Abstract
Rilpivirine is a nonnucleoside reverse transcriptase inhibitor (NNRTI) recently developed as a drug of choice for initial antiretroviral treatment of HIV-1 infection. Disturbances in lipid metabolism and, ultimately, in adipose tissue distribution and function are common concerns as secondary effects of antiretroviral treatment. Efavirenz, the most commonly used NNRTI, causes mild dyslipidemic effects in patients and strongly impaired adipocyte differentiation in vitro. In this study, we provide the first demonstration of the effects of rilpivirine on human adipocyte differentiation, gene expression, and release of regulatory proteins (adipokines and cytokines) and compare them with those caused by efavirenz. Rilpivirine caused a repression of adipocyte differentiation that was associated with impaired expression of the master adipogenesis regulators peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT enhancer binding protein alpha (C/EBPα), and sterol regulatory element binding transcription factor 1 (SREBP-1) and their target genes encoding lipoprotein lipase and the adipokines leptin and adiponectin. Rilpivirine also repressed adiponectin release by adipocytes, but only at high concentrations, and did not alter leptin release. Rilpivirine induced the release of proinflammatory cytokines (interleukin-6 and -8, monocyte chemoattractant protein 1 [MCP-1], plasminogen activator inhibitor type 1 [PAI-1]) only at very high concentrations (10 μM). A comparison of the effects of rilpivirine and efavirenz at the same concentration (4 μM) or even at lower concentrations of efavirenz (2 μM) showed that rilpivirine-induced impairment of adipogenesis and induction of proinflammatory cytokine expression and release were systematically milder than those of efavirenz. It is concluded that rilpivirine causes an antiadipogenic and proinflammatory response pattern, but only at high concentrations, whereas efavirenz causes similar effects at lower concentrations.
INTRODUCTION
Metabolic disturbances are common in HIV-1-infected patients undergoing combined antiretroviral treatment (cART). The main alterations are dyslipidemia (including hypertriglyceridemia and hypercholesterolemia) insulin resistance and, in some cases, overt lipodystrophy, as evidenced by altered distribution and function of adipose tissue in affected individuals. These alterations are the result of a complex interaction of the adverse effects of the drug combinations used in antiretroviral therapy and events associated with the underlying HIV-1 infection. Although no single drug component of the cART regimen may account for the distinct features of altered metabolism in patients, nucleoside-analog reverse transcriptase inhibitors (NRTIs), especially stavudine and zidovudine, are thought to promote lipoatrophy and mitochondrial dysfunction, whereas protease inhibitors (PIs) are considered to favor insulin resistance and dyslipidemia (25).
Nonnucleoside analog inhibitors of reverse transcriptase, in combination with drugs from other families, are drugs of choice for initial antiretroviral treatment of HIV-1-infected patients. Efavirenz is the preferred NNRTI for use in antiretroviral regimens (11, 13), owing to the high antiretroviral efficacy it has shown in pivotal clinical trials (19, 22). NNRTIs have not traditionally been associated with the appearance of lipodystrophy. In fact, they are often perceived as drugs that do not cause adipose alterations, although efavirenz is known to favor an altered pattern of circulating lipids (18). However, clinical trials reported recently that efavirenz could favor lipoatrophy when included as a component in a cART cocktail (14, 19).
Rilpivirine (TMC278) is a recently developed NNRTI (12). Two clinical trials using rilpivirine in naïve patients in association with distinct drug backbones and in comparison with efavirenz have been reported to date (3, 17). Both concurred in the observation that rilpivirine had an antiviral efficacy similar to that of efavirenz and was associated with fewer lipid metabolism-related side effects. These studies basically concluded that treatment with rilpivirine caused a much lower increase in blood triglycerides, total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol than did efavirenz treatment. Further studies confirmed these findings (26). On the other hand, a recent report indicated no change in limb fat in patients under rilpivirine or efavirenz in a clinical trial setting (23). However, the U.S. Food and Drug Administration (FDA) approval document of rilpivirine issued in 2011 included fat redistribution among its warnings and precautions (24).
Studies on the effects of antiretroviral drugs on adipose cells have proven to be useful for initial in vitro assessment of the potential of drugs to disturb adipose tissue and lipid metabolism. Available studies on the action of NNRTIs on adipocytes have shown that efavirenz causes a profound impairment in adipocyte differentiation and promotes the secretion of proinflammatory adipokines and cytokines (7, 9, 10). The potential effects of rilpivirine on adipose cells have not been studied. We report here that rilpivirine alters adipogenic differentiation and induces expression of proinflammatory cytokines by adipose cells but to a lower extent than efavirenz at similar concentrations of treatment.
MATERIALS AND METHODS
Cell culture and drug treatment.
Human adipocyte precursor cells from healthy individuals, obtained from Advancell (Barcelona, Spain), were cultured as previously reported (20). After cells had reached 80% confluence, differentiation was induced by treating cells with Dulbecco's modified Eagle's medium (DMEM)-F12 medium containing 33 μM biotin, 17 μM sodium pantothenate, 200 nM insulin, 25 nM dexamethasone, 0.5 mM IBMX (3-isobutyl-1-methylxanthine), 2 μM rosiglitazone, and 0.2 nM triiodothyronine. After 4 days and every 5 days thereafter, the medium was replaced with culture medium with the same composition but without IBMX, rosiglitazone, and dexamethasone. In untreated cells, maximal differentiation, estimated on the basis of the maximal number of cells accumulating lipid droplets, was attained 15 days after induction of differentiation. For studies on the effects of drugs during adipocyte differentiation, treatment with rilpivirine or efavirenz was initiated on day 0 and continued throughout the differentiation process. Fresh drugs were included every time the medium was replaced. The extent of morphological differentiation was quantified by measuring the percentage of the cell culture surface occupied by adipocytes in relation to controls, defined as 100%.
Cytotoxicity assays.
Potential cytotoxic effects of drugs on differentiating human preadipocytes were determined after exposing differentiating preadipocytes to drugs for 5 days using a CytoTox96 kit (Promega, Madison, WI), which measures the appearance of lactate dehydrogenase (LDH) in the cell culture medium. Data were calculated as a percentage of LDH activity in the medium after exposure to drugs relative to the maximum release after total lysis of cells, as described by the manufacturer.
RNA extraction and transcript quantification.
Total RNA was extracted from cells using an RNAeasy minikit (Qiagen). Reverse transcription was performed in 20 μl using random hexamer primers (Applied Biosystems) and 0.5 μg total RNA. PCRs were conducted in an ABI/Prism 7700 sequence detector system using 25 μl of reaction mixture containing 1 μl of cDNA, 12.5 μl of TaqMan Universal PCR Master Mix, 250 nM probes, and 900 nM primers from Assays-on-Demand gene expression assay mix (TaqMan; Applied Biosystems). The following Assay-on-Demand probes were used: cytochrome c oxidase subunit IV (COX4I1), Hs00266371; CCAAT enhancer binding protein alpha (C/EBPα), Hs00269972; lipoprotein lipase (LPL), Hs00173425; peroxisome proliferator-activated receptor gamma (PPARγ), Hs00234592; sterol regulatory element binding transcription factor 1 (SREBP-1), Hs00231674; monocyte chemoattractant protein-1 (MCP-1), Hs00234140; interleukin-6 (IL-6), Hs00174131; adiponectin, Hs00605917; leptin, Hs00174877; and 18S rRNA, Hs99999901. Primers and probe for the detection of cytochrome c oxidase subunit II (COII) were designed using the Assay-by-Design system (custom TaqMan gene expression assays; Applied Biosystems); primer sequences were 5′-CAA ACC ACT TTC ACC GCT ACA C-3′ (forward) and 5′-GGA CGA TGG GCA TGA AAC TGT-3′ (reverse), and the sequence of the 6-carboxyfluorescein (FAM)-labeled probe was 5′-AAA TCT GTG GAG CAA ACC-3′. Controls with no RNA, primers, or reverse transcriptase were included in each set of experiments. Each sample was run in duplicate, and the mean value of the duplicate was used to calculate the relative amount of individual mRNAs. Each mean value was normalized to that of the 18S rRNA gene using the comparative (2−ΔCT) method following the manufacturer's instructions.
Quantification of adipokines and cytokines in the cell culture medium.
Adipokines and cytokines released by adipocytes and accumulated in the cell culture medium were measured during the last 5 days of culture. A total of 25 μl of medium from adipocyte cultures before harvest was used. Adiponectin, leptin, MCP-1, interleukin-6, interleukin-8, total plasminogen activator inhibitor type 1 (PAI-1), hepatocyte growth factor (HGF), and nerve growth factor (NGF) were quantified using a multiplex analysis system based in fluorescently labeled microsphere beads linked to specific antibodies (Linco Research/Millipore). Quantification was performed with Luminex100ISv2 equipment. The multiplex system used (HADCYT-61K; Millipore) also allowed the quantification of interleukin-1b, resistin, and tumor necrosis factor alpha, with results below the detection limits in the adipocyte culture medium in all the conditions tested (0.1 pg/ml for interleukin-1b, 0.1 pg/ml for tumor necrosis factor alpha, and 4.5 pg/ml for resistin). Where appropriate, statistical analysis was performed by Student's t test; significance is indicated in the text.
RESULTS
The cytotoxic effects of rilpivirine on human preadipocytes were quantitatively assessed (Table 1). For exposure to drugs up to 5 days, the results indicated that rilpivirine was not cytotoxic toward preadipocytes at concentrations up to 4 μM; a modest cytotoxic effect was observed at 10 μM rilpivirine. In sharp contrast, 10 μM efavirenz led to massive cytotoxicity (approaching 100%) in this cell system.
Table 1.
Cytotoxic effects of rilpivirine on human preadipocytesa
| Drug | Mean ± SEM percent cytotoxicity at a drug concn (μM) of: |
|||||
|---|---|---|---|---|---|---|
| 0.01 | 0.1 | 1 | 2 | 4 | 10 | |
| Rilpivirine | −0.2 ± 0.03 | 0.9 ± 0.08 | −1.1 ± 0.12 | 0.1 ± 0.02 | 0.1 ± 0.01 | 5.1 ± 0.3* |
| Efavirenz | ND | ND | −0.1 ± 0.01 | 0.2 ± 0.01 | 0.1 ± 0.02 | 98 ± 7 |
Preadipocytes were treated with the indicated drugs for 5 days. Data are expressed as means ± SEMs of the percent cytotoxicity (LDH activity in the cell culture medium) relative to the 100% cytotoxicity control (LDH activity after total lysis of cells). The asterisk indicates a significantly different value (P < 0.05) from 0. ND, not determined.
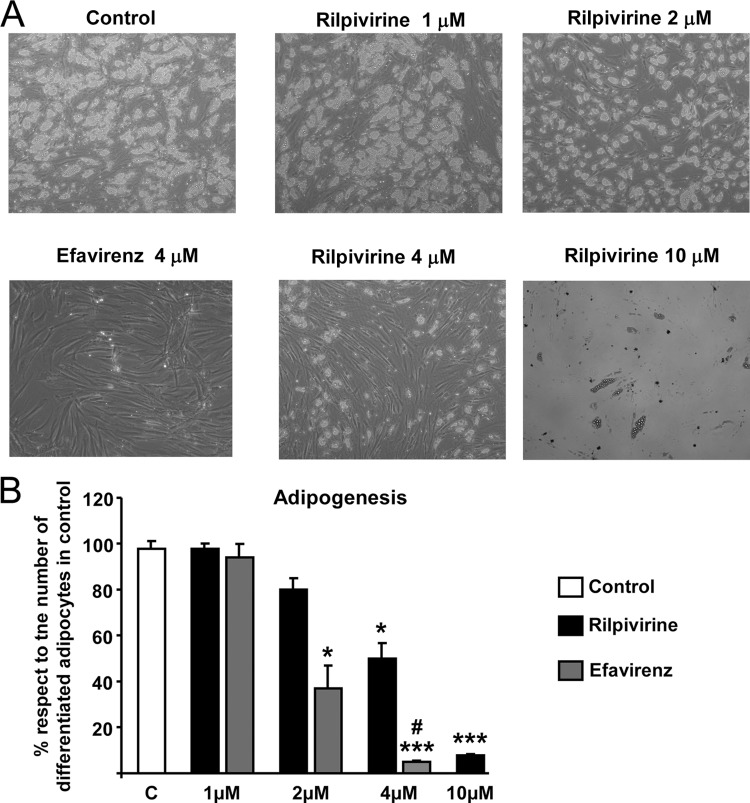
A concentration-response study of rilpivirine effects on adipogenic differentiation of human preadipocytes was performed over a concentration range of 0.01 to 10 μM. Because an assessment of cell morphology indicated that most of the changes elicited by rilpivirine occurred between 1 and 10 μM, the effects of intermediate concentrations (2 and 4 μM) were also analyzed. The results indicated that exposure of cells to concentrations of rilpivirine up to 2 μM had no effect on the acquisition of an adipocyte morphology, as evidenced by unaltered lipid accumulation in cells. In contrast, 2 μM efavirenz caused a significant decrease in adipogenic differentiation (Fig. 1). A small but significant impairment in adipocyte differentiation was observed at 4 μM rilpivirine, whereas this same concentration of efavirenz completely prevented adipocyte differentiation. A similarly massive impairment in adipogenic differentiation of preadipocytes was observed with 10 μM rilpivirine.
Fig 1.
Effects of rilpivirine on human preadipocytes differentiating in culture. Human preadipocytes were differentiated in culture in the presence of the indicated concentrations of drugs. (A) Representative microphotographs of adipocyte cell cultures differentiating in the presence of the indicated concentrations of drugs; (B) bars are means ± standard errors of the means (SEMs) (n = 4 or 5 replicates) of the extent of morphological adipocyte differentiation expressed relative to values from untreated control cells, defined as 100% (see Materials and Methods). *, P < 0.05; and **, P < 0.01 for each drug treatment versus control. #, P < 0.05 for rilpivirine versus efavirenz treatment at the same concentration. Duration of treatment was 15 days.
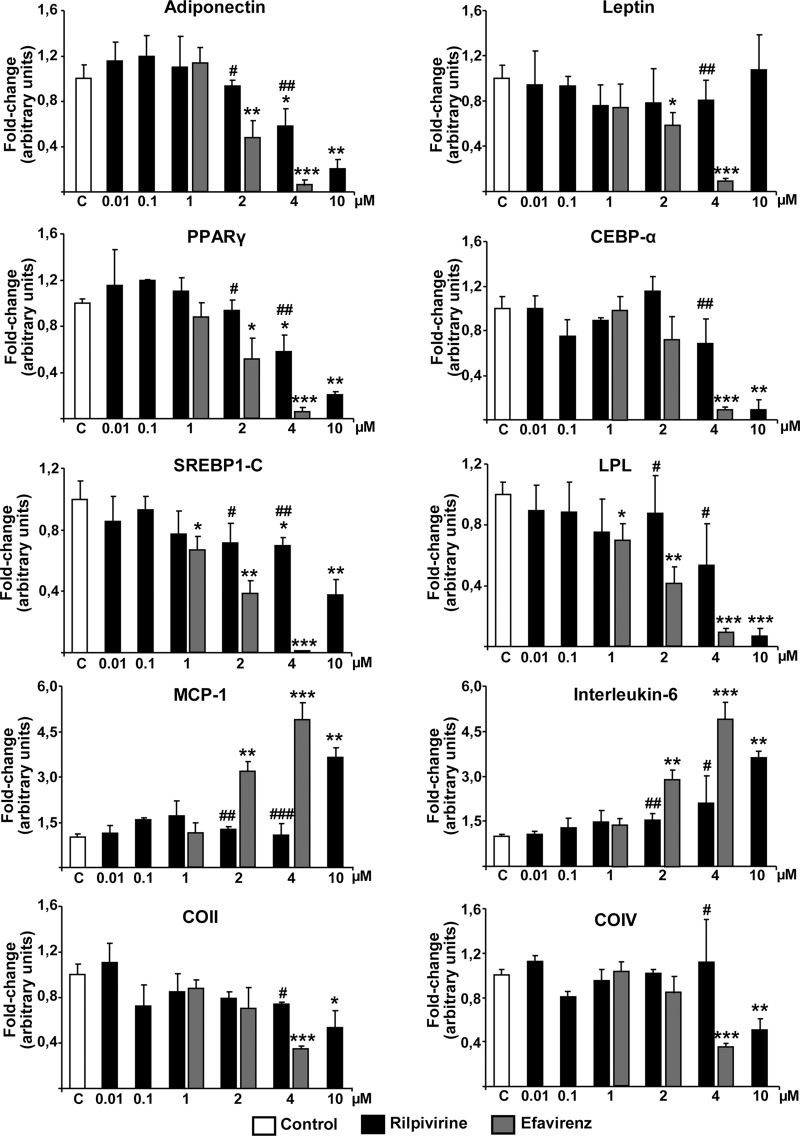
In general, the expression of genes related to acquisition of the differentiated adipocyte phenotype was unaltered by exposure of cells to rilpivirine at concentrations below 4 μM (Fig. 2). Expression of LPL, a marker of the metabolic pathways associated with lipid accumulation in adipogenic differentiation, was significantly reduced by high concentrations of rilpivirine (10 μM) compared with that of controls. The expression of genes for the master transcription factors of adipogenesis, PPARγ and C/EBPα, was also moderately reduced at 4 μM rilpivirine and profoundly impaired at 10 μM. Expression of the lipid metabolism transcriptional regulator, SREBP-1C, was also impaired by rilpivirine, but only at 10 μM. The expression of genes for the adipokine, adiponectin, followed a similar profile, whereas no significant changes were found for leptin gene expression at any rilpivirine concentration tested. A comparison with efavirenz exposure showed that 2 μM efavirenz caused a mild impairment in the expression of most of the adipogenesis-related genes analyzed. Exposure to 4 μM efavirenz caused a dramatic reduction in the expression of adipogenic genes that was much more profound than that induced by 4 μM rilpivirine.
Fig 2.
Effects of rilpivirine on the expression of genes related to adipogenic function, inflammation, and mitochondriogenesis in human adipocytes differentiating in culture. Human preadipocytes were differentiated in culture in the presence of the indicated concentrations of drugs over 15 days. Data on mRNA levels are presented as means ± SEMs from 4 to 5 independent experiments expressed relative to values from untreated control cells (defined as 1). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 for each drug treatment versus control. #, P < 0.05 for rilpivirine versus efavirenz treatment at the same concentration. Duration of treatment was 15 days.
We also analyzed the expression of genes for proinflammatory cytokines. Both MCP-1 and IL-6 gene expressions were significantly induced by rilpivirine only at the highest concentration (10 μM). In contrast, both 2 μM and 4 μM efavirenz caused a marked increase in MCP-1 and IL-6 gene expression relative to that of untreated controls and cells treated with the same concentration of rilpivirine. Finally, the potential effects of rilpivirine on mitochondrial toxicity were tested by determining expression of the gene for COII (mitochondrial DNA-encoded subunit II of cytochrome oxidase) and COIV (nuclear DNA-encoded subunit IV of cytochrome c oxidase). Both transcripts were moderately but significantly reduced by 10 μM rilpivirine. Again, 4 μM efavirenz caused a more marked reduction in COII and COIV transcript levels than both controls and 4 μM rilpivirine.
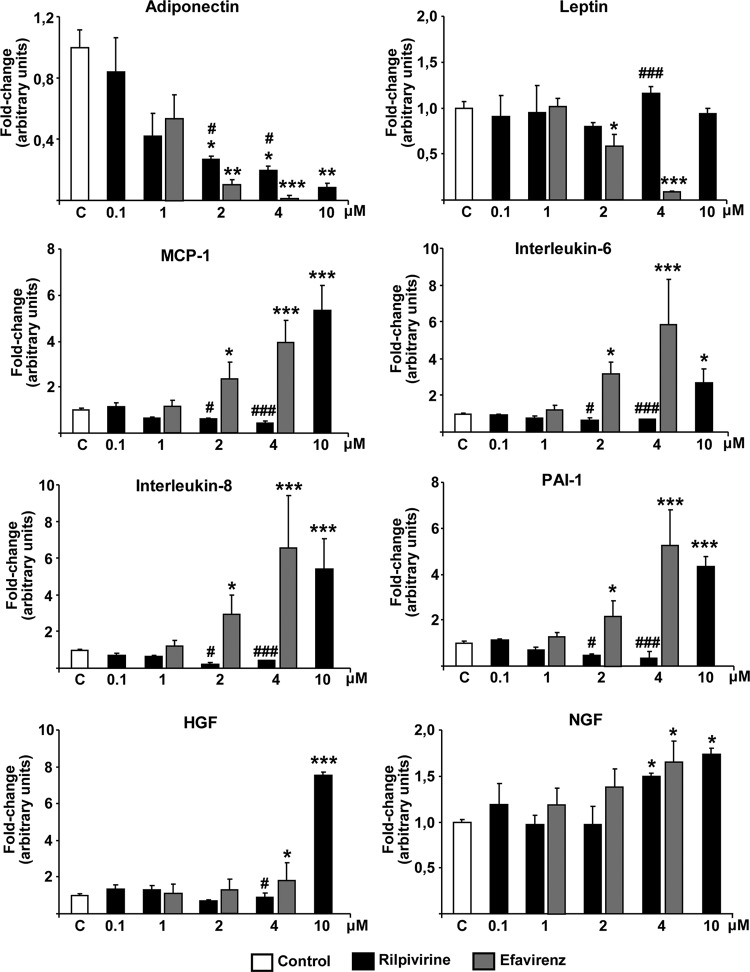
We further analyzed the effects of rilpivirine on the release of adipokines and cytokines into the medium (Fig. 3). Rilpivirine induced a concentration-dependent decrease in the levels of adiponectin in adipocyte culture medium, with 2 μM being the lowest concentration at which adiponectin levels were significantly reduced compared to those in controls. A comparison of the effects of efavirenz and rilpivirine at equal concentrations of 4 μM showed that adiponectin release into the medium was reduced to almost undetectable levels in cells treated with efavirenz, whereas 20% of control levels of adiponectin remained in cells exposed to rilpivirine. Treatment of cells with 2 μM efavirenz caused also a much more marked reduction in adiponectin levels than that caused by 2 μM rilpivirine. Rilpivirine had no effect on leptin release by adipocytes at any concentration tested; in contrast, 2 μM efavirenz significantly reduced leptin release and 4 μM efavirenz almost completely eliminated it. Similar results were obtained for the release of the proinflammatory cytokines, MCP-1, IL-6, IL-8, and PAI-1. Rilpivirine had no effect on the release of any of these cytokines at concentrations up to 4 μM, producing a marked, significant induction only at 10 μM, whereas efavirenz at 4 μM strongly induced the release of these proinflammatory cytokines, increasing their secretion to levels comparable to (MCP-1, IL-8, and PAI-1) or even greater than (IL-6) those elicited by 10 μM rilpivirine. Even 2 μM efavirenz caused a minor but significant induction of release of MCP-1, IL-6, IL-8, and PAI-1. We also analyzed the effects of rilpivirine on the release of HGF and NGF, regulatory factors known to be produced by adipocytes, into the culture medium. HGF release was significantly induced only by 10 μM rilpivirine or 4 μM efavirenz. A similar modest although statistically significant increase in NGF in adipocyte culture medium relative to that of controls was observed in cells exposed to 4 μM rilpivirine or 4 μM efavirenz.
Fig 3.
Effects of rilpivirine on the release of adipokines and cytokines by human adipocytes in culture. Human preadipocytes were differentiated in culture in the presence of the indicated concentrations of drugs. Values represent concentrations of adipokines and cytokines in the cell culture medium from the last 5 days of culture and are presented as means ± SEMs from 4 to 5 independent experiments expressed relative to values from untreated control cells (defined as 1). Mean values of cytokines and adipokines in the medium from control (untreated) cells were as follows: adiponectin, 0.24 ± 0.3 μg/ml; leptin, 9.0 ± 0.8 ng/ml; MCP-1, 3.2 ± 0.4 ng/ml; IL-6, 66 ± 9 pg/ml; IL-8, 29 ± 4 pg/ml; PAI-1, 0.41 ± 0.06 ng/ml; HGF, 85 ± 10 pg/ml; NGF, 8.1 ± 1.1 pg/ml. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 for each drug treatment versus control. #, P < 0.05 for rilpivirine versus efavirenz treatment at the same concentration.
DISCUSSION
We determined that rilpivirine was completely noncytotoxic to preadipocytes at most concentrations tested and during the 5-day exposure to the drug tested, producing only a modest cytotoxic effect at the highest concentrations analyzed. These observations indicate that preadipocytes were somewhat less sensitive to rilpivirine than were the nonadipose cells tested to date, in which significant cytotoxicity has been reported at the low micromolar range (median 50% cytotoxic concentration of rilpivirine, 8.1 μM) (6).
The present study established that rilpivirine may impair acquisition of adipocyte morphology, expression of genes related to adipogenesis, and release of adipokines, such as adiponectin, when preadipocytes are exposed to the drug throughout the adipogenic differentiation process. Rilpivirine also caused a concerted reduction in the expression of master transcription factors of adipogenesis and lipid accumulation (PPARγ, C/EBPα, SREBP-1C) and their metabolic (LPL) and adipokine (adiponectin) targets. However, these effects were significant in most cases only at concentrations of 4 or 10 μM. Although the effects of rilpivirine were qualitatively similar to those of efavirenz (7, 10) (present findings), the concentrations of efavirenz required to induce them were systematically lower. Thus, at the same 4 μM concentration, efavirenz impaired adipocyte differentiation to a dramatically greater degree than did rilpivirine. In fact, 4 μM efavirenz fully suppressed adipogenesis, adipogenic gene expression, and release of adiponectin, whereas 4 μM rilpivirine, despite causing a significant reduction relative to controls, maintained substantial levels of these factors. The complete abrogation of leptin gene expression and release by 4 μM efavirenz was in marked contrast with the normal levels of leptin that prevailed in the presence of 4 or 10 μM rilpivirine. Moreover, most of the effects of efavirenz impairing adipogenic gene expression were found to be significant at 2 μM exposure, a concentration at which rilpivirine either did not cause any effect or impaired expression of adipogenic genes was milder.
The contrast between the effects of rilpivirine and efavirenz was even more marked when the expression of proinflammatory genes and the actual release of proinflammatory cytokines were analyzed. Whereas rilpivirine at concentrations up to 4 μM did not induce any proinflammatory response, 2 μM and 4 μM efavirenz induced the systematic expression and release of the proinflammatory cytokines IL-6, IL-8, MCP-1, and PAI-1, whereas 4 μM efavirenz also increased the release of HGF, an angiogenic factor known to be released by adipose tissue under proinflammatory conditions (1). Rilpivirine caused a similar effect only when its concentration was raised to 10 μM, a concentration at which some degree of cytotoxicity occurs. NGF, a neurotrophin released by adipocytes and induced under conditions of metabolic syndrome (2), was only modestly induced by rilpivirine and efavirenz.
The differential effects of rilpivirine and efavirenz on the release of regulatory molecules by adipose tissue may be especially relevant in relation to the effects of these drugs on systemic metabolism and the healthier effect of rilpivirine on lipid profile relative to that of efavirenz. High levels of proinflammatory cytokines and abnormally lowered adiponectin levels are known to elicit insulin resistance and promote dyslipidemia and lipodystrophy (16, 21). In the absence of systematic assays of adipokine and cytokine levels in clinical studies of rilpivirine, our present data are consistent with a more metabolic-friendly scenario for rilpivirine than for efavirenz.
Overall, the present data indicate that rilpivirine causes deleterious effects in adipocytes that are qualitatively similar to those observed for efavirenz in vitro but much less intense. However, in vitro studies using cells in culture, such as this one, have obvious limitations with respect to their relevance to the treatment of patients. The average rilpivirine plasma concentration after the common 25-mg, once-daily treatment pattern is approximately 0.2 μM (4, 5), indicating that the concentration range at which rilpivirine exerts most of the deleterious effects reported here under in vitro conditions may not be reached in vivo. Moreover, it should be noted that rilpivirine in cell culture studies is added to serum-free adipocyte culture medium; thus, dose for dose, the effective concentration of rilpivirine in cell culture may be higher than the actual free rilpivirine concentration in blood, where serum proteins may bind substantial amounts of drug (15). In contrast with other NNRTIs, including efavirenz, where information on differential tissue distribution in patients is available (8), there is no data relating to the potential accumulation of rilpivirine at higher concentrations in adipose tissue than in blood.
In summary, this first study on the effects of rilpivirine on adipose cells suggests that rilpivirine, at moderate concentrations, would not be expected to exert profound deleterious effects on adipose tissue development or the endocrine function of adipose tissue (adipokine and cytokine release) in treated patients. However, caution should be maintained, considering that lipodystrophy and overall alterations of lipid metabolism in HIV-1-infected patients analyzed to date are caused by complex interactions of a given drug with other cART components, as well as with underlying HIV-1 infection-related events.
ACKNOWLEDGMENTS
This research was supported by grants from Ministerio de Ciencia e Innovación (SAF2008-01896 and SAG2011-23636), Fondo de Investigaciones Sanitarias (PI08-1715), Red de Investigación en SIDA (RD06/006/0022), Instituto de Salud Carlos III, and an independent grant from Janssen-Cilag.
Janssen-Cilag had no role in the study design, data collection, interpretation of data, or preparation of the manuscript.
Footnotes
Published ahead of print 19 March 2012
REFERENCES
- 1. Bell LN, et al. 2008. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am. J. Physiol. Endocrinol. Metab. 294:E336–E344 [DOI] [PubMed] [Google Scholar]
- 2. Bulló M, Peeraully MR, Trayhurn P, Folch J, Salas-Salvadó J. 2007. Circulating nerve growth factor levels in relation to obesity and the metabolic syndrome in women. Eur. J. Endocrinol. 157:303–310 [DOI] [PubMed] [Google Scholar]
- 3. Cohen CJ, et al. 2011. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet 378:229–237 [DOI] [PubMed] [Google Scholar]
- 4. Crauwels HM, et al. 2010. Effect of intrinsic and extrinsic factors on the pharmacokinetics of TMC278 in antiretroviral-naive, HIV-1-infected patients in ECHO and THRIVE. J. Int. AIDS Soc. 13(Suppl 4):P186 [Google Scholar]
- 5. Crauwels H, Vingerhoets J, Ryan R, Witek J, Anderson D. 9 November 2011. Pharmacokinetic parameters of once-daily rilpivirine following administration of efavirenz in healthy subjects. Antivir. Ther. [Epub ahead of print.] doi:10.3851/IMP1959 [DOI] [PubMed] [Google Scholar]
- 6. de Bethune MP, et al. 2005. TMC278, a new potent NNRTI with an increased barrier to resistance and favourable pharmacokinetic profile, abstr 556. Abstr. 12th Conf. Retroviruses Opportunistic Infect [Google Scholar]
- 7. Díaz-Delfín J, et al. 2011. Effects of nevirapine and efavirenz on human adipocyte differentiation, gene expression, and release of adipokines and cytokines. Antiviral Res. 91:112–119 [DOI] [PubMed] [Google Scholar]
- 8. Dupin N, et al. 2002. HIV and antiretroviral drug distribution in plasma and fat tissue of HIV-infected patients with lipodystrophy. AIDS 16:2419–2424 [DOI] [PubMed] [Google Scholar]
- 9. El Hadri K, et al. 2004. In vitro suppression of the lipogenic pathway by the nonnucleoside reverse transcriptase inhibitor efavirenz in 3T3 and human preadipocytes or adipocytes. J. Biol. Chem. 279:15130–15141 [DOI] [PubMed] [Google Scholar]
- 10. Gallego-Escuredo JM, et al. 2010. Differential effects of efavirenz and lopinavir/ritonavir on human adipocyte differentiation, gene expression and release of adipokines and proinflammatory cytokines. Curr. HIV Res. 8:545–553 [DOI] [PubMed] [Google Scholar]
- 11. Gazzard BG, et al. 2008. British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy. HIV Med. 9:563–608 [DOI] [PubMed] [Google Scholar]
- 12. Goebel F, et al. 2006. Short-term antiviral activity of TMC278—a novel NNRTI—in treatment-naive HIV-1-infected subjects. AIDS 20:1721–1726 [DOI] [PubMed] [Google Scholar]
- 13. Hammer SM, et al. 2008. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-U.S.A. panel. JAMA 300:555–570 [DOI] [PubMed] [Google Scholar]
- 14. Haubrich RH, et al. 2009. AIDS Clinical Trials Group (ACTG) A5142 Study Team. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS 23:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janssen PA, et al. 2005. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J. Med. Chem. 48:1901–1909 [DOI] [PubMed] [Google Scholar]
- 16. Kadowaki T, et al. 2006. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 116:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molina JM, et al. 2011. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet 378:238–246 [DOI] [PubMed] [Google Scholar]
- 18. Pérez-Molina JA, Domingo P, Martínez E, Moreno S. 2008. The role of efavirenz compared with protease inhibitors in the body fat changes associated with highly active antiretroviral therapy. J. Antimicrob. Chemother. 62:234–245 [DOI] [PubMed] [Google Scholar]
- 19. Riddler SA, et al. 2008. AIDS Clinical Trials Group Study A5142 Team. Class-sparing regimens for initial treatment of HIV-1 infection. N. Engl. J. Med. 358:2095–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlüter A, Yubero P, Iglesias R, Giralt M, Villarroya F. 2002. The chlorophyll-derived metabolite phytanic acid induces white adipocyte differentiation. Int. J. Obes. Relat. Metab. Disord. 26:277–1280 [DOI] [PubMed] [Google Scholar]
- 21. Stankov MV, Behrens GM. 2010. Contribution of inflammation to fat redistribution and metabolic disturbances in HIV-1 infected patients. Curr. Pharm. Des. 16:3361–3371 [DOI] [PubMed] [Google Scholar]
- 22. Staszewski S, et al. 1999. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N. Engl. J. Med. 341:1865–1873 [DOI] [PubMed] [Google Scholar]
- 23. Tebas P, et al. 2011. Results from the pooled DEXA substudies of the double-blind, randomised, phase III trials comparing rilpivirine (RPV, TMC278) versus efavirenz (EFV) in treatment-naïve, HIV-infected adults. Antivir. Ther. 16(Suppl 2):A19 [Google Scholar]
- 24. U. S. Food and Drug Administration 2011. Approval of Edurant, rilpivirine, a new NNRTI for the treatment of HIV in treatment naive patients. FDA, Washington, DC: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm256151.htm [Google Scholar]
- 25. Villarroya F, Domingo P, Giralt M. 2007. Lipodystrophy in HIV 1-infected patients: lessons for obesity research. Int. J. Obes. (Lond.) 31:1763–1776 [DOI] [PubMed] [Google Scholar]
- 26. Wilkin A, et al. 2012. Long-term efficacy, safety, and tolerability of rilpivirine (RPV, TMC278) in HIV type 1-infected antiretroviral-naive patients: week 192 results from a phase IIb randomized trial. AIDS Res. Hum. Retrovir. 28:437–446 [DOI] [PubMed] [Google Scholar]