Abstract
Acutely ill patients with candidemia frequently suffer from renal insufficiency. Voriconazole's intravenous formulation with sulfobutylether beta-cyclodextrin (SBECD) is restricted in patients with renal insufficiency. We evaluated the use of intravenous voriconazole formulated with SBECD in candidemic patients with renal insufficiency and compared treatment outcome and safety to those who received a short course of amphotericin B deoxycholate followed by fluconazole. We reviewed data on treatment outcome, survival, safety, and tolerability from the subset of patients with moderate (creatinine clearance [CrCl], 30 to 50 ml/min) or severe (CrCl, <30 ml/min) renal insufficiency enrolled in a trial of voriconazole compared to amphotericin B deoxycholate followed by fluconazole for treatment of candidemia in 370 patients. Fifty-eight patients with renal impairment were identified: 41 patients on voriconazole and 17 on amphotericin B/fluconazole. The median duration of treatment was 14 days for voriconazole (median, 7 days intravenous) and 11 days for amphotericin B/fluconazole, 3 days of which were for amphotericin B. Despite the short duration of exposure, worsening of renal function or newly emerged renal adverse events were reported in 53% of amphotericin B-treated patients compared to 39% of voriconazole-treated patients. During treatment, median serum creatinine decreased in the voriconazole arm, whereas creatinine increased in the amphotericin B/fluconazole arm, before return to baseline at week 3. All-cause mortality at 14 weeks was 49% in the voriconazole arm compared to 65% in the amphotericin B/fluconazole arm. Intravenous voriconazole formulated with SBECD was effective in patients with moderate or severe renal insufficiency and candidemia and was associated with less acute renal toxicity than amphotericin B/fluconazole.
INTRODUCTION
Acutely ill patients with candidemia often have comorbidities, such as renal failure, hepatic insufficiency, or respiratory failure. In a large randomized trial, voriconazole was shown not to be inferior to a regimen of amphotericin B deoxycholate followed by fluconazole for first-line treatment of candidemia in nonneutropenic patients and was associated with fewer adverse events (AE) than the comparator regimen (6). Based on these data, voriconazole has been approved for treatment of candidemia in nonneutropenic patients, but use of the intravenous (i.v.) formulation with sulfobutylether beta-cyclodextrin (SBECD) is not recommended in patients with renal insufficiency. Information on patients with impaired renal function is scarce. Three small studies on critically ill patients requiring hemodialysis showed accumulation of SBECD over time without observation of any toxic effects (3, 4, 10).
We reviewed the use of i.v. voriconazole formulated with SBECD followed by oral voriconazole in a subset of candidemic patients with moderate or severe renal insufficiency from a recent comparative study and compared the treatment outcome and safety to those of patients who received the standard treatment, consisting of a short course of amphotericin B deoxycholate followed by fluconazole (amphotericin B/fluconazole).
MATERIALS AND METHODS
We retrospectively reviewed data from the previously published prospective trial of voriconazole compared to a regimen of amphotericin B followed by fluconazole for first-line treatment of candidemia in 370 nonneutropenic patients (6). The patients were randomized (2:1) to receive either voriconazole or the regimen of amphotericin B followed by fluconazole. Patients with significant renal impairment at initial evaluation (creatinine, >2.5 mg/dl) were excluded from enrollment; all patients included in this analysis were part of the previously published trial (6). Voriconazole was administered intravenously at 6 mg/kg of body weight every 12 h for 24 h and then at 3 mg/kg every 12 h. After 3 days, patients could be switched to oral voriconazole at 200 mg twice daily. Amphotericin B was administered at 0.7 to 1.0 mg/kg/day, infused over 2 to 6 h. Investigators were required to discontinue amphotericin B and switch to either oral or intravenous fluconazole at 400 mg/day after giving the initial therapy for a minimum of 3 days and a maximum of 7 days, with the exception of patients infected with an isolate resistant to fluconazole. Patients unable to tolerate 3 days of amphotericin B could be switched to fluconazole even before day 4. The patients received treatment for at least 2 weeks after the last positive blood culture, with a maximum duration of 8 weeks. The primary efficacy analysis of this randomized, physician-unblinded study was based on clinical and mycological responses 12 weeks after the end of therapy, assessed by an independent, blinded data review committee (DRC).
From this database, we collected treatment outcome, survival, safety, and tolerability data from all patients with moderate to severe renal insufficiency at study entry. Serum creatinine and creatinine clearance (CrCl) were assessed at least twice weekly during antifungal therapy, at the end of therapy, and 2 weeks after the end of therapy. Creatinine clearance was calculated using the Cockroft-Gault equation. For the present analysis, baseline renal insufficiency was defined as a CrCl of <50 ml/min. Moderate renal insufficiency was defined as a baseline CrCl of 30 to 50 ml/min and severe renal insufficiency as a CrCl of <30 ml/min. For each individual patient, the course of serum creatinine (the difference between actual and baseline serum creatinine values) per treatment week was analyzed. The highest serum creatinine value per treatment week per patient was taken. For the assessment of risk factors for renal impairment and outcome of infection, the data on patients with baseline renal impairment were compared to the data on all study patients with normal baseline renal function.
Statistical analysis.
Univariate and multivariate analyses were performed to evaluate any association between worsening of renal function (creatinine doubling or a creatinine rise of >1 mg/dl) or mortality and the variables age, sex, ethnicity, baseline renal function, randomization arm, body weight, Acute Physiology and Chronic Health Evaluation (APACHE) II score, intensive care unit (ICU) admission at baseline, and Asian ethnicity, the last in view of the potential effect of the high incidence of slow voriconazole metabolizers predominantly present in Asians. Categorical data were analyzed using chi-square tests or logistic regression analysis as appropriate. Multivariate analyses were performed by logistic regression analysis. All analyses were conducted using SPSS (PASW Statistics 18) statistical software.
RESULTS
A total of 370 patients with blood culture-confirmed candidemia were enrolled in the overall study. Of these 370 patients, 58 (15.7%) had renal impairment (CrCl, <50 ml/min) at baseline. As severe renal failure (serum creatinine, >2.5 mg/dl) was an exclusion criterion in the original study protocol, most patients with baseline renal impairment had a creatinine clearance of <50 ml/min, while serum creatinine was between 1 and 2.5 mg/dl. Only 2 of 370 patients had been randomized into the trial despite a baseline serum creatinine of >2.5 mg/dl and thus constituted protocol violations. According to the protocol, these patients were included in the modified intent-to-treat analyses. Table 1 shows the baseline characteristics of the patients with renal insufficiency.
Table 1.
Baseline characteristics of study patients with moderate or severe renal insufficiency (CrCl, <50 ml/min) at study entry
| Characteristic | Value |
|
|---|---|---|
| Voriconazole (n = 41) | Amphotericin B/fluconazole (n = 17) | |
| Sex (male) (%) | 44 | 53 |
| Age (median, range) | 70 (18–90) | 71 (36–87) |
| APACHE II-score (median, range) | 15 (5–41) | 19 (7–29) |
| Surgical patient (%) | 46 | 53 |
| In ICU at baseline (%) | 54 | 41 |
| Mechanical ventilation (%) | 29 | 47 |
| Renal impairmenta | ||
| Moderate [no. (%)] | 32 (78) | 13 (76) |
| Severe [no. (%)] | 9 (22) | 4 (24) |
| Causative microorganism | ||
| C. albicans [no. (%)] | 17 (41) | 10 (58) |
| C. glabrata [no. (%)] | 5 (12) | 3 (18) |
| C. parapsilosis [no. (%)] | 6 (15) | 0 |
| C. tropicalis [no. (%)] | 8 (20) | 1 (6) |
| C. krusei [no. (%)] | 1 (2) | 0 |
| Mixed and other [no. (%)] | 4 (10) | 3 (18) |
Moderate renal impairment, baseline creatinine clearance of 30 to 50 ml/min; severe renal impairment, baseline creatinine clearance of <30ml/min
The median duration of antifungal treatment of the subset of patients with baseline renal insufficiency was 13 days (range, 1 to 52 days); in patients randomized to voriconazole, the median duration of treatment was 14 days (range, 2 to 52 days), of which 7 days (2 to 32) were intravenous therapy containing SBECD. In the amphotericin B/fluconazole group, the duration of treatment was 11 days (1 to 48), of which 3 days (1 to 11) were intravenous amphotericin B, followed by fluconazole. Patients with severe renal impairment received 7 days (2 to 15) of i.v. voriconazole (9 patients) or 4 days (2 to 7) of i.v. amphotericin B (4 patients).
Course of renal function.
The course of serum creatinine over time in patients with moderate or severe renal insufficiency at study entry is shown in Fig. 1. During randomized antifungal treatment, median serum creatinine decreased compared to that at baseline in the voriconazole arm, whereas serum creatinine increased in the amphotericin B/fluconazole arm before return to baseline. The changes in creatinine clearance showed similar differences between randomization arms, i.e., an increase during antifungal treatment with voriconazole and an initial decrease in the amphotericin B/fluconazole arm (data not shown).
Fig 1.
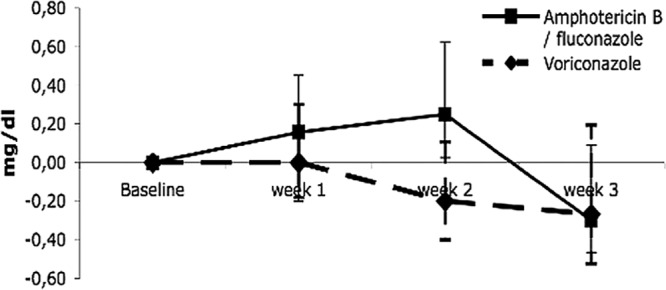
Median change in serum creatinine relative to baseline for 3 weeks after randomization. The bars indicate the 25th and 75th percentiles.
In the univariate analysis of the entire trial cohort of 370 patients, age, body weight, and randomized treatment with amphotericin B/fluconazole were associated with risk for a creatinine rise of >1 mg/dl (Table 2). In the multivariate analysis, randomization to voriconazole remained associated with a lower risk of creatinine rise, indicating that randomization to amphotericin B/fluconazole was associated with a risk of a creatinine rise of >1 mg/dl with an odds ratio (OR) of 1.99 (range, 1.088 to 3.642; P = 0.026).
Table 2.
Univariate and multivariate analyses on all 370 study patients
| Characteristica | Unadjusted OR (95% CI) | P | Adjusted OR (95%CI) | P |
|---|---|---|---|---|
| Risk for mortality | ||||
| Randomization arm (vori vs amB) | 0.77 (0.49–1.19) | 0.238 | 0.847 (0.517–1.385) | 0.508 |
| Age (continuous) | 1.03 (1.02–1.04) | <0.001 | 1.037 (1.021–1.052) | <0.001 |
| APACHE II (≥20 vs <20) | 3.62 (2.12–6.18) | <0.001 | 2.562 (1.355–4.845) | 0.004 |
| Baseline renal insufficiency | 2.17 (1.23–3.82) | 0.007 | 0.913 (0.458–1.821) | 0.797 |
| ICU at baseline (ICU vs non-ICU) | 2.74 (1.77–4.23) | <0.001 | 2.489 (1.483–4.178) | 0.001 |
| Body wt (continuous) | 0.999 (0.976–0.998) | 0.027 | 0.975 (0.960–0.990) | 0.001 |
| Ethnicity (Asian vs other) | 1.555 (0948–2.551) | 0.08 | 1.431 (0.768–2.665) | 0.259 |
| Sex (female vs male) | 0.87 (0.57–1.34) | 0.53 | 0.731 (0.448–1.194) | 0.211 |
| Risk for creatinine doubling | ||||
| Randomization arm (vori vs amB) | 0.64 (0.37–1.08) | 0.095 | 0.657 (0.381–1.134) | 0.132 |
| Age (continuous) | 1.00 (0.99–1.01) | 0.96 | 1.004 (0.988–1.020) | 0.594 |
| APACHE II (≥20 vs <20) | 1.43 (0.77–2.66) | 0.26 | 1.444 (0.712–2.930) | 0.309 |
| Baseline renal insufficiency | 0.54 (0.23–1.25) | 0.15 | 0.452 (0.189–1.134) | 0.091 |
| ICU at baseline (ICU vs non-ICU) | 1.32 (0.78–2.23) | 0.295 | 1.106 (0.671–2.168) | 0.531 |
| Body wt (continuous) | 1.00 (0.99–1.01) | 0.93 | 0.999 (0.984–1.015) | 0.904 |
| Ethnicity (Asian vs other) | 1.030 (0.554–1.916) | 0.925 | 1.061 (0.519–2.167) | 0.871 |
| Sex (female vs male) | 1.14 (0.68–1.93) | 0.62 | 0.219 (0.710–2.114) | 0.466 |
| Risk for creatinine increase (>1mg/dl) | ||||
| Randomization arm (vori vs amB) | 0.53 (0.30–0.96) | 0.03 | 0.502 (0.275–0.919) | 0.026 |
| Age (continuous) | 1.02 (1.001–1.034) | 0.04 | 1.016 (0.996–1.034) | 0.117 |
| APACHE II (≥20 vs <20) | 1.90 (0.99–3.63) | 0.051 | 1.672 (0.774–3.612) | 0.191 |
| Baseline renal insufficiency | 1.24 (0.58–2.62) | 0.58 | 1.071 (0.448–2.563) | 0.877 |
| ICU at baseline (ICU vs non-ICU) | 1.02 (0.58–1.81) | 0.94 | 0.808 (0.414–1.575) | 0.531 |
| Body wt (continuous) | 1.02 (1.00–1.03) | <0.05 | 1.019 (1.003–1.036) | 0.023 |
| Ethnicity (Asian vs other) | 0.959 (0.480–1.917) | 0.906 | 1.514 (0.676–3.388) | 0.313 |
| Sex (female vs male) | 0.77 (0.43–1.40) | 0.39 | 0.867 (0.467–1.613) | 0.653 |
vori, voriconazole; amB, amphotericin B.
In the univariate analysis, patients with normal baseline renal function who were randomized to amphotericin B/fluconazole experienced creatinine doubling significantly more often than those receiving voriconazole (27% [28/105] versus 17% [35/207]; OR, 1.787; 95% confidence interval [CI], 1.016 to 3.144; P = 0.04). However, in the multivariate analysis, this effect was not significant (OR, 1.765; 95% CI, 0.989 to 3.149; P = 0.055).
In a separate analysis of the subgroup of patients with baseline renal impairment, none of the variables (age, sex, ethnicity, body weight, baseline renal function, randomization arm, APACHE II score, and ICU at baseline) had a statistically significant effect on creatinine doubling (data not shown).
Adverse events. (i) Renal adverse events.
In patients with renal impairment at baseline, renal adverse events (AE), such as oliguria, anuria, doubling of serum creatinine, hypokalemia, and hyperkalemia, were reported in 54% (22/41) of patients randomized to voriconazole and in 53% (9/17) of patients on amphotericin B/fluconazole (P = 0.96). However, significantly fewer new renal AE were reported in patients with normal baseline renal function receiving voriconazole (74/207 [36%]) than in patients with normal baseline renal function receiving amphotericin B/fluconazole (61/105 [58%]; P = <0.01).
(ii) Serious adverse events.
In patients with renal insufficiency at baseline, serious adverse events were reported in 31/41 (76%; voriconazole) versus 14/17 (82%; amphotericin B/fluconazole) patients (P = 0.58). However, only a few serious adverse events were reported to be potentially related to intravenous voriconazole or SBECD; they were septic shock (n = 1), ventricular arrhythmias (n = 1), cardiac arrest (n = 1), and elevated liver enzymes (n = 1). Serious adverse events reported to be potentially related to amphotericin B/fluconazole were dyspnea (n = 1), cardiac arrest (n = 1), and renal failure (n = 1).
(iii) Nonrenal adverse events.
In neither randomization arm was there a significant relationship between baseline renal function and the incidence of adverse events, such as hepatic toxicity, infusion-related events, or cardiovascular events, except for the occurrence of multiorgan failure in patients with baseline renal impairment who were randomized to amphotericin B/fluconazole (voriconazole, 0/41, versus amphotericin B/fluconazole, 2/17; P = 0.025).
Outcome of candidemia.
In patients with baseline renal impairment, treatment of candidemia was successful at the end of randomized study drug treatment (EOT) in 26/41 (63.4%) patients randomized to voriconazole and 11/17 (64.7%) of those randomized to amphotericin B/fluconazole (P = 0.93). In all 370 randomized study patients, the success rates were similar: 178/248 (72%; voriconazole) and 86/122 (70%; amphotericin B/fluconazole; P = 0.80).
Also, there were no significant differences in sustained success rates at 12 weeks of follow-up. For voriconazole patients with baseline renal impairment, the success rate was 34.1% (14/41) versus 40.7% (101/248) for all voriconazole-treated patients (P = 0.43). For patients with baseline renal impairment randomized to amphotericin B/fluconazole, the sustained success rate was 23.5% (4/17) versus 41.0% (50/122) for all patients on amphotericin B/fluconazole (P = 0.17).
Mortality.
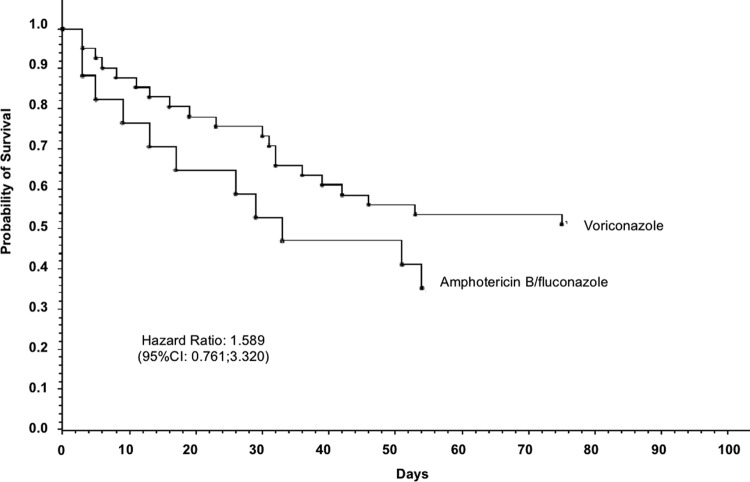
All-cause mortality at 14 weeks among patients with baseline renal impairment was 20/41 (49%) in the voriconazole arm compared to 11/17 (65%) in the amphotericin B/fluconazole arm (Fig. 2).
Fig 2.
Probability of survival in patients with baseline renal impairment.
In the univariate analysis, baseline renal impairment was associated with a higher risk of mortality (OR, 2.17; 95% CI, 1.23 to 3.82); however, this association disappeared when the other variables were adjusted for (OR, 0.797). In the multivariate analysis, an APACHE II score of ≥20 (OR, 2.562), ICU admission at baseline (OR, 2.489), age (OR, 1.04 per year), and lower body weight were factors significantly associated with risk for mortality (Table 2).
Notably, in patients with severe renal insufficiency at baseline, mortality was 56% (5/9) in patients on voriconazole and 100% (4/4) in patients on amphotericin B/fluconazole (P = 0.11). As judged by the blinded DRC, invasive candidiasis was probably contributory to death in 12% (5/41) of voriconazole-treated patients and in 24% (4/17) of amphotericin B/fluconazole-treated patients (P = 0.28) (Table 3).
Table 3.
Mortality in patients with and without baseline renal insufficiency
| Patient group | Mortality at 14 wk [no./total (%)] |
P value | |
|---|---|---|---|
| Voriconazole | Amphotericin B/fluconazole | ||
| All patients | 88/248 (36) | 51/122 (42) | 0.23 |
| Without baseline renal insufficiency | 68/207 (33) | 40/105 (38) | 0.36 |
| Baseline renal insufficiency | 20/41 (49)a | 11/17 (65)b | 0.27 |
| Moderate renal insufficiency | 15/32 (47) | 7/13 (54) | 0.67 |
| Severe renal insufficiency | 5/9 (56) | 4/4 (100) | 0.11 |
| Candidemia-related mortality | 5/41 (12) | 4/17 (24) | 0.28 |
P = 0.051 compared to patients without baseline renal insufficiency randomized to voriconazole.
P = 0.039 compared to patients without baseline renal insufficiency randomized to amphotericin B/fluconazole.
DISCUSSION
This is the largest study on the effects of i.v. voriconazole in patients with renal insufficiency to date. The i.v. formulation of voriconazole includes SBECD as a solubilizer (12). The clearance of SBECD is linearly related to creatinine clearance, and in subjects with normal renal function, its terminal half-life is 1.6 h. Earlier studies reported that the maximum concentration of drug in serum (Cmax) of SBECD increases by 50% in subjects with a creatinine clearance of 30 to 50 ml/min compared to healthy subjects (1). In patients on hemodialysis receiving 200 to 400 mg of voriconazole twice daily i.v., SBECD trough levels were 271 to 581 μg/ml compared to <100 μg/ml in the case of normal renal function (10). Whereas SBECD is eliminated by hemodialysis, SBECD trough levels in hemodialysis patients still exceed those of patients with normal renal function by a factor of 6 (4, 10).
The acute toxicity of SBECD has been low in animal experiments (7). Repeated doses of i.v. SBECD up to 2,000 mg/kg resulted in changes in renal histopathology, such as cytoplasmic vacuolation in the renal tubules of rodents (7; http://www.fda.gov/ohrms/dockets/ac/01/briefing/3792b2_01_Pfizer.pdf). However, functional renal changes were not observed after prolonged exposures to cyclodextrin compounds up to 600 mg/kg per day for 6 months (7).
There are no clinical data regarding the potential toxicity of SBECD in humans. The manufacturer required that, in patients with moderate or severe renal insufficiency (creatinine clearance, <50 ml/min), oral voriconazole should be administered, unless an assessment of the benefit versus risk to the patient justifies the use of intravenous voriconazole (http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021266s023,021267s024,021630s013lbl.pdf). In three reports on patients receiving voriconazole while on hemodialysis, no toxic effects were observed (3, 4, 10).
A retrospective study assessed the impact of i.v. voriconazole on renal function in critically ill ICU patients treated for at least 3 days with i.v. voriconazole (2, 8). Of 69 patients treated with i.v. voriconazole, 26 had impaired renal function at baseline. Renal toxicity was observed in 13 patients (30.2%) with normal baseline renal function compared to 4 patients (15.4%) with impaired baseline renal function (P = 0.257).
In the present study, 41 patients receiving intravenous voriconazole in SBECD despite renal insufficiency at baseline were followed prospectively. The mycological and clinical outcomes in these subjects were not significantly different from those in patients randomized to amphotericin B/fluconazole. Notably, mortality in patients receiving amphotericin B/fluconazole despite baseline renal impairment was very high (65%), significantly higher than in patients without renal insufficiency receiving amphotericin B/fluconazole (P = 0.039).
Consistent with this observation, randomization to amphotericin B/fluconazole was significantly associated with a risk of a creatinine rise of >1 mg/dl in the entire trial cohort of 370 patients. This underscores the notion that even a short course of amphotericin B deoxycholate may be associated with severe adverse effects (6, 9).
Given the unknown consequences of SBECD in patients with renal insufficiency, it is noteworthy that the numbers of overall adverse effects were not increased compared to patients with renal failure treated with amphotericin B/fluconazole. Thus, whereas the present data do not support a more liberal use of voriconazole in SBECD in patients with renal insufficiency, its use appeared to be appropriate in selected patients. Voriconazole may be considered the drug of choice in patients with fluconazole-resistant invasive candidiasis at sites were echinocandins reach insufficient concentrations, e.g., in the central nervous system or the eye. Importantly, in patients with aspergillosis, voriconazole has been recommended as the drug of choice (5, 11). In these patients, oral administration of antifungal drugs often is difficult to accomplish due to mucositis and poor resorption.
In conclusion, data on the use of intravenous voriconazole in patients with renal insufficiency remain scarce. In this retrospective analysis, treatment outcome and survival were no less than expected, and few serious adverse events were reported to be potentially related to intravenous voriconazole or SBECD. The data reviewed here suggest that intravenous voriconazole may be appropriate for renally impaired patients with fungal infections for which voriconazole is considered to be the drug of choice.
ACKNOWLEDGMENTS
The original study was sponsored by Pfizer Inc. The present analysis was done by A. M. L. Oude Lashof and B. J. Kullberg at Nijmegen Institute for Infection, Inflammation, and Immunity. B. J. Kullberg and A. M. L. Oude Lashof received no compensation for this work.
Footnotes
Published ahead of print 26 March 2012
REFERENCES
- 1. Abel S, et al. 2008. Pharmacokinetics, safety and tolerance of voriconazole in renally impaired subjects: two prospective, multicentre, open-label, parallel-group volunteer studies. Clin. Drug Invest. 28:409–420 [DOI] [PubMed] [Google Scholar]
- 2. Alvarez-Lerma F, et al. 2008. Impact of intravenous administration of voriconazole in critically ill patients with impaired renal function. J. Chemother. 20:93–100 [DOI] [PubMed] [Google Scholar]
- 3. Burkhardt O, Thon S, Burhenne J, Welte T, Kielstein JT. 2010. Sulphobutylether-beta-cyclodextrin accumulation in critically ill patients with acute kidney injury treated with intravenous voriconazole under extended daily dialysis. Int. J. Antimicrob. Agents 36:93–94 [DOI] [PubMed] [Google Scholar]
- 4. Hafner V, et al. 2010. Pharmacokinetics of sulfobutylether-beta-cyclodextrin and voriconazole in patients with end-stage renal failure during treatment with two hemodialysis systems and hemodiafiltration. Antimicrob. Agents Chemother. 54:2596–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbrecht R, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 6. Kullberg BJ, et al. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 366:1435–1442 [DOI] [PubMed] [Google Scholar]
- 7. Luke DR, Tomaszewski K, Damle B, Schlamm HT. 2010. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD). J. Pharm. Sci. 99:3291–3301 [DOI] [PubMed] [Google Scholar]
- 8. Pfaller MA, et al. 2001. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J. Clin. Microbiol. 39:3254–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saliba F, Dupont B. 2008. Renal impairment and amphotericin B formulations in patients with invasive fungal infections. Med. Mycol. 46:97–112 [DOI] [PubMed] [Google Scholar]
- 10. von Mach MA, Burhenne J, Weilemann LS. 2006. Accumulation of the solvent vehicle sulphobutylether beta cyclodextrin sodium in critically ill patients treated with intravenous voriconazole under renal replacement therapy. B.M.C. Clin. Pharmacol. 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walsh TJ, et al. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 12. Walsh TJ, et al. 2000. New targets and delivery systems for antifungal therapy. Med. Mycol. 38(Suppl. 1):335–347 [PubMed] [Google Scholar]