Abstract
Luliconazole is a novel topical antifungal imidazole with broad-spectrum and potent antifungal activity. The drug is under clinical development in the United States for management of dermatophytosis with a short-term treatment regimen. The present study was undertaken to investigate the clinical benefit of short-term therapy with luliconazole cream in guinea pig models of tinea corporis and tinea pedis induced with Trichophyton mentagrophytes. The dose-dependent therapeutic efficacy of topical luliconazole cream (0.02 to 1%), measured by macroscopic improvement of skin lesions and by fungal eradication as determined by a culture assay, was demonstrated using a tinea corporis model. The improvement in skin lesions seen with luliconazole cream was observed even at a concentration of 0.02%, and its efficacy at 0.1% was equal to that of 1% bifonazole cream. The efficacy of short-term therapy with 1% luliconazole cream, which is used for clinical management, was investigated using the tinea corporis model (4- and 8-day treatment regimens) and the tinea pedis model (7- and 14-day treatment regimens). The 1% luliconazole cream completely eradicated the fungus in half or less of the treatment time required for 1% terbinafine cream and 1% bifonazole cream, as determined by a culture assay for both models. These results clearly indicate that 1% luliconazole cream is sufficiently potent for short-term treatment for dermatophytosis compared to existing drugs. Luliconazole is expected to be useful in the clinical management of dermatophytosis.
INTRODUCTION
Superficial mycoses are not fatal, but they constitute a serious problem for patients' quality of life in view of the considerable discomfort and/or cosmetic deformity they cause. These diseases are found worldwide and affect 20 to 25% of the world's population (14). Dermatophytosis is the most common infection among the superficial mycoses (1, 6–8, 11, 13, 26). According to an epidemiological survey of ambulatory visits in the United States, the incidences of dermatophytoses such as tinea unguium, tinea corporis, and tinea pedis were as high as 23.2%, 20.4%, and 18.8%, respectively, during 1995 to 2004 (24). Trichophyton rubrum, an anthropophilic fungus, is the most prevalent causative agent of dermatophytosis in developed countries. The incidence of this fungus has not changed in recent decades (1, 14, 24, 25, 27, 29), although many antifungal drugs with potent action against this species have been introduced into the market during this period (3, 23). One reason for unsuccessful antifungal management is the relapse of skin manifestations due to poor adherence to long-term treatment regimens using topical antifungal drugs (2, 11, 13). Under these circumstances, a drug with a more potent therapeutic efficacy capable of shortening treatment duration and leading to improvement in the patient adherence rate would be beneficial.
Luliconazole (LLCZ) {(−)-(E)-[(4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene](1H-imidazol-1-yl) acetonitrile} is a novel, optically active antifungal imidazole (21). The compound has a unique chemical structure (Fig. 1) which is augmented by the introduction of an imidazole moiety into the ketene dithioacetate structure. By this modification, an unusually high potency against filamentous fungi, including dermatophytes, was achieved while maintaining the broad antifungal spectrum of an imidazole. The in vitro antifungal activity of LLCZ against Trichophyton spp. was found to be the highest among existing topical antifungal drugs (17, 18, 30). Luliconazole was developed as a topical antifungal drug and approved in Japan in 2005. Currently, a 1% cream and a 1% solution of LLCZ are available for the treatment of superficial mycoses such as dermatophytosis, candidiasis, and pityriasis versicolor. Topical LLCZ has been introduced in India (approved in 2010) and presumably will also be available in Asian countries such as China and Vietnam, among others. In the United States, the clinical development of LLCZ for the treatment of dermatophytosis with a short-term regimen was recently begun, based on the clinical data obtained in Japan (33). The drug is now in phase II clinical trials to evaluate its efficacy in curing tinea pedis in half the treatment time required for commonly prescribed products. Although the excellent in vitro antifungal activity of LLCZ strongly suggests that it would be useful for short-term therapy, preclinical evidence to support this hypothesis via in vivo studies using an animal model is limited (21, 31).
Fig 1.
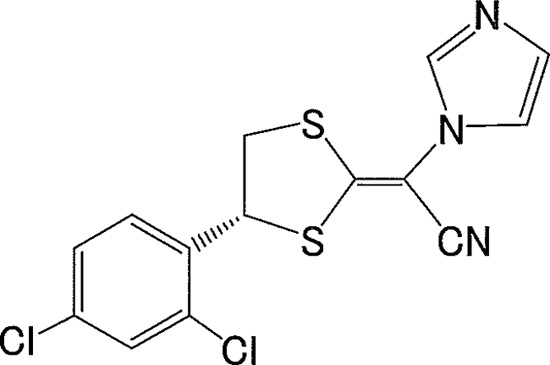
Chemical structure of luliconazole.
The present study investigated the clinical efficacy of short-term LLCZ treatment in the management of dermatophytosis, using guinea pig models of tinea corporis and tinea pedis. Trichophyton mentagrophytes was used as a pathogenic agent, because this zoophilic fungus is able to produce a stable infection in guinea pigs and has previously been used to establish tinea corporis and tinea pedis models (12, 15, 22, 26).
MATERIALS AND METHODS
Antifungal drugs.
Luliconazole was synthesized and prepared in a cream formulation at concentrations of 0.02, 0.1 0.5, and 1% at the Research Center of Nihon Nohyaku Co. Ltd. (Osaka, Japan). The recipe for the cream formulation was the same as that of the commercial product sold in Japan (Pola Pharma Inc., Tokyo, Japan) and used in clinical trials in the United States. The reference drugs, 1% terbinafine (TRB) cream (Novartis Pharma K.K., Tokyo, Japan) and 1% bifonazole (BFZ) cream (Bayer Yakuhin Ltd., Osaka, Japan), were commercial versions available for clinical use.
Animals.
Male specific-pathogen-free (SPF) Hartley guinea pigs were purchased from Nippon SLC Ltd. (Shizuoka, Japan). The animals were acclimatized to laboratory conditions for more than 7 days, including the quarantine period, and used when they were 6 weeks old. They were housed in individual cages during the experiment, at 21 ± 2°C and a relative humidity of 50% ± 20%. The room was ventilated 12 to 15 times per hour by an All Fresh Air system and lit for 12 h per day. The animals were allowed free access to food (Lab-G stock; Nosan Corporation, Kanagawa, Japan) and local tap water, which was filtered twice through 5-μm-pore-size filters.
The animal care and use conformed to the standards established by the animal welfare committee of the institute and complied with the legal requirements for the humane treatment and management of animals (The Act on Welfare and Management of Animals [Act No. 105 of 1973, Act No. 68 of 2005], Japan).
Test organisms and inoculum preparation.
Trichophyton mentagrophytes TIMM1189 and TIMM2789, obtained from the Teikyo University Institute of Medical Mycology (Tokyo, Japan), were used for the tinea corporis and tinea pedis models, respectively. The MICs of LLCZ, BFZ, and TRB against these organisms, measured by a standardized microdilution method using RPMI 1640 medium (5, 28), were 0.002, 0.5, and 0.0078 μg ml−1, respectively, for T. mentagrophytes TIMM1189 and 0.002, 4, and 0.016 μg ml−1, respectively, for TIMM2789. To prepare inoculums for animal infections, the test organisms were precultured on a Sabouraud dextrose agar (SDA; Difco, MD) slant at 27°C for 1 to 2 weeks until maturity. Sterile saline containing 0.1% (vol/vol) Tween 80 was added to the slant, and the conidia were suspended by gently rubbing them with a loop. The suspension was filtered through sterilized gauze to remove the hyphal fragments. The number of conidia in the filtrate was measured using a Thoma hemacytometer, and the concentration was adjusted to 4.0 × 107 conidia ml−1 for T. mentagrophytes TIMM1189 and to 1.0 × 108 conidia ml−1 for T. mentagrophytes TIMM2789, using sterile saline containing 0.1% (vol/vol) Tween 80.
Animal models.
Tinea corporis was induced in the dorsal skin by the method reported by Niwano et al. (22), with slight modifications. The dorsal skin was clipped, and the skin sites (diameter, 2 cm bilaterally) were stripped with adhesive tape three times (no. 159; Teraoka, Tokyo, Japan). The T. mentagrophytes TIMM1189 inoculum (0.05 ml per site) was then applied to the skin surface. Meanwhile, the animals were prepared to host the fungal infection. The drug treatment commenced 3 or 5 days after the inoculation. The infected site remained uncovered throughout the experiment.
Tinea pedis was induced in the planta of the hind leg by using a modified version of the method pioneered by Fujita and Matsumiya (12). The planta was cleaned with a cotton swab moistened with sterile saline, and then the surface of the skin was lightly abraded with sandpaper. A sterile adhesive bandage (Band-Aid; Johnson & Johnson Co., Ltd., Tokyo, Japan) soaked with 0.15 ml of the T. mentagrophytes TIMM2789 inoculum was applied to the planta and covered with a film (Saran Wrap; Asahi Kasei Corporation, Tokyo, Japan). To avoid excessive pressure to the infected site, a form pad (Reston 1560M; Sumitomo 3M Ltd., Tokyo, Japan) was placed on the site with adhesive elastic tape (Elastplast; Smith & Nephew Wound Management KK, Tokyo, Japan). The bandage was removed 7 days after the commencement of the inoculation, and the animals were maintained for another 21 days to establish the infection.
Experimental design.
A dose-response study of the LLCZ cream was performed using the tinea corporis guinea pig model. A total of 40 guinea pigs were used. Three days after inoculation, animals without any accidental skin damage (e.g., by self-scratching) were selected and assigned into 7 groups of 5 animals each. Different concentrations of LLCZ cream (0 [base], 0.02, 0.1, 0.5, and 1%) or 1% BFZ cream was applied to the skin surface (0.2 ml per site) once daily, without covering the surface, for 7 consecutive days. Animals in the nontreated group received no drug treatment. The animals were observed macroscopically throughout the experiment, and a culture assay for the infected skin sites was performed on day 14 postinoculation (5 days after completion of the drug treatment).
A short-term study of the 1% LLCZ cream treatment was conducted with both the tinea corporis and tinea pedis guinea pig models. Both 1% TRB cream and 1% BFZ cream were used for comparison. In the tinea corporis guinea pig model, a total of 45 guinea pigs were used. Five days after the inoculation, animals with developing skin lesions (score of 1) without any accidental skin damage were selected and assigned into one of the following seven groups of five animals each: nontreated group, cream base group (8-day treatment), 1% LLCZ cream groups (4- and 8-day treatments), 1% TRB cream groups (4- and 8-day treatments), and 1% BFZ cream group (8-day treatment). The drugs were applied topically to the skin (0.2 ml per site) once daily, without covering the surface, for 4 or 8 consecutive days. The animals were observed macroscopically throughout the experiment, and a culture assay was performed on day 17 postinoculation on skin from the site of infection (5 or 9 days after completion of drug treatment). In the tinea pedis model, a total of 40 guinea pigs were used. Animals presenting with skin lesions (scaling) were assigned to one of the following eight groups of four animals each (four groups each for 7- and 14-day treatments): nontreated group, 1% LLCZ cream groups, 1% TRB cream groups, and 1% BFZ cream groups. In total, 0.1 ml of each drug was applied topically to the planta once daily for 7 or 14 consecutive days. A culture assay of the planta was performed 14 days after the completion of each treatment. Another experiment was performed using the tinea pedis model with the same treatment regimen but using a deactivator-supplemented medium to prevent any carryover effect in the culture assay.
Macroscopic observation.
The skin lesions caused by tinea corporis were observed macroscopically daily throughout the experiment and were assessed using the following score system: 0, no signs or normal; 1, a small number of distinct erythematous papules at the site; 2, moderate erythema spread over the entire site, accompanied partly by an inflammatory response or abrasion of the epidermis in some parts; 3, patches of intense erythema with scaling and crusting; and 4, severe erythema with extensive and intense crusting. The average score for each group was calculated to determine the average lesion score.
Culture assay.
The animals were euthanized with ether, and skin tissue specimens, including samples of the epidermis and dermis, were collected from the infected dorsal skin or planta by use of sterile scissors. The tissues were rinsed with 0.1% (vol/vol) benzalkonium chloride followed by sterile saline. The skin tissues were cut into small pieces (approximately 5 mm square), and 10 blocks of the samples were randomly selected for culture. These samples were implanted on an SDA plate containing cycloheximide (500 μg ml−1), chloramphenicol (50 μg ml−1), and sisomicine (50 μg ml−1). For the culture study using the deactivator-supplemented medium (20), skin blocks were implanted on an SDA plate containing the following deactivators: 1% egg lecithin (Kanto Chemical Co., Inc., Tokyo, Japan) and 0.7% Tween 80 (polyoxyethylene sorbitan monooleate; Kanto Chemical Co., Inc., Tokyo, Japan). The plates were incubated at 27°C for 14 days, and the formation of colonies on the plates was observed. A skin block yielding a colony was regarded as fungus positive. The percentage of fungus-positive feet for each group was calculated as the culture-positive rate. To evaluate the amount of fungus associate with residual infections, the number of culture-positive skin blocks was rated 0 to 10, according to the corresponding number of culture-positive skin tissues among the 10 skin tissues implanted per plate. The average score for each group was calculated to determine the fungal burden score.
Statistical analysis.
The SAS System for Windows (release 8.02; SAS Institute, Inc., Cary, NC) was used for statistical analysis. The average lesion score on the last day of macroscopic observation and the fungal burden score in the culture assay were analyzed by Dunnett's multiple-comparison test or Steel's multiple-comparison rank sum test (nonparametric Dunnett's multiple-comparison test), based on the homogeneity of variance tested by Bartlett's test (P < 0.01). The culture-positive rate in the culture assay was analyzed by Fisher's exact probability test. The differences of the means of the groups compared with the nontreated group and/or the cream base group were considered significant when the P value was below 0.05.
RESULTS
Dose-response study.
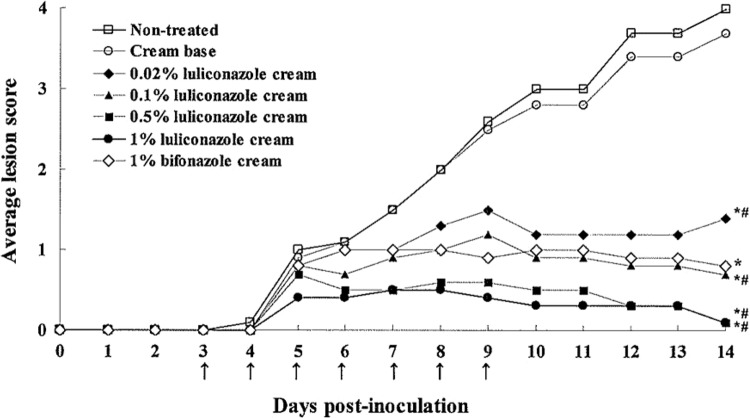
Figure 2 shows the results of macroscopic observation for the tinea corporis model. Luliconazole cream prepared at various concentrations improved skin lesions in a dose-dependent manner. Compared to the nontreated group and/or the cream base group, there was a statistically significant improvement in lesions treated with 0.02% LLCZ cream, and 0.1% LLCZ cream was comparable in efficacy to the 1% BFZ cream. The results of the culture assay performed on day 14 postinoculation are shown in Table 1. The culture-positive rate and the fungal burden score of the nontreated group reached maximum values of 100% and 10.0, respectively. The LLCZ cream eliminated the fungi in a dose-dependent manner, and statistically significant differences in fungal burden were observed even after treatment with a concentration of 0.02% LLCZ compared to the nontreated and/or cream base group. The LLCZ cream completely cured the mycological infection at concentrations of 0.5% and above.
Fig 2.
Macroscopic improvement of skin lesions in experimental tinea corporis in guinea pigs treated with different concentrations of luliconazole cream or 1% bifonazole cream (dose-response study). Topical treatment (↑) was started on day 3 postinoculation and continued once daily for 7 consecutive days. *, P < 0.05 versus nontreated group; #, P < 0.05 versus cream base group (day 14 postinoculation; Steel's multiple-comparison rank sum test; n = 10).
Table 1.
Mycological efficacies of different concentrations of luliconazole cream and reference drugs in experimental tinea corporis in guinea pigs
| Study and treatment group | Culture-positive rate (%) | Fungal burden score (mean ± SE) |
|---|---|---|
| Dose-response studye | ||
| Nontreated | 100 | 10.0 ± 0.0 |
| Cream base | 100 | 9.5 ± 0.3 |
| Luliconazole cream | ||
| 0.02% | 90 | 3.0 ± 0.7c |
| 0.1% | 60a,b | 1.2 ± 0.4c,d |
| 0.5% | 0a,b | 0c,d |
| 1.0% | 0a,b | 0c,d |
| 1.0% bifonazole cream | 90 | 3.0 ± 1.0c |
| Short-term treatment studyf | ||
| Nontreated | 100 | 10.0 ± 0.0 |
| Cream base | 100 | 9.9 ± 0.1 |
| 4-day treatment | ||
| 1% luliconazole cream | 20a,b | 0.2 ± 0.1c,d |
| 1% terbinafine cream | 70 | 1.3 ± 0.3c |
| 8-day treatment | ||
| 1% luliconazole cream | 0a,b | 0c,d |
| 1% terbinafine cream | 60a | 1.4 ± 0.5c |
| 1% bifonazole cream | 100 | 6.3 ± 0.4 |
P < 0.05 versus nontreated group, by Fisher's exact probability test (n = 10).
P < 0.05 versus cream base group, by Fisher's exact probability test (n = 10).
P < 0.05 versus nontreated group, by Steel's multiple-comparison rank sum test (n = 10).
P < 0.05 versus cream base group, by Steel's multiple-comparison rank sum test (n = 10).
Topical treatment was started on day 3 postinoculation and continued once daily for 7 consecutive days. The culture assay was performed on day 14 postinoculation.
Topical treatment was started on day 5 postinoculation and continued once daily for 4 or 8 consecutive days. The culture assay was performed on day 17 postinoculation.
Short-term treatment study.
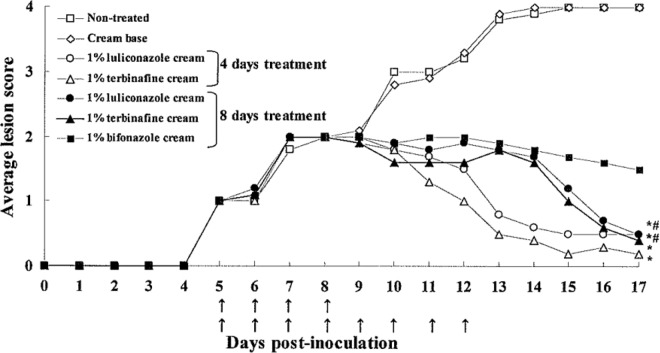
The treatment duration required for 1% LLCZ cream to cure a fungal infection was assessed in the tinea corporis and tinea pedis models. In the tinea corporis model, the commencement of drug treatment was changed from day 3 to day 5 postinoculation to generate more severe lesions, because the 1% LLCZ cream was found to be extremely potent in the dose-response study. The results of macroscopic observation for the tinea corporis model are shown in Fig. 3. For both the 1% LLCZ cream and 1% TRB cream treatment groups, the average lesion score for the 8-day treatment group was higher than that for the 4-day treatment group after day 11 due to accumulated cream vehicle materials on the surface of the treated skin, not inflammation caused by microorganisms. The 1% LLCZ cream significantly improved the skin lesions in the 4-day treatment and 8-day treatment groups compared to those in controls, and the efficacy ratings were similar to those for the 1% TRB cream groups (4- and 8-day treatments). In the culture assay (Table 1), the 1% LLCZ cream eliminated the fungi from the skin at the site of infection with the 4-day treatment regimen and completely cured the infection with the 8-day treatment regimen with regard to the culture-positive rate and fungal burden score. For comparison, the 1% TRB cream showed moderate efficacy, and the 1% BFZ cream showed only slight efficacy, even with an 8-day treatment. The efficacies of short-term therapy with different drugs were also compared in the tinea pedis model, as shown in Table 2. The treatment with 1% LLCZ cream resulted in a complete mycological cure with a 7-day treatment regimen with regard to the culture-positive rate and fungal burden score. The 1% TRB cream significantly reduced the amount of fungi with a 7-day treatment regimen; however, the complete eradication of fungi by this drug required more than 14 days. The 1% BFZ cream did not show significant antifungal effects even after 14 days of treatment. The results of the culture assay using the deactivator-supplemented medium are also shown in Table 2. The culture-positive rates and fungal burden scores for the 1% LLCZ cream and reference drugs in the presence of the deactivator-supplemented medium were similar to those with the conventional SDA medium.
Fig 3.
Macroscopic improvement of skin lesions in experimental tinea corporis in guinea pigs treated with 1% luliconazole cream, 1% terbinafine cream, or 1% bifonazole cream in 4- and 8-day treatment regimens (short-term treatment study). Topical treatment (↑) was started on day 5 postinoculation and continued once daily for 4 or 8 consecutive days. *, P < 0.05 versus nontreated group; #, P < 0.05 versus cream base group (day 17 postinoculation; Steel's multiple-comparison rank sum test; n = 10).
Table 2.
Mycological efficacies of 1% luliconazole cream and reference drugs in experimental tinea pedis in guinea pigsa
| Study and treatment group | Culture-positive rate (%) | Fungal burden score (mean ± SE) |
|---|---|---|
| Short-term treatment study | ||
| 7-day treatment | ||
| Nontreated | 100 | 9.9 ± 0.1 |
| 1% luliconazole cream | 0* | 0# |
| 1% terbinafine cream | 62.5 | 1.5 ± 0.5# |
| 1% bifonazole cream | 100 | 6.3 ± 0.6 |
| 14-day treatment | ||
| Nontreated | 100 | 10.0 ± 0.0 |
| 1% luliconazole cream | 0* | 0# |
| 1% terbinafine cream | 25 | 0.3 ± 0.2# |
| 1% bifonazole cream | 100 | 4.1 ± 1.0 |
| Short-term treatment study using deactivator-supplemented medium | ||
| 7-day treatment | ||
| Nontreated | 100 | 10.0 ± 0.0 |
| 1.0% luliconazole cream | 0* | 0# |
| 1.0% terbinafine cream | 75 | 1.4 ± 0.6# |
| 1.0% bifonazole cream | 100 | 8.0 ± 0.7# |
| 14-day treatment | ||
| Nontreated | 100 | 9.5 ± 0.4 |
| 1.0% luliconazole cream | 0* | 0# |
| 1.0% terbinafine cream | 0* | 0# |
| 1.0% bifonazole cream | 100 | 6.0 ± 0.9 |
Drug was applied topically once daily for 7 or 14 consecutive days. The culture assay was performed 14 days after the completion of the drug treatment, with or without a deactivator-supplemented medium. *, P < 0.05 versus nontreated group, by Fisher's exact probability test (n = 8); #, P < 0.05 versus nontreated group, by Steel's multiple-comparison rank sum test (n = 8).
DISCUSSION
The two animal models employed in the present study are highly reproducible and are used to study the pathogenesis and antifungal treatment of dermatophytosis (4, 15, 26). The tinea corporis model reliably results in infection after only one inoculation and has proven to be time-saving for testing the antifungal activity of drugs. One is able to select the severity of the model used by choosing the appropriate number of days after inoculation for analysis, because the inflammatory response at the infected site increases daily after the inoculation, e.g., the skin lesions on day 5 after the inoculation employed in the short-term treatment study were more severe than those on day 3 in the dose-response study. In contrast to the tinea corporis model, the tinea pedis guinea pig model requires more skilled techniques for inoculation and a longer period to develop an infection (a total of 4 weeks), although it is a stable and substantiated model that closely mimics human tinea pedis. We used the conventional tinea corporis model to test the dose-response relationships of LLCZ creams and the efficacy of short-term treatment of 1% LLCZ cream and then adopted the true tinea pedis model for further confirmation of the efficacy of the short-term therapy. The reference drugs used in this study were 1% BFZ cream and 1% TRB cream. Bifonazole was the first drug to be used for once-daily treatment of superficial mycoses and has been the drug of choice for topical antifungal infections for the last 2 decades in Japan (19, 32). Terbinafine is another potent antifungal agent with proven short-term efficacy for the treatment of tinea (9, 10).
The LLCZ cream showed a dose-dependent efficacy in the tinea corporis model. Significant improvement of lesions was observed after treatment with a concentration of 0.02%, and the efficacy of LLCZ at 0.1% was equal to that of 1% BFZ cream. These data are in accordance with results obtained in randomized clinical trials for dose optimization, in which LLCZ cream (0.1 to 1%) showed significant antimycotic activity against tinea pedis at doses as low as 0.1% (34). Additionally, the efficacy of short-term treatment with 1% LLCZ cream was examined and compared with that of 1% TRB cream and 1% BFZ cream, using the tinea corporis and tinea pedis models. In both models, treatment with the 1% LLCZ cream resulted in a complete mycological cure in half or less of the treatment period required for the 1% TRB cream and the 1% BFZ cream. The strong antimycotic activity of 1% LLCZ cream against tinea pedis in the guinea pig has also been reported by Uchida et al. (31), in whose study complete eradication of fungi after a 7-day treatment regimen was demonstrated by a culture assay performed up to 16 weeks after the completion of treatment.
Although T. mentagrophytes was used as the pathogenic agent in this study, the susceptibility of T. rubrum, the major causative agent of dermatophytosis, to LLCZ is higher than that of T. mentagrophytes (17, 18). Therefore, 1% LLCZ cream would be expected to be more potent in clinical use than the potency exhibited in the present study. In fact, the mycological efficacy of a 2-week treatment of tinea pedis with 1% LLCZ cream was comparable or superior to that of a 4-week treatment with 1% BFZ in a clinical trial in Japan (33).
It is generally recognized that azoles are more fungistatic than allylamine (e.g., TRB) or thiocarbamate (e.g., liranaftate). However, LLCZ has exceptionally strong antifungal activity. The MICs of LLCZ against T. mentagrophytes TIMM1189 and TIMM2789 were 4 and 8 times lower, respectively, than that of TRB and 250 and 2,000 times lower, respectively, than that of BFZ. In addition, LLCZ has been shown to have preferable pharmacokinetic properties in the skin compared to TRB (16). In short, the drug concentration in the stratum corneum of the tinea skin of guinea pigs treated with 1% LLCZ cream was significantly higher than that found in those treated with 1% TRB cream. Luliconazole was also significantly easier to release from the stratum corneum than TRB. Thus, the strong therapeutic efficacy of LLCZ cream as a topical antifungal drug can be attributed not only to its excellent in vitro antifungal activity but also to its optimal pharmacokinetic properties in the skin.
In a culture study of topical antifungal drugs, carryover effects should be taken into account when evaluating drugs showing high rates of retention in the skin (20). To confirm that there was no carryover effect of LLCZ in this study, we performed a culture study using drug deactivators in the tinea pedis model, in which the infected site has a thick stratum corneum. A carryover effect was not observed for LLCZ or the reference drugs under the test conditions used in this study. The skin samples were collected 2 weeks after the completion of drug treatment, and therefore most of the drug retained in the stratum corneum may have been eliminated by the turnover of the horny layer.
In conclusion, the results of the present study clearly indicate that 1% LLCZ cream is sufficiently potent for short-term treatment of dermatophytosis compared to existing drugs. The use of 1% LLCZ cream for short-term treatment of dermatophytosis may encourage patient adherence to the treatment regimen and contribute to reducing the relapse rate.
ACKNOWLEDGMENTS
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Footnotes
Published ahead of print 5 March 2012
REFERENCES
- 1. Borman AM, Campbell CK, Fraser M, Johnson EM. 2007. Analysis of dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med. Mycol. 45:131–141 [DOI] [PubMed] [Google Scholar]
- 2. Bouchara JP, Mignon B, Chatuvedi V. 2008. Dermatophytes and dermatophytosis: a reappraisal for the twenty-first century. Mycopathologia 166:235–237 [DOI] [PubMed] [Google Scholar]
- 3. Brennan B, Leyden JJ. 1997. Overview of topical therapy for common superficial fungal infections and role of new topical agents. J. Am. Acad. Dermatol. 36:s3–s8 [DOI] [PubMed] [Google Scholar]
- 4. Capilla J, Clemons KV, Stevens DA. 2007. Animal models: an important tool in mycology. Med. Mycol. 45:657–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard-second ed. M38-A2. CLSI, Wayne, PA [Google Scholar]
- 6. Committee for Epidemiology of Japanese Society for Medical Mycology 2001. 1997 epidemiological survey of dermatophytosis in Japan. Jpn. J. Med. Mycol. 42:11–18 [DOI] [PubMed] [Google Scholar]
- 7. Committee for Epidemiology of Japanese Society for Medical Mycology 2006. An epidemiological survey of dermatomycoses in Japan, 2002. Jpn. J. Med. Mycol. 47:103–111 [Google Scholar]
- 8. Drakensjö IT, Chryssanthou E. 2011. Epidemiology of dermatophyte infections in Stockholm, Sweden: a retrospective study from 2005–2009. Med. Mycol. 49:484–488 doi:10.3109/13693786.2010.540045 [DOI] [PubMed] [Google Scholar]
- 9. Evans EGV, Seaman RAJ, James IGV. 1994. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br. J. Dermatol. 130:83–87 [DOI] [PubMed] [Google Scholar]
- 10. Finlay AY. 1994. Global overview of Lamisil. Br. J. Dermatol. 130:1–3 [DOI] [PubMed] [Google Scholar]
- 11. Foster KW, Ghannoum MA, Elewski BE. 2004. Epidemiological surveillance of cutaneous fungal infection in United States from 1999 to 2002. J. Am. Acad. Dermatol. 50:748–752 [DOI] [PubMed] [Google Scholar]
- 12. Fujita S, Matsumiya T. 1987. Experimental tinea pedis induced by non-abrasive inoculation of Trichophyton mentagrophytes arthrospores on the plantar part of a guinea pig foot. J. Med. Vet. Mycol. 25:202–213 [DOI] [PubMed] [Google Scholar]
- 13. Gupta AK, Cooper EA. 2008. Update in antifungal therapy of dermatophytosis. Mycopathologia 166:353–367 [DOI] [PubMed] [Google Scholar]
- 14. Havlickova B, Czaika VA, Friedrich M. 2008. Epidemiological trends in skin mycoses world wide. Mycoses 51:2–15 [DOI] [PubMed] [Google Scholar]
- 15. Koga H. 2009. Animal model for superficial mycosis. Jpn. J. Med. Mycol. 50:85–89 [DOI] [PubMed] [Google Scholar]
- 16. Koga H, et al. 2006. Luliconazole, a novel topical imidazole: results of the preclinical studies, abstr P-0090. Abstr. 16th Cong. Int. Soc. Hum. Anim. Mycol., Paris, France [Google Scholar]
- 17. Koga H, Nanjoh Y, Makimura K, Tsuboi R. 2009. In vitro antifungal activities of luliconazole, a new topical imidazole. Med. Mycol. 47:640–647 [DOI] [PubMed] [Google Scholar]
- 18. Koga H, Tsuji Y, Inoue K, Kanai K, Majima T. 2006. In vitro antifungal activity of luliconazole against clinical isolates from patients with dermatomycoses. J. Infect. Chemother. 12:163–165 [DOI] [PubMed] [Google Scholar]
- 19. Lackner TE, Clissold SP. 1989. Bifonazole: a review of its antimicrobial activity and therapeutic use in superficial mycoses. Drugs 38:204–225 [DOI] [PubMed] [Google Scholar]
- 20. Nakashima T, Nozawa A, Ito T, Majima T, Yamaguchi H. 2002. Development of a new medium useful for the recovery of dermatophytes from clinical specimens by minimizing the carryover effect of antifungal agents. Microbiol. Immunol. 46:83–88 [DOI] [PubMed] [Google Scholar]
- 21. Niwano Y, et al. 1998. In vitro and in vivo antidermatophyte activities of NND-502, a novel optically active imidazole antimycotic agent. Antimicrob. Agents Chemother. 42:967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niwano Y, Tabuchi T, Kanai K, Uchida K, Yamaguchi H. 1995. Therapeutic efficacy of lanoconazole ointment in guinea pig model of tinea corporis, a comparative study with ointment and cream preparations. Jpn. J. Antibiot. 48:150–154 [PubMed] [Google Scholar]
- 23. Odom RB. 1997. Update on topical therapy for superficial fungal infections: focus on butenafine. Am. Acad. Dermatol. 36:S1–S2 [DOI] [PubMed] [Google Scholar]
- 24. Panackal AA, Halpern EF, Watson AJ. 2009. Cutaneous fungal infections in the United States: analysis of the National Ambulatory Medical Care Survey (NAMCS) and National Hospital Ambulatory Medical Care Survey (NHAMCS), 1995–2004. Int. J. Dermatol. 48:704–712 [DOI] [PubMed] [Google Scholar]
- 25. Panasiti V, et al. 2007. Epidemiology of dermatophytic infections in Rome, Italy: a retrospective study from 2002 to 2004. Med. Mycol. 45:57–60 [DOI] [PubMed] [Google Scholar]
- 26. Saunte DM, et al. 2008. Experimental guinea pig model of dermatophytosis: a simple and useful tool for the evaluation of new diagnostics and antifungals. Med. Mycol. 46:303–313 [DOI] [PubMed] [Google Scholar]
- 27. Seebacher C, Bouchara J-P, Mignon B. 2008. Update on the epidemiology of dermatophyte infections. Mycopathologia 166:335–352 [DOI] [PubMed] [Google Scholar]
- 28. Standardization Committee of Japanese Society of Medical Mycology 1999. Report of the Standardization Committee of Japanese Society of Medical Mycology 1995–1997. Jpn. J. Med. Mycol. 40:239–257 [Google Scholar]
- 29. Svejgaard EL. 1995. Epidemiology of dermatophytes in Europe. 1995. Int. J. Dermatol. 34:525–528 [DOI] [PubMed] [Google Scholar]
- 30. Uchida K, Nishiyama Y, Yamaguchi H. 2004. In vitro antifungal activity of luliconazole (NND-502), a novel imidazole antifungal agent. J. Infect. Chemother. 10:216–219 [DOI] [PubMed] [Google Scholar]
- 31. Uchida K, Tanaka T, Yamaguchi H. 2003. Achievement of complete mycological cure by topical antifungal agent NND-502 in guinea pig model of tinea pedis. Microbiol. Immunol. 47:143–146 [DOI] [PubMed] [Google Scholar]
- 32. Watanabe S, et al. 1996. Fundamental and clinical studies on the efficacy of bifonazole in patients with tinea pedis at 10 years after approval. Jpn. J. Antibiot. 49:1095–1108 [PubMed] [Google Scholar]
- 33. Watanabe S, et al. 2006. A comparative clinical study between 2 weeks of luliconazole 1% cream treatment and 4 weeks of bifonazole 1% cream treatment for tinea pedis. Mycoses 49:236–241 [DOI] [PubMed] [Google Scholar]
- 34. Watanabe S, et al. 2007. Dose-finding comparative study of 2 weeks of luliconazole cream treatment for tinea pedis—comparison between three groups (1%, 0.5%, 0.1%) by a multicenter randomised double-blind study. Mycoses 50:35–40 [DOI] [PubMed] [Google Scholar]