Glaucoma is an optic neuropathy in which the retinal ganglion cells and their axons, which package and transmit visual impulses from the photoreceptors and associated retinal interneurons to the brain, die individually or in small groups, typically over many years (Figs. 1 and 2) (1). The resulting functional deficit is the second leading cause of irreversible visual loss in the U.S., and the most common cause among African-Americans. In its most prevalent form, the condition is strongly dependent upon age, race, and family history. Approximately 3–5% of White Americans, 10% of African-Americans, and 20% of Afro-Caribbeans over the age of 70 years have the disease (2–5). As lifespan and the proportion of the population comprising the aged increase, so will the number and proportion afflicted.
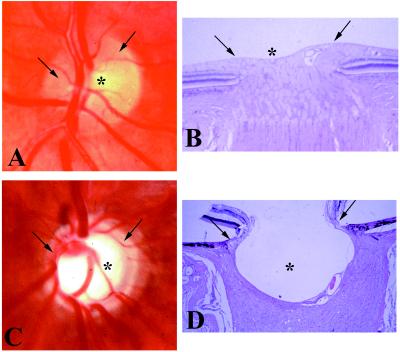
Figure 1.
Human optic nerve head. (A and C) In vivo photograph. (B and D) Postmortem photomicrograph. (Hematoxylin and eosin stain; ×20.) (A and B) Normal. (C and D) Advanced glaucoma. Note the small, shallow central physiologic “cup,” (asterisk) and the full neuroretinal rim (arrow) (which comprises approximately 106 axons) in the normal nerve head. Compare with the deep, wide central excavation, the nasal displacement of the blood vessels, and the narrowing of the neuroretinal rim, indicating loss of most optic nerve fibers and their retinal ganglion cells, in the glaucomatous eye.
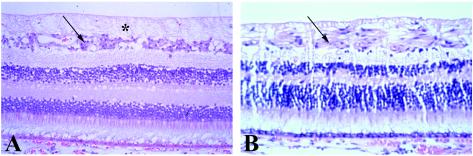
Figure 2.
Human retina, postmortem photomicrograph. (Hematoxylin and eosin stain; ×40.) (A) Normal. (B) Advanced glaucoma. Note loss of retinal ganglion cells (arrow in A) and retinal nerve fiber layer (axons of retinal ganglion cells; asterisk in A) and their replacement by glial tissue (arrow in B) in the glaucomatous retina.
Intraocular pressure (IOP) plays a causal, albeit not necessarily exclusive, role in most cases of glaucomatous visual loss; the higher the IOP the greater the risk (4, 6). There is controversy about whether the primary insult occurs at the level of the axon or the cell body, and the pathophysiology of pressure-induced glaucomatous optic neuropathy is unclear and fiercely debated (7–11). Leading candidate theories include obstruction of axoplasmic flow within the retinal ganglion cell axons at the lamina cribrosa (the connective tissue plate at the back of the eye where the axons coalesce to form the optic nerve “head” or “disk,” from whence they continue as the optic nerve through the orbit and into the brain); compromise of the optic nerve microcirculation at the level of the lamina; and alterations in the laminar glial cells and connective tissues. Secondary insults (e.g., excitotoxicity caused by glutamate or glycine released from injured neurons, growth factor deprivation, oxidative damage caused by over-production of reactive oxygen species) may contribute. Whatever the initial injury site and the initial and subsequent mechanisms (Table 1 lists various possibilities), the end stage of the process is death of the retinal ganglion cell by means of the triggering of an apoptosis program (12, 13), akin to that which eliminates 50% of the retinal ganglion cells during normal developmental organization of the visual pathway (9).
Table 1.
Site and pathophysiology of glaucomatous optic neuropathy
| Where is the primary site of injury? |
| Intraocular retinal ganglion cell axon |
| Laminar retinal ganglion cell axon |
| Retinal ganglion cell body |
| What is the primary mechanism and subsequent sequence of injury? |
| Obstructed axoplasmic flow |
| Oxidative/free radical insult |
| Starvation/growth factor deprivation |
| Excitotoxicity |
| Nitric oxide (NO) toxicity |
| Apoptosis (retinal ganglion cell suicide/programmed cell death) |
All present glaucoma therapy is directed at lowering IOP (6). However, assaulting or bypassing the anterior eye tissues, which produce and drain the fluid aqueous humor, and thereby determine and regulate IOP, completely neglects the retinal ganglion cells and their axons, whose dysfunction and death are directly responsible for the visual loss. Only recently has knowledge of the mechanisms of neuronal death and its prevention, delay, or even reversal after a variety of insults reached the point where we can seriously entertain the possibility of glaucoma therapy directed at the retinal ganglion cell bodies or axons themselves. Studies in cultured neurons (including retinal ganglion cells) and in vivo models of mechanical (crush, transection), ischemic, and pharmacologic (e.g., intraocular glutamate injection) optic nerve or brain injury have suggested various strategies for neuroprotection, neurorescue, and neuroregeneration (14–21) (Table 2). However, none of these strategies had been tested, much less shown effective, in a glaucomatous animal model.
Table 2.
Potential strategies for preventing retinal ganglion cell death and restoring normal function
| Protection of undamaged but at-risk retinal ganglion cells and axons from noxious stimuli released by proximate damaged tissue and/or prevention of initiation of the apoptosis program |
| N-Methyl-d-aspartate (NMDA)/other excitatory amino acid mantagonists (block excitotoxicity) |
| Anti-oxidants/free radical scavengers (decrease levels of toxic moxygen radicals) |
| NO synthase inhibitors (block formation of reactive peroxynitrite from NO and superoxide) |
| Neurotrophins/growth factors |
| Ca2+ channel blockers (prevent entry of toxic levels of calcium into the cell body or axon) |
| Rescue of marginally damaged retinal ganglion cells and axons |
| Lazaroids/21-aminosteroids (block lipid peroxidation) |
| Up-regulation of anti-death genes (bcl-2, bcl-xL) |
| Anti-oxidants/free radical scavengers |
| Ca2+ channel blockers |
| NO synthase inhibitors |
| Neurotrophins/growth factors |
| Regeneration/regrowth/replacement of axons |
| Spanner neural grafts |
| Growth factors |
| Transglutaminases/interleukin-2 dimerizers/oligodendrocytotoxins |
| Neuroimmunomodulation—appropriately activated macrophages, T cells |
| Gene therapy (e.g., using viral vectors) vs. small molecule (drug) therapy to modulate the various pathways listed |
| Difficult and risks of gene and drug delivery to the posterior ocular segment |
| Duration and specificity of gene expression and drug effects whether delivery is local or systemic |
The paper by Neufeld, Sawada, and Becker in this issue of the Proceedings (22), demonstrating protection of the optic nerve/retinal ganglion cells from the neurotoxic effects of chronically elevated IOP in a live animal model, by inhibition of the inducible isoform of nitric-oxide synthase (NOS-2), is the first to do so, and will likely be considered classic in years to come. The authors had previously demonstrated the presence of NOS-2 in astrocytes in the optic nerve head and lamina cribrosa of glaucomatous human eyes and chronically hypertensive rat eyes, and its absence in normotensive eyes in these species. They postulated that excessive NO produced and released by these reactive astrocytes led to death and loss of retinal ganglion cell axons, and then the ganglion cells themselves. However, it was not clear whether the increased NOS-2 activity and consequently increased NO production caused the neuronal damage by one of the mechanisms described above, or whether it was consequent, or perhaps even unrelated, to the neuronal damage. By demonstrating that chronic oral administration of aminoguanidine, a relatively specific inhibitor of NOS-2, prevents excavation and axonal degeneration in the optic disk, and loss of retinal ganglion cells, in rats with iatrogenically elevated IOP, they have both validated NO toxicity as an important pathophysiological component of IOP-induced glaucomatous optic neuropathy and identified a potential therapeutic strategy. Furthermore, the significance of their findings may go far beyond glaucoma, with broad pathophysiologic and therapeutic implications for neurodegenerative and neurovascular diseases in general. The roads are of course long and winding from identifying IOP-inducible NO production and consequent optic neurotoxicity to understanding the complete pathophysiological sequence of events: from rat to human; from glaucoma to diseases such as Parkinson’s, Alzheimer’s, multiple sclerosis, amyotrophic lateral sclerosis, or stroke; and from pathophysiology to therapy. Nonetheless, “the journey of a thousand miles begins with a single step.”
Acknowledgments
I am grateful to Dr. Morton Smith for the optic nerve head and retinal photomicrographs and to Eli Zamir for preparation of the electronic images. This commentary was supported in part by National Eye Institute Grant EY02698.
Footnotes
A commentary on this article begins on page 9944.
References
- 1.Shields M B, Ritch R, Krupin T. In: The Glaucomas, Vol. II, Clinical Science. 2nd Ed. Ritch R, Shields M B, Krupin T, editors. St. Louis: Mosby; 1996. pp. 717–725. [Google Scholar]
- 2.Tielsch J M, Sommer A, Katz J, Royall R M, Quigley H A, Javitt J. J Am Med Assoc. 1991;266:369–374. [PubMed] [Google Scholar]
- 3.Leske M C, Connell A M S, Schachat A P, Hyman L. Arch Ophthalmol. 1994;112:821–829. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 4.Quigley H A, Enger C, Katz J, Sommer A, Scott R, Gilbert D. Arch Ophthalmol. 1994;112:644–649. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 5.Klein B E, Klein R, Sponsel W E, Franke T, Cantor L B, Martone J, Menage M J. Ophthalmology. 1992;99:1499–1504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 6.Garratt S. Primary Open-Angle Glaucoma. Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology; 1996. [Google Scholar]
- 7.Schumer R A, Podos S M. Arch Ophthalmol. 1994;112:37–44. doi: 10.1001/archopht.1994.01090130047015. [DOI] [PubMed] [Google Scholar]
- 8.Nickells R W. J Glaucoma. 1997;5:345–356. [PubMed] [Google Scholar]
- 9.Levin L A. Curr Opin Ophthalmol. 1997;8:9–15. doi: 10.1097/00055735-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez M R, Gong H. In: The Glaucomas, Vol. I, Basic Sciences. 2nd Ed. Ritch R, Shields M B, Krupin T, editors. St. Louis: Mosby; 1996. pp. 213–249. [Google Scholar]
- 11.Dreyer E B, Zurakowski D, Schumer R A, Podos S M, Lipton S A. Arch Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- 12.Kerrigan L A, Zack D J, Quigley H A, Smith S D, Pease M E. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 13.Quigley H A, Nickells R W, Kerrigan L A, Pease M E, Thibault D J, Zack D J. Invest Ophthalmol Visual Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 14.Schwartz M, Belkin M, Solomon A. J Glaucoma. 1996;5:427–432. [PubMed] [Google Scholar]
- 15.Yoles E, Schwartz M. Surv Ophthalmol. 1998;42:367–372. doi: 10.1016/s0039-6257(97)00123-9. [DOI] [PubMed] [Google Scholar]
- 16.Eitan S, Schwartz M. Science. 1993;261:106–108. doi: 10.1126/science.8100369. [DOI] [PubMed] [Google Scholar]
- 17.Eitan S, Solomon A, Lavie V, Yoles E, Hirschberg D L, Belkin M, Schwartz M. Science. 1994;264:1764–1768. doi: 10.1126/science.7911602. [DOI] [PubMed] [Google Scholar]
- 18.Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen I R, Schwartz M. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 19.Hirschberg D L, Schwartz M. J Neuroimmunol. 1995;61:89–96. doi: 10.1016/0165-5728(95)00087-i. [DOI] [PubMed] [Google Scholar]
- 20.Lazarov-Spiegler O, Solomon A S, Zeev-Brann A B, Hirschberg D L, Lavie V, Schwartz M. FASEB J. 1996;10:1296–1302. doi: 10.1096/fasebj.10.11.8836043. [DOI] [PubMed] [Google Scholar]
- 21.Vidal-Sanz M, Bray G M, Aguayo A J. J Neurocytol. 1991;20:940–952. doi: 10.1007/BF01190471. , and erratum (1992) 21, 234. [DOI] [PubMed] [Google Scholar]
- 22.Neufeld A H, Sawada A, Becker B. Proc Natl Acad Sci USA. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]