar]
- 26. Park MS, Gao C, Stern HA. 2011. Estimating binding affinities by docking/scoring methods using variable protonation states. Proteins 79:304–314 [DOI] [PubMed] [Google Scholar]
- 27. Peters WRB. 1999. Handbook of animal models of infection. Academic Press, London, United Kingdom [Google Scholar]
- 28. Rogers WO, et al. 2009. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar. J. 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosenthal PJ. 2002. Hydrolysis of erythrocyte proteins by proteases of malaria parasites. Curr. Opin. Hematol. 9:140–145 [DOI] [PubMed] [Google Scholar]
- 30. Schlitzer M. 2007. Malaria chemotherapeutics part I: History of antimalarial drug development, currently used therapeutics, and drugs in clinical development. ChemMedChem 2:944–986 [DOI] [PubMed] [Google Scholar]
- 31. Shelley JC, et al. 2007. Epik: a software program for pK(a) prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 21:681–691 [DOI] [PubMed] [Google Scholar]
- 32. Skinner-Adams TS, et al. 2007. Identification of phosphinate dipeptide analog inhibitors directed against the Plasmodium falciparum M17 leucine aminopeptidase as lead antimalarial compounds. J. Med. Chem. 50:6024–6031 [DOI] [PubMed] [Google Scholar]
- 33. Skinner-Adams TS, et al. 2010. Plasmodium falciparum neutral aminopeptidases: new targets for antimalarials. Trends Biochem. Sci. 35:53–61 [DOI] [PubMed] [Google Scholar]
- 34. Stack CM, et al. 2007. Characterization of the Plasmodium falciparum M17 leucyl aminopeptidase. A protease involved in amino acid regulation with potential for antimalarial drug development. J. Biol. Chem. 282:2069–2080 [DOI] [PubMed] [Google Scholar]
- 35. Trager W, Jensen JB. 1976. Human malaria parasites in continuous culture. Science 193:673–675 [DOI] [PubMed] [Google Scholar]
- 36. Uhlemann AC, Krishna S. 2005. Antimalarial multi-drug resistance in Asia: mechanisms and assessment. Curr. Top. Microbiol. Immunol. 295:39–53 [DOI] [PubMed] [Google Scholar]