Abstract
A combination of drugs in experimental chemotherapy of Chagas' disease may increase the effectiveness of treatment. To evaluate the possible mechanisms that influence the improvement of therapy, we investigated the pharmacokinetic interaction between benznidazole and itraconazole in a murine model treated orally with single doses of 5 mg of each compound separately or together. Blood samples from treated mice were collected at different intervals for 48 h, and a high-performance liquid chromatography (HPLC)-UV method was used to quantify both drugs in the plasma. A decrease of 1.5-fold in the maximum drug concentration in the plasma (Cmax) and an increase of 2.66-fold in the volume of distribution (V) and 7.5-fold in the elimination half-life (t1/2β) of benznidazole when coadministered with itraconazole were observed. The parameters area under the curve (AUC0-t), area under the curve extrapolated to infinity (AUC0-∞), time to maximum concentration of drug in serum (Tmax), and clearance (CL) for benznidazole were not significantly different in this therapeutic regime. None of the evaluated parameters for ITC demonstrated a significant difference between isolated and associated administration. These results suggest that the main effect of this interaction leads to accumulation of benznidazole in the biological system. This effect may contribute to the improved therapeutic efficacy of this combination of drugs, in addition to synergism of the different mechanisms of action of benznidazole and itraconazole against Trypanosoma cruzi in vivo.
INTRODUCTION
Chagas' disease is also known as American trypanosomiasis, a potentially severe illness caused by the protozoan parasite Trypanosoma cruzi, present mainly in Latin America, where it is typically transmitted to humans by the feces and/or urine of infected triatomine bugs.
The disease has spread to other countries on different continents by blood transfusion and vertical transmission. It is estimated that approximately 10 million people are infected worldwide, mostly in Latin America, where Chagas' disease is endemic. Three million are in Brazil. In 2008, Chagas' disease killed >10,000 people, and more than 25 million are at risk of infection and could develop the disease if they do not receive effective treatment (29).
The search for an appropriate therapy for treatment of Chagas' disease has been a challenge since its discovery. Benznidazole (BNZ), a nitro compound and current specific chemotherapy for Chagas' disease empirically introduced for clinical use in the early 1970s, is considered far from ideal due to multiple side effects and limited efficacy. These limitations may be related to unfavorable pharmacokinetic properties, such as a relatively short half-life and limited tissue penetration (28), which limit its action in the chronic phase when the parasites are mostly confined to deep tissues in which replication occurs (24, 25).
Itraconazole (ITC), a triazole specific inhibitor of fungal sterol C-14α-dimethylase, has a suppressive effect against T. cruzi infections in human and experimental animals, with the additional advantage of few side effects. However, the compound does not cure the infection or interrupt disease progression (25) as a single therapy.
Some studies have shown that new therapies, including combinations of drugs with different mechanisms of action, may improve the effectiveness of treatment and/or reduce the intensity of adverse reactions (17, 23). Antifungal triazoles, such as ketoconazole, when associated with BNZ, produce a positive synergistic effect in experimental infection with T. cruzi (3). New strategies for Chagas' disease treatment using drug associations have been suggested, including nifurtimox or benznidazole in combination with other ergosterol pathway inhibitors (fluconazole or itraconazole) (6).
To elucidate the possible differences in the effectiveness of treatment resulting from the association of BNZ with ITC in experimental chemotherapy of Chagas' disease, this work aimed to monitor and compare the pharmacokinetic profiles of both drugs in a murine model when used alone or in combination.
Since pharmacokinetic studies require the existence of a precise and accurate bioanalytical assay, a method involving high-performance liquid chromatography coupled with a UV detector (HPLC-UV) for simultaneous quantification of BNZ and ITC in the plasma of mice was developed and validated.
This study aims to analyze and understand the pharmacokinetic parameters possibly responsible for the improved therapeutic efficacy of this association and to contribute to more clearly defining treatment regimens involving drug association in Chagas' disease.
MATERIALS AND METHODS
Chemicals and reagents.
The analytical standards benznidazole (N-benzyl-2-nitro-1-imidazole acetamide [97.0%]) and itraconazole {(±)-1-sec-butyl-4-[p-[4-[p[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-lmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl-D2-1,2,4-triazolin-5-one (98.0%)} and the internal standard (IS) diazepam (98.0%) were purchased from Sigma-Aldrich (St. Louis, MO). HPLC grade acetonitrile was obtained from Tedia (Rio de Janeiro, Brazil) and analytical grade reagents from Merck (Rio de Janeiro, Brazil).
Benznidazole tablets (Rochagan; Roche) and itraconazole capsules (Sporanox; Janssen-Cilag) were commercially purchased (Belo Horizonte, Brazil). Ultrapure water was produced in the laboratory by distillation, followed by purification with a MilliQ Simplicity (Millipore) system.
Analytical method.
BNZ and ITC were dissolved in dimethyl sulfoxide (DMSO) to prepare stock solutions, both at 3,000 μg/ml. The IS stock solution was prepared under similar conditions at 1,800 μg/ml. Work solutions obtained from dilutions of the stock solutions were stable when prepared in DMSO and were stored in polypropylene tubes at −20°C.
To prepare the standard calibration in plasma and the quality control in low, medium, and high concentrations, 10 μl of IS stock solution and 10 μl of each working solution of BNZ and ITC were spiked with 270 μl of blank plasma to final concentrations of 60 μg/ml for IS, 0.5 to 100.0 μg/ml for BNZ, and 1.0 to 75.0 μg/ml for ITC. The calibration curves were plotted, including the peak areas of both drugs versus the expected concentrations, and the linear regression was calculated by the minimum-square method.
The simultaneous measurement of BNZ and ITC in the plasma of mice was previously validated according to the U.S. FDA bioanalytical method validation guidelines (10, 18).
Chromatographic conditions.
The HPLC chromatographic system used consisted of a Waters Alliance E2695 separation module (Waters, Manchester, United Kingdom) fitted with a Waters 2489 UV detector. The chromatographic separation of BNZ and ITC was performed using a C18 analytical column (4 μm; 150 mm by 4.6 mm) and a C18 precolumn (3 μm; 2 mm by 4.6 mm) (Gemini-NX Phenomenex) to protect the column from residual particles. The mobile phase was a mixture (60:40 [vol/vol]) of acetonitrile and water, pumped isocratically at 1 ml/min. The UV wavelengths selected to quantify BNZ and ITC were 324 and 263 nm, respectively, at a temperature of 40°C and an injection volume of 25 μl.
Animals.
Female Swiss albino mice, 4 months old and weighing 45 to 50 g, were used and maintained according to the guidelines established by the Brazilian College of Animal Experimentation (COBEA), kept in a controlled room with regular alternating cycles of light and dark at a temperature of 23 ± 2°C, and given food and water at libitum. The experiments were approved by the Ethical Committee on Animal Experimentation of the Universidade Federal de Ouro Preto, Brazil (protocol no. 2010/08).
Treatment schedule and experimental design.
The experiment consisted of three groups: animals treated with BNZ, animals treated with ITC, and animals treated with both drugs. All mice received a single dose of 5 mg of ITC in a homogeneous suspension in water and/or BNZ in a homogeneous suspension in gum arabic, both administered by gavage.
To determine drug levels in the plasma of mice, heparinized polypropylene tubes were used for collection of approximately 600 μl of blood obtained from the orbital sinuses of the animals at selected times after treatment.
Considering that this study was carried out in a small experimental model, a single animal had to be used for each sampling. Thus, treatment and sample collection were performed in quintuplicate in the three experimental groups. The sampling times for construction of the pharmacokinetic curves were 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 24, 36, and 48 h.
Sample preparation and drug extraction.
Plasma was separated from blood samples by centrifugation (900 × g) and stored at −20°C for later quantification of the drugs by HPLC.
The IS (10 μl; 60 μM) and 20 μl of DMSO were added to 270 μl of each mouse plasma sample to get the same amount of solvent in the calibration curves. The drugs were extracted from the plasma by liquid-liquid extraction with addition of 1 ml of ethyl ether to the samples, vortexing for 20 min, and centrifugation at 1,500 × g for 10 min. The organic phase was collected and filtered through a Millipore membrane (13 mm by 0.45 μm). This procedure was repeated one time, and the resultant filtrate was evaporated to dryness under a nitrogen stream. The dried residue was reconstituted with 300 μl of mobile phase and vortexed for 1 min. The supernatant was transferred to a 300-μl vial insert, and a volume of 25 μl was injected into the chromatographic system.
Pharmacokinetics and statistical analysis.
The study was carried out comparing the pharmacokinetic profiles of both drugs employed separately and in combination under the same conditions. Possible interference between the drugs in the processes of absorption, bioavailability, and metabolism were checked. To fit the concentration of drugs, it was assumed that the total volume of plasma from each animal corresponds to 4.9% of its body weight (4).
We used a noncompartmental pharmacokinetic analysis in this investigation. This independent model, based on the application of the statistical-moment theory for the analysis of the concentration-time curves, was used to obtain its representative parameters. The rate of absorption of the drugs was determined by estimation of its maximum concentration in plasma (Cmax) and the time spent in reaching it (Tmax). The values were read directly from data on the mean of the plasma curves. The amounts of drugs absorbed after oral administration were determined by calculating the area under the curve (AUC0-t) and the area under the first-moment curve (AUMC0-t) by the trapezoidal rule to the last measurable concentration. The area under the curve extrapolated to infinity (AUC0-∞) was calculated as AUC0-t + Ct/λz, where Ct is the last measurable concentration and λz is the elimination rate constant, and the value of the AUMC extrapolated to infinity (AUMC0-∞) was calculated as AUMC0-t + (Ct × t/λz) + (Ct/λz2) obtained as the slope of linear regression of the log-transformed concentration values versus time in the terminal phase. The mean residence time (MRT), clearance (CL), and volume of distribution (V) were calculated as follows: MRT = AUMC0-∞/AUC0-∞, CL = dose/AUC, and V = MRT × CL. The λz value was obtained as the slope of linear regression of the log-transformed concentration values versus time in the terminal phase. The half-life of elimination (t1/2β) was determined as ln2/λz.
The pharmacokinetic parameters in each experimental group were expressed as means ± standard errors (SE), and significant differences of the mean values were assessed by analysis of variance (ANOVA) using Graph Pad Prism 5.01 software. The level of significance was set at a P value of <0.05 and 95% confidence interval.
RESULTS
Analytical method.
The method developed for the simultaneous quantification of BNZ and ITC in mouse plasma was validated and shown to be specific, with no interference from the plasma constituents, and linear, with correlation coefficients of >0.99 in the concentration range that cover the limits of quantification, 0.5 μg/ml for BNZ and 1.0 μg/ml for ITC. The method showed good reproducibility, with precision within the acceptable limits of the coefficient of variation and accuracy according to FDA criteria. The extraction procedure showed adequate recovery of the analytes (Table 1).
Table 1.
Validation parameters of the HPLC-UV method developed for simultaneous quantification of BNZ and ITC in plasma of mice (mean, 5 repetitions)
| Validation parameter | Value |
|
|---|---|---|
| BNZ | ITC | |
| Sensitivity | ||
| Limit of quantification (μg/ml) | 0.5 | 1.0 |
| Linearity | ||
| Correlation coefficient | 0.9985 | 0.9924 |
| Mean regression equation | y = 0.0522x + 0.0229 | y = 0.0128x + 0.0111 |
| Reproducibility | ||
| Mean accuracy (%)b | 96.67 | 93.37 |
| Mean precision (RSD) (%)a | 3.87 | 5.43 |
| Recovery | ||
| Mean recovery (%) | 92.46 | 93.92 |
Mean precision of intra- and interday analyses; RSD, relative standard deviation.
Mean accuracy of intra- and interday analyses.
Pharmacokinetic assay.
The effect of simultaneous administration of BNZ and ITC was studied in a single oral dose of 5 mg for both compounds. When BNZ was employed as single therapy, it was possible to detect its concentration in the plasma of mice for 12 h after administration with a peak concentration of 151.3 μg/kg (Table 2). The elimination half-life (t1/2β) for BNZ was determined as 1.6 h, and the values of AUC0-t (t = 12 h) and AUC0-∞ were 539.1 and 547.5 μg · h/ml, respectively.
Table 2.
Pharmacokinetic parameters of BNZ and ITC administered alone and in association in mice after a single oral dose of 5 mg of each drug
| Parameter | Valuea when administered: |
|||
|---|---|---|---|---|
| Alone |
In association |
|||
| BNZ | ITC | BNZ | ITC | |
| Cmax (μg/kg) | 151.28 ± 6.03b | 11.76 ± 1.33 | 101.19 ± 5.10b | 10.55 ± 0.96 |
| Tmax (h) | 2.2 ± 0.2 | 4.6 ± 0.24 | 2.4 ± 0.24 | 5.0 ± 0.32 |
| AUC0-t (μg · h/ml) | 539.14 ± 14.54 | 65.93 ± 2.99 | 612.33 ± 50.87 | 77.45 ± 9.17 |
| AUC0-∞ (μg · h/ml) | 547.53 ± 11.96 | 79.51 ± 4.69 | 673.90 ± 64.24 | 94.21 ± 12.61 |
| AUMC0-t (μg · h2/ml) | 2,635.39 ± 65.66b | 526.67 ± 41.65 | 7,465.97 ± 1,345.0b | 949.05 ± 231.0 |
| AUMC0-∞ (μg · h2/ml) | 2,757.49 ± 86.19b | 753.16 ± 74.53 | 10,817.19 ± 2,112.0b | 1,377.09 ± 386.6 |
| MRT (h) | 5.05 ± 0.22b | 9.38 ± 0.42 | 15.47 ± 1.7b | 13.52 ± 2.17 |
| CL (ml/h/kg) | 416.74 ± 10.92 | 3,417.30 ± 149.50 | 375.2 ± 28.9 | 3,077.97 ± 404.4 |
| V (ml/kg) | 2,112.33 ± 136.90b | 31,870.60 ± 970.80 | 5,621.29 ± 209.5b | 38,710.66 ± 2,604.0 |
| λz (1/h) | 0.51 ± 0.09b | 0.23 ± 0.04 | 0.05 ± 0.00b | 0.21 ± 0.04 |
| t½β (h) | 1.64 ± 0.41b | 3.39 ± 0.47 | 12.18 ± 0.78b | 3.89 ± 0.79 |
Mean ± SE of five experiments.
Significant differences in each experimental group at P < 0.05.
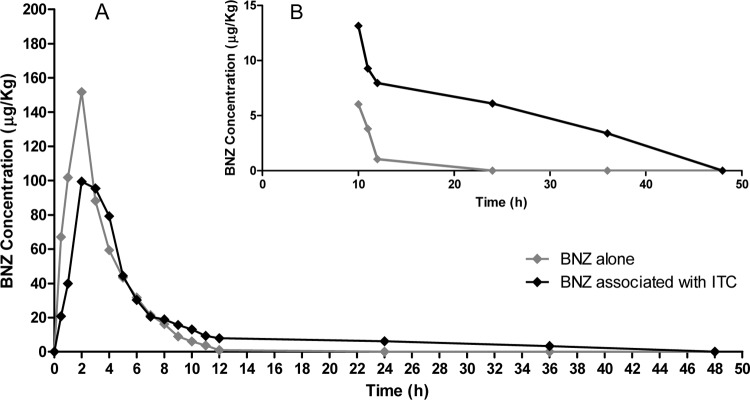
In coadministration with ITC, there was a significant decrease in the Cmax of BNZ from 151.3 to 101.2 μg/kg (1.5-fold; P < 0.0032) and increases in the AUMC0-t from 2,635.39 to 7,465.97 μg · h2/ml (2.83-fold; P < 0.0001), in the AUMC0-∞ from 2,757.49 to 10,817.19 μg · h2/ml (3.92-fold; P < 0.0001), in the MRT from 5.05 to 15.47 h (3.06-fold; P = 0.0006), in V from 2,112.33 to 5,621.29 ml/kg (2.66-fold; P = 0.0001), and in the t1/2β for BNZ from 1.6 to 12.2 h (7.5-fold; P < 0.0001). The values for AUC0-t, AUC0-∞, and Tmax did not significantly change. The mean of five time-concentration curves of BNZ in the presence or absence of ITC in mouse plasma are shown in Fig. 1A, which highlights the final portion of this curve, illustrating the prolonged half-life of BNZ as an effect of its interaction with ITC (Fig. 1B).
Fig 1.
(A) Mean plasma concentration profiles of BNZ administered alone and in association with ITC in mice (n = 5) after a single oral dose of 5 mg of each drug. (B) Highlight of the final portion of the curve displayed in panel A.
However, BNZ had no effect on the pharmacokinetics of ITC. There was a slight increase in the AUC0-t and AUC0-∞ for ITC in coadministration and a slight decrease in the Cmax for ITC compared with single treatment, but these differences were not statistically significant (Table 2). The mean concentration-time profiles in mouse plasma following the administration of ITC alone and in association with BNZ are given in Fig. 2.
Fig 2.
Mean plasma concentration profiles of ITC administered alone and in association with BNZ in mice (n = 5) after a single oral dose of 5 mg of each drug.
DISCUSSION
This study was carried out to evaluate in a murine model the effects of simultaneous administration of two drugs previously used separately in experimental chemotherapy of Chagas' disease. The results show that the coadministration of BNZ and ITC significantly decreases the Cmax and increases the MRT, V, and t1/2β of BNZ in the plasma of mice.
BNZ pharmacokinetics is characterized as being linear with rapid oral absorption (21, 22). The decrease in the Cmax of BNZ observed in this study (33.1%) when administered together with ITC could be related to several factors that influence the process of drug absorption, including the physiological conditions of the animals or possible changes in the biopharmaceutical characteristics of BNZ when administered in association with ITC. Since the limiting step for absorption of BNZ is its dissolution in vivo (15), alteration of doses and/or excipients can lead to a decrease in its rate of absorption (1).
Independent of the compartmental pharmacokinetic model, V was calculated as a relationship between the MRT and CL, both obtained with trapezoidal area calculations. The total clearance of a drug corresponds to the sum of all clearances that contribute to its elimination from the body and can be estimated directly from the plasma drug concentration-time curve, using the relationship between dose and AUC.
Since the AUC values of BNZ were increased when administered in combination with ITC, but not statistically significantly, the CL showed a slight decrease. However, the calculation of the statistical-moment AUMC, obtained by plotting concentration-time versus time, and the related parameter MRT increased considerably (3.92- and 3.06-fold, respectively). Consequently, the increase in V (2.66-fold) indicates changes in the balance of BNZ rates from plasma and tissues. The largest amount of BNZ distributed beyond the plasma in the associated therapy with ITC may also explain the decrease in Cmax observed after absorption (11).
In the case of ITC, its pharmacokinetics is influenced by its low solubility in water, approximately 100,000-fold less soluble than BNZ in neutral pH, which limits both the speed and level of absorption (16, 19, 22). The lower AUC values for ITC in this study are possibly a consequence of this fact.
BNZ is extensively metabolized to compounds involved in the trypanocidal effect. This activation to reactive metabolites requires enzymatic reduction of the nitro group to 2-amino-imidazole and 2-hydroxy-imidazole, and these processes are fundamentally mediated by cytochrome P450 (CYP) reductase and CYP (22, 28).
Likewise, ITC is mainly metabolized in the liver by CYP enzymes (13), and the majority of drug-drug interactions with azole antifungals are caused by inhibition of drug-metabolizing enzymes (8). Several studies have demonstrated that ITC is an effective inhibitor of the metabolism of many drugs in humans (digoxin, diazepam, and warfarin) (14) and mice (triazolam, midazolam, and ciprofloxacin) (2, 20, 27).
The regimen of drug associations used here resulted in variations in the profile of plasma elimination of BNZ, shown by a significant increase in the t1/2β (7.5-fold). Since BNZ is a substrate of the same CYP enzymes, and considering that the elimination of both drugs is essentially dependent on this metabolism, the long half-life of BNZ may be due to the effect of coadministration of ITC causing an accumulation of BNZ in the biological system. Nevertheless, to assert that the metabolism of BNZ can be effectively inhibited by ITC, as observed in several combinations, requires enzymatic inhibition studies with selective markers of the CYP isoforms and rigorous monitoring to understand the true influence of this metabolism on pharmacokinetic parameters with regard to the risk of adverse reactions. It is known that liver nitroreductive metabolism of BNZ is also responsible for the modulation of its toxicity and side effects (5).
The higher therapeutic efficacy expected for the BNZ and ITC association could be attributed to the synergistic effect resulting from the administration of two drugs with different mechanisms of action. However, the benefit of the treatment employing ITC separately is limited to reduction of parasitemia, and it is unable to provide a parasitological cure in experimental models and in humans, as demonstrated in clinical assays, where interruption of disease progress was not observed (26). Thus, an increase in the therapeutic efficacy of this proposed association, as our group has demonstrated in mice (data not shown), may be related to the specific activity of BNZ in addition to an effect promoted by ITC via the pharmacokinetic interaction.
The bioanalytical method employed (18) has the ability to detect and quantify the residual concentration of BNZ up to 36 h after associated treatment. Since the experimental treatment used in chemotherapy of acute Chagas' disease lasts 20 days, the accumulation of BNZ, if used in association with ITC, tends to increase with each administration. This suggests that when it reaches the steady state between absorption and elimination, the greatest cumulative amount of drug may substantially increase the AUC, the pharmacokinetic parameter that most decisively influences the drug's efficacy in parasitic diseases (9). However, it is not possible to perform pharmacokinetic evaluation at steady state using small animals as experimental models due to their low total blood volume and the short time between the collections of the necessary blood samples. Similarly, the compartmental pharmacokinetic assessment was probably limited by the animal model used. The inability to obtain full AUC in mice led to working with groups, which introduces variability. Thus, the use of bigger experimental models simultaneously infected with T. cruzi and proven to be a good experimental model for Chagas' disease (7), such as dogs, and study of the associated chemotherapy (12) could offer better results.
Besides, a proposal for experimental studies using different doses of BNZ combined with ITC, could be associated with reduction in the time of treatment usually employed in chemotherapy of Chagas' disease to increase the efficacy of this combination therapy.
Conclusion.
The present work describes a drug-drug interaction, using a validated HPLC-UV method for the determination of BNZ and ITC in the plasma of mice, resulting mainly in lower elimination (prolonged half-life) of BNZ, which presented a profile of accumulation in the animal model. This preclinical monitoring showed that the proposed combination therapy should take into account the animal species and the pharmacokinetic model to establish rational therapeutic regimens of dose and period of treatment in the experimental chemotherapy of Chagas' disease.
ACKNOWLEDGMENTS
This work was supported by Capes (proequipments Ed), FAPEMIG (Rede Mineira de Bioterismo and Rede Toxifar), and CNPq.
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Abdou HM. 1995. Dissolution, p 593–604 In Mischer A. (ed), Remington: the science and practice of pharmacy, 19th ed Mack Publishing Company Press, Easton, PA [Google Scholar]
- 2. Abou-Audaa HS, Mustafa AA, Al-Humayyd MS. 2008. Pharmacokinetic interaction of ketoconazole and itraconazole with ciprofloxacin. Biopharm. Drug Dispos. 29:29–35 [DOI] [PubMed] [Google Scholar]
- 3. Araújo MS, Martins-Filho OA, Pereira ME, Brener Z. 2000. A combination of benznidazole and ketoconazole enhances efficacy of chemotherapy of experimental Chagas' disease. J. Antimicrob. Chemother. 45:819–824 [DOI] [PubMed] [Google Scholar]
- 4. Auletta CS. 1995. Acute subchronic and chronic toxicology, p 51–103 In Derelanko MJ, Hollinger MA. (ed), Handbook of toxicology. CRC Press, Boca Raton, FL [Google Scholar]
- 5. Castro JA, de Mecca MM, Bartel LC. 2006. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis). Hum. Exp. Toxicol. 25:471–479 [DOI] [PubMed] [Google Scholar]
- 6. Coura JR. 2009. Present situation and new strategies for Chagas Disease chemotherapy—a proposal. Mem. Inst. Oswaldo Cruz 104:549–554 [DOI] [PubMed] [Google Scholar]
- 7. de Lana M, Chiari E, Tafuri WL. 1992. Experimental Chagas' disease in dogs. Mem. Inst. Oswaldo Cruz. 87:59–71 [DOI] [PubMed] [Google Scholar]
- 8. Dvorak Z. 2011. Drug-drug interactions by azole antifungals: beyond a dogma of CYP3A4 enzyme activity inhibition. Toxicol. Lett. 202:129–132 [DOI] [PubMed] [Google Scholar]
- 9. Edwards G, Krishna S. 2004. Pharmacokinetic and pharmacodynamic issues in the treatment of parasitc infections. Eur. J. Clin. Microbiol. Infect. Dis. 23:233–242 [DOI] [PubMed] [Google Scholar]
- 10. Food and Drug Administration 2001. Guidance for industry. Bioanalytical method validation. U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research Center for Veterinary Medicine, Rockville, MD: http://www.fda.gov/downloads/Drugd/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf [Google Scholar]
- 11. Garibaldi M, Nagashima R, Levy G. 1969. Relationship between drug concentration in plasma or serum and amount of drug in the body. J. Pharm. Sci. 58:193–197 [Google Scholar]
- 12. Guedes PM, et al. 2002. The dog as model for chemotherapy of the Chagas' disease. Acta Trop. 84:9–17 [DOI] [PubMed] [Google Scholar]
- 13. Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE. 2004. Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab. Dispos. 32:1121–1131 [DOI] [PubMed] [Google Scholar]
- 14. Janssen-Ortho, Inc 2011. Sporanox. Product monograph of Janssen-Ortho, Inc., Toronto, Ontario: www.janssen.ca/product/188 [Google Scholar]
- 15. Kasim NA, et al. 2004. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 1:85–96 [DOI] [PubMed] [Google Scholar]
- 16. Lamas MC, et al. 2006. Development of parenteral formulations and evaluation of the biological activity of the trypanocide drug benznidazole. Int. J. Pharm. 307:239–243 [DOI] [PubMed] [Google Scholar]
- 17. Maldonado RA, Molina J, Payares G, Urbina JA. 1993. Experimental chemotherapy with combinations of ergosterol biosynthesis inhibitors in murine models of Chagas' disease. Antimicrob. Agents Chemother. 37:1353–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moreira-Silva R, Oliveira TL, Carvalho MG, Souza J, Lana M. 2011. Validation of a new HPLC-UV method for preclinical monitoring of benznidazole-itraconazole drug association. XXIII International Symposium on Pharmaceutical and Biomedical Analysis João Pessoa, PB, Brazil http://www.pba2011.com [Google Scholar]
- 19. Peeters J, Neeskens P, Tollenaere JP, Van Remoortere P, Brewster ME. 2002. Characterization of the interaction of 2-hydroxypropyl-β-cyclodextrin with itraconazole at pH 2, 4 and 7. J. Pharm. Sci. 91:1414–1422 [DOI] [PubMed] [Google Scholar]
- 20. Olkkola KT, Backman JT, Neuvonen PJ. 1994. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or intraconazole. Clin. Pharmacol. Ther. 55:481–485 [DOI] [PubMed] [Google Scholar]
- 21. Raaflaub J, Ziegler WH. 1979. Single-dose pharmacokinetics of the the trypanosmicide benznidazole in man. Arzneimittelforschung 29:1611–1614 [PubMed] [Google Scholar]
- 22. Raether W, Hanel H. 2003. Nitroheterocyclic drugs with broad spectrum activity. Parasitol. Res. 90(Suppl.):S19–S39 [DOI] [PubMed] [Google Scholar]
- 23. Urbina JA, et al. 1993. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 37:580–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Urbina JA. 2001. Specific treatment of Chagas disease: current status and new developments. Curr. Opin. Infect. Dis. 14:733–741 [DOI] [PubMed] [Google Scholar]
- 25. Urbina JA, Docampo R. 2003. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 19:495–501 [DOI] [PubMed] [Google Scholar]
- 26. Urbina JA. 2010. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop. 115:55–68 [DOI] [PubMed] [Google Scholar]
- 27. Varhe A, Olkkola KT, Neuvonen PJ. 1994. Oral triazolam is potentially hazardous to patients receiving systemic antimycotics ketoconazole or intraconazole. Clin. Pharmacol. Ther. 56:601–607 [DOI] [PubMed] [Google Scholar]
- 28. Workman P, White RAS, Walton MI, Owen LN, Twentyman PR. 1984. Preclinical pharmacokinetics of benznidazole. Br. J. Cancer 50:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization 2010. Neglected tropical diseases. First WHO report on neglected tropical diseases. Working to overcome the global impact of neglected tropical diseases World Health Organization, Geneva, Switzerland: http://www.who.int/neglected_diseases/2010report/en/ [Google Scholar]