Abstract
In response to concerns raised about the quality of parenteral vancomycin products, the U.S. Food and Drug Administration (FDA) is investigating the product quality of all FDA-approved parenteral vancomycin products available in the United States. Product quality was evaluated independently at two FDA Office of Testing and Research (FDA-OTR) sites. In the next phase of the investigation, being done in collaboration with the National Institute of Allergy and Infectious Diseases, the in vivo activity of these products will be evaluated in an appropriate animal model. This paper summarizes results of the FDA investigation completed thus far. One site used a validated ultrahigh-pressure liquid chromatography method (OTR-UPLC), and the second site used the high-performance liquid chromatography (HPLC) method for related substances provided in the British Pharmacopeia (BP) monograph for vancomycin intravenous infusion. Similar results were obtained by the two FDA-OTR laboratories using two different analytical methods. The products tested had 90 to 95% vancomycin B (active component of vancomycin) by the BP-HPLC method and 89 to 94% vancomycin by OTR-UPLC methods. Total impurities were 5 to 10% by BP-HPLC and 6 to 11% by OTR-UPLC methods. No single impurity was >2.0%, and the CDP-1 level was ≤2.0% across all products. Some variability in impurity profiles of the various products was observed. No adverse product quality issues were identified with the six U.S. vancomycin parenteral products. The quality parameters of all parenteral vancomycin products tested surpassed the United States Pharmacopeia acceptance criteria. Additional testing will characterize in vivo performance characteristics of these products.
INTRODUCTION
Vancomycin, a tricyclic glycopeptide antibiotic developed by Eli Lilly and Company, was first approved in 1958. As Eli Lilly no longer manufactures vancomycin for injection, the Lilly drug product is not available. Currently, six intravenous vancomycin products are available in the United States. Five are available as lyophilized sterile powders for injection in single- and multiple-use vials and require reconstitution and further dilution before use. The sixth product is available as a premixed, ready-to-use solution for intravenous use that is stored frozen until use. With the exception of the premixed solution, all parenteral vancomycin products are generics. The five lyophilized products contain no excipients other than sodium hydroxide and hydrochloric acid used for pH adjustment in some preparations. The generic parenteral vancomycin products contain the same active ingredient as that in the original Eli Lilly product. Additionally, as required by our approval process, the manufacturer of each generic product would have to show that their product met purity, potency, quality, and identity standards required to market vancomycin in the United States.
The U.S. Food and Drug Administration (FDA) has initiated a two-tiered investigation to evaluate the product quality of parenteral vancomycin products available in the U.S. market. In the first phase, the FDA evaluated impurity levels in all six FDA-approved vancomycin parenteral products. This paper summarizes the results of the FDA investigation completed thus far. Findings from additional in vitro testing are described in the accompanying paper by Hadwiger et al. (10a). In the second phase of the FDA's evaluation, additional testing will be conducted, including testing in an appropriate animal model, to evaluate the in vivo activity of the six FDA-approved parenteral vancomycin products available in the United States. This testing will be done in collaboration with the National Institute of Allergy and Infectious Diseases.
The FDA studies are in response to concerns raised about the quality of parenteral generic vancomycin products. In an article by Vesga et al., the in vitro and in vivo activities of certain generic vancomycin products were compared to those of the Lilly product (16). Antibacterial activity was studied in vitro by broth microdilution and time-kill curves. The pharmacodynamic activity of bacterial killing was evaluated in vivo using the neutropenic murine thigh infection model and a clinical isolate of Staphylococcus aureus. Of the three generic vancomycin products used in the study by Vesga et al., two are United States-approved generics while the third is a generic available in Colombia but not approved for use in the United States. As this study was conducted from 2002 to 2009, the authors had access to the Lilly product before marketing of the product was discontinued.
The authors postulated that the observed difference in activities of the generics compared to that of the Lilly product could be related to the amount of a specific impurity, crystalline degradation product (CDP-1). The findings of the study by Vesga et al. have not been independently verified to date. The authors further suggested that the observed differences in activity may be related to differences in the manufacturing processes and product quality of the generic vancomycin formulations compared to the Lilly product (16). The same authors have also raised concerns about the activity of other generic antibacterial products compared to their respective innovators (14, 17).
To provide accurate information regarding FDA-approved generic products, this paper also discusses the scientific and legal requirements for drug approval relating to product quality.
MATERIALS AND METHODS
The FDA's Office of Testing and Research (FDA-OTR) evaluated the purity and impurity level of the six vancomycin parenteral drug products marketed in the United States. Tests were conducted independently at two different FDA-OTR sites. One site used a validated ultrahigh-pressure liquid chromatography method developed by FDA-OTR (OTR-UPLC). The second site used the high-pressure liquid chromatography (HPLC) method for related substances provided in the British Pharmacopeia (BP) monograph for vancomycin intravenous infusion. Method details are included in Tables 1 and 2. The BP method was selected rather than the United States Pharmacopeia (USP) HPLC method because it provides better resolution of impurities. Unlike the USP method, the BP method also provides a disregard limit for calculating impurities by disregarding any impurity less than 0.1%.
Table 1.
Comparison of USP method with UPLC and BP-HPLC methodsa
| Condition | USP method for impurities (vancomycin hydrochloride for injection) | BP method for related substances (vancomycin intravenous infusion) | OTR-UPLC purity method |
|---|---|---|---|
| Instrument | Specific instrument not provided | Specific instrument not provided | Waters Acquity UPLC system |
| Column | L1 (4.6 by 250 mm, 5 μm) | End-capped ODS silica gel (4.6 by 250 mm, 5 μm), e.g., Hypersil ODS | Waters BEH C18 (2.1 by 100 mm, 1.7 μm with Waters BEH C18 (2.1 by 5 mm, 1.7 μm) guard column |
| Temp | Does not state in method | Ambient | 35°C |
| Mobile phase A | 92:7:1 (pH 3.2 TEA buffer-acetonitrile-THF) | 92:7:1 (pH 3.2 TEA buffer-acetonitrile-THF) | 5 mM KH2PO4 (pH 3.2) |
| Mobile phase B | 70:29:1 (pH 3.2 TEA buffer-acetonitrile-THF) | 70:29:1 (pH 3.2 TEA buffer-acetonitrile-THF) | Acetonitrile |
| Gradient | See Table 2 | See Table 2 | Linear acetonitrile gradient (5 to 15%) over 4.0 min |
| Flow rate (ml/min) | 2.0 | 1.0 | 0.55 |
| Wavelength for detection (nm) | 280 | 280 | 230 |
| Injection vol (μl) | 20 | 20 | 5 |
| Criteria for impurities | For vancomycin B, NLT 80.0%; for any other impurity, NMT 9.0% | For each impurity, NMT 4.0%; total impurities, NMT 12.0%; disregard limit, area of vancomycin peak in lowest concentration solution | No criteriab |
Abbreviations: ODS, octadecylsilyl; TEA, triethylamine; THF, tetrahydrofuran; NLT, no less than; NMT, no more than.
The OTR-UPLC method is not associated with BP/USP monographs.
Table 2.
Gradient times for USP and BP methods
| USP method |
BP method |
||||
|---|---|---|---|---|---|
| Time (min) | % A | % B | Time (min) | % A | % B |
| 0 | 100 | 0 | 0 | 100 | 0 |
| 12 | 100 | 0 | 13 | 100 | 0 |
| 20 | 0 | 100 | 21 | 0 | 100 |
| 22 | 0 | 100 | 25 | 0 | 100 |
| 23 | 100 | 0 | 26 | 100 | 0 |
| 30 | 100 | 0 | 35 | 100 | 0 |
Ten lots of sterile parenteral vancomycin hydrochloride representing products from the six U.S. suppliers were obtained from a drug distributor (Washington Wholesale Drug Exchange-Bradley Drugs, Bethesda, MD). The vancomycin reference standard used was the USP vancomycin hydrochloride RS obtained from USP, Rockville, MD (lot no. MOH006, catalog no. 1709007). The CDP-1 impurity standard was prepared in-house according to the literature and characterized by accurate measurement of mass by liquid chromatography-mass spectrometry (LC-MS) (12).
RESULTS
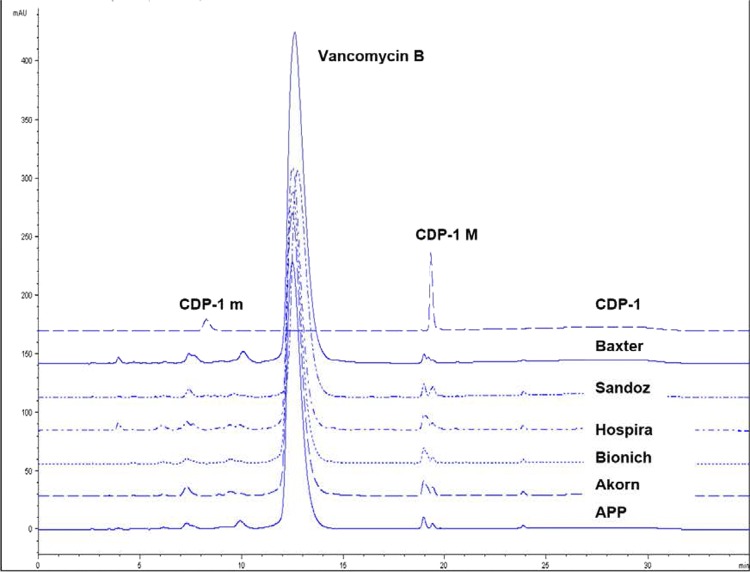
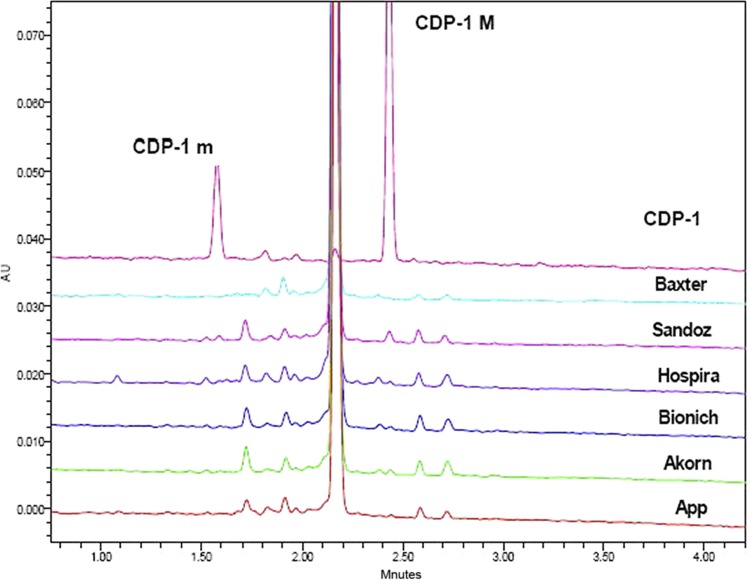
Similar results were obtained by the two different FDA-OTR laboratories using two different analytical methods. Lots were tested by either method to gather data. Thus, not all lots were tested by both methods. The six FDA-approved vancomycin parenteral products had 90 to 95% vancomycin B (active component of vancomycin) by BP-HPLC and 89 to 94% vancomycin B by OTR-UPLC (Table 3). Correspondingly, the total amount of impurities was 5 to 10% by BP-HPLC and 6 to 11% by OTR-UPLC. Although the amounts of the largest impurity differed among the various products, no single impurity was greater than 2.0%. Some variability in the impurity profiles of the various products was observed. The amount of vancomycin B, the total impurities, the amount of the largest impurity, and the total amount of CDP-1 present in each sample, calculated as an area percentage, are shown in Table 3. Figures 1 and 2 show the chromatographic profiles obtained by the BP-HPLC and OTR-UPLC methods, respectively.
Table 3.
Test results for U.S. vancomycin for injection suppliers
| Source | Lot no. | Sample details | Method (calculations based on area %) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BP-HPLC |
OTR-UPLC |
|||||||||
| % vancomycin B | % total impurities | % largest impurity | % CDP-1 (maximum amt) | % vancomycin B | % total impurities | % largest impurity | % CDP-1 (maximum amt) | |||
| Sandoz Incorporated | AY1780 | 1-g vial | 93 | 7 | 1.4 | 1.8 | 94 | 6 | 1.5 | 1.1 |
| Baxter Health Care Corporation | NC063966 | 1-g intravenous bag | 92 | 8 | 1.7 | 0.3 | ||||
| 2G3552 | 200-ml intravenous bag | 95 | 5 | 2.0 | 0.6 | |||||
| Hospira Inc. | 923103A | 1-g vial | 90 | 10 | 0.5 | 1.4 | 89 | 11 | 1.7 | 0.5 |
| APP Pharmaceuticals LLC | 6100850 | 1-g vial | 94 | 6 | 1.9 | 1.2 | ||||
| 6100475 | 93 | 7 | 1.5 | None detected | ||||||
| Bioniche Pharma USA LLC | 10F13112A | 1-g vial | 93 | 7 | 1.6 | 0.2 | ||||
| 10H18782A | 94 | 6 | 1.2 | 2.0 | ||||||
| Akorn Strides, LLC | 7004195 | 1-g vial | 91 | 9 | 2.0 | 0.6 | ||||
| 7004471 | 94 | 6 | 1.1 | 1.9 | ||||||
Fig 1.
Representative chromatograms using the BP-HPLC method (CDP-1M coelutes with another impurity). The main peak just before 12.30 min is vancomycin B. Chromatogram profiles are stacked for clarity. The retention time of the vancomycin peak in the test products was consistent with the USP reference standard.
Fig 2.
Representative chromatograms for CDP-1 and marketplace products using the OTR-UPLC method. The main peak just before 2.20 min is vancomycin B. Chromatogram profiles are stacked for clarity. The retention time of the vancomycin peak in the test products was consistent with the USP reference standard.
The USP acceptance criterion for the content of vancomycin in the monograph on vancomycin hydrochloride for injection is not less than 80.0% for vancomycin B and not greater than 9.0% for any other peak, i.e., individual impurities (15). The USP monographs provide standards of identity, strength, quality, and purity for most drug substances and drug products that are legally marketed in the United States. Thus, all six FDA-approved parenteral vancomycin products surpassed USP acceptance criteria for purity by having higher levels of vancomycin B and far lower levels of individual impurities than the monograph allows.
A key finding in the testing performed at the FDA is that the level of CDP-1 present in the U.S. vancomycin products was low and that levels were similar across the products. CDP-1 coexists as two conformers, CDP-1M and CDP-1m, the respective major and minor forms (11). CDP-1 content (calculated as the sum of the CDP-1M and CDP-1m peak area percentages) in any product was no more than 2.0% by the BP-HPLC method and 1.1% by the OTR-UPLC method. CDP-1 conformers, particularly CDP-1M in the BP-HPLC method, coelute with other impurities. As these impurity results include impurities in addition to CDP-1, the actual amount of CDP-1 present is expected to be lower than the values reported in Table 3.
DISCUSSION
Antibacterial drugs can be manufactured by chemical synthesis, by fermentation, or by chemical modification of a fermentation product. Chemical synthesis allows drugs to be manufactured to a high degree of purity through careful control of the chemical reactions and purification processes. In contrast, fermentation processes produce many structurally similar components and degradation products in addition to the desired component. These impurities tend to comigrate with the desired component during product isolation and purification, even after multiple purification steps. Hence, fermentation-derived antibacterial drugs tend to have higher levels of impurities than do chemically synthesized antibacterial drugs. Vancomycin is a fermentation product that does not undergo further chemical modification.
As early as 1983, chemical analysis of the Eli Lilly vancomycin product showed the presence of unidentified peaks that were presumed to be related substances or degradation products. Many of these impurities have now been characterized, and the mechanisms for formation have been elucidated (9, 11, 13). The main related-substance impurities of vancomycin are monodechlorovancomycin, demethylvancomycin, desamidovancomycin B (CDP-1M and CDP-1m), aglucovancomycin, and desvancosaminylvancomycin. In general, related-substance impurities exhibit reduced activity compared to vancomycin B (13). For example, monodechlorovancomycin, a by-product formed during fermentation, has only half the activity of vancomycin B. Aglucovancomycin and desvancosaminylvancomycin, degradation products formed by the loss of sugar moieties, also have far less activity than does vancomycin B. CDP-1, the rearranged isoaspartic analogue of vancomycin, has no antibacterial activity (11–13). Antagonism of vancomycin by individual impurities is not known.
The two regulatory assays used by the FDA to evaluate parenteral vancomycin are the HPLC assay for purity and the USP <81> microbiological assay for potency. These assays are used throughout the product's life cycle from product release through shelf life to ensure a consistent level of purity and potency in marketed products. The HPLC assay is used for determining purity as it allows quantitative measurement of vancomycin B and individual related-substance impurities. The USP <81> microbiological assay is used for determining vancomycin potency or activity as measured against the compendial standard. The microbiological assay measures the growth inhibition of a culture of Bacillus subtilis ATCC 6633, the test organism. In contrast to the HPLC assay, the microbiological assay is not a suitable method for evaluating product purity/impurity or stability, as structurally related impurities with reduced activity compared to that of vancomycin B could also contribute to the assay results.
Although Vesga et al. postulated that the observed difference in in vivo bactericidal activities could be due to lower levels of vancomycin B and high levels of CDP-1 in the drug products tested, they did not quantify the amount of CDP-1. The authors based pharmaceutical equivalency on the results of the microbiological potency assay, which, as discussed previously, is suited by itself neither for evaluating the purity and impurity level of vancomycin nor for determination of pharmaceutical equivalence. The FDA evaluates pharmaceutical equivalence based on comprehensive chemical characterization of the active ingredient and numerous other factors required in the generic drug approval process described below. Our results presented here show that all products currently on the U.S. market surpass the USP standards for vancomycin B and impurity levels. In addition, the CDP-1 isomers were quantified to ensure that they were not at levels that could affect the performance of the product.
In order to market a generic drug in the United States, a pharmaceutical company is required to submit an abbreviated new drug application (ANDA) to the FDA. The ANDA applicant must identify the innovator drug (commonly referred to as the reference listed drug [RLD]) on which it seeks to rely (1). In addition, with limited exceptions, a drug product described in an ANDA must contain the same active ingredient, conditions of use, route of administration, dosage form, strength, and (with certain permissible differences) labeling as those of the RLD which it references. The ANDA applicant also must demonstrate that the proposed generic drug is bioequivalent to the listed drug (2). Drugs administered by injection containing the same active and inactive ingredients in the same concentrations as those in the RLD are considered to be qualitatively and quantitatively the same as the RLD. In some cases, a generic injection product can differ in preservative, buffer, or antioxidant from the RLD, but only if the differences are well characterized and evidence is provided that safety or efficacy is not affected (3). The five lyophilized products contain no excipients other than sodium hydroxide and hydrochloric acid used for pH adjustment in some preparations. They do not contain preservatives, buffers, or antioxidants.
In addition to the above, an ANDA must include detailed information and supporting data regarding the physical and chemical characteristics, composition, method of manufacture, specifications, and stability of the drug substance and the drug product (4, 5). Once a drug is approved, a drug manufacturer must adhere to the approved manufacturing process. Federal regulations require an applicant to notify the FDA about each change in each condition established in an approved application beyond the variations already provided for in the application, which include manufacturing changes (6, 7). This helps to ensure continued product consistency, safety, and effectiveness after approval.
The FDA finds products to be pharmaceutically equivalent if they have identical dosage forms that contain identical amounts of the identical active drug ingredient that deliver identical amounts of the active drug ingredient over the identical dosing period and meet the identical compendial or other applicable standards of identity, strength, quality, and purity, including potency and, where applicable, content uniformity, disintegration times, and/or dissolution rates (8). Only drug products that meet the ANDA approval requirements and are both bioequivalent and pharmaceutically equivalent to the RLD are considered by the FDA to be therapeutically equivalent to the RLD and therefore substitutable (10).
The six vancomycin parenteral products tested by the FDA met these criteria and were shown to have vancomycin B and CDP-1 levels that were very similar to each other and surpassed the USP standards for vancomycin B and impurity levels. The rigors of the FDA review process, together with the FDA's continued monitoring of currently marketed, FDA-approved vancomycin parenteral products, provide little reason to believe that there are significant quality differences between the USP reference standard and the FDA-approved vancomycin parenteral products available in the United States.
Conclusions.
As noted in our results, we have not identified any adverse product quality issues with the six vancomycin parenteral products available in the U.S. market. The quality parameters of all six FDA-approved vancomycin parenteral products available in the United States surpassed the USP acceptance criteria. Overall, no significant product quality differences were observed among the five generic and one brand-name parenteral vancomycin products. Some variability between the products is expected, but the level of CDP-1 was no more than 2.0% across all products. Although Vesga et al. had proposed that high levels of CDP-1 were inhibiting vancomycin activity by competitive binding to d-Ala-d-Ala in the bacterial cell wall, the FDA's findings of small amounts of CDP-1 make this very unlikely. At this juncture, in collaboration with the National Institute of Allergy and Infectious Diseases, the FDA is planning to evaluate the in vivo activity of the six parenteral vancomycin products available in the U.S. market, including testing in an appropriate animal model.
ACKNOWLEDGMENTS
The views expressed in this report are those of the authors and do not necessarily represent the views of the U.S. Food and Drug Administration.
There was no financial support for any authors.
Footnotes
Published ahead of print 6 February 2012
REFERENCES
- 1. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(3). [Google Scholar]
- 2. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a). [Google Scholar]
- 3. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(9)(iii). [Google Scholar]
- 4. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(9). [Google Scholar]
- 5. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.94(a)(9)(i) [citing 21 CFR 314.50(d)(1)]. [Google Scholar]
- 6. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.97. [Google Scholar]
- 7. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 314. Applications for FDA approval to market a new drug. 21 CFR 314.70. [Google Scholar]
- 8. Code of Federal Regulations 2011. Title 21. Food and Drugs. Chapter I. Food and Drug Administration. Subchapter D. Drugs for human use. Part 320. Bioavailability and bioequivalence requirements. 21 CFR 320.1(c). [Google Scholar]
- 9. Diana J, Visky D, Hoogmartens J, Van Schepdael A, Adams E. 2006. Investigation of vancomycin and related substances by liquid chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 20:685–693 [DOI] [PubMed] [Google Scholar]
- 10. Food and Drug Administration Accessed 5 July 2011 Approved drug products with therapeutic equivalence evaluations, 31st ed (orange book). Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM071436.pdf [Google Scholar]
- 10a. Hadwiger ME, Sommers CD, Mans DJ, Patel V, Boyne MT., II 2012. Quality assessment of U.S. marketplace vancomycin for injection products using high-resolution liquid chromatography-mass spectrometry and potency assays. Antimicrob. Agents Chemother. 56:2824–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris CM, Kopecka H, Harris TM. 1983. Vancomycin: structure and transformation to CDP-1. J. Am. Chem. Soc. 105:6915–6922 [Google Scholar]
- 12. Marshall FJ. 1965. Structure studies on vancomycin. J. Med. Chem. 8:18–22 [DOI] [PubMed] [Google Scholar]
- 13. Nagarajan R. 1991. Antibacterial activities and modes of action of vancomycin and related glycopeptides. Antimicrob. Agents Chemother. 35:605–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez CA, Agudelo M, Zuluaga AF, Vesga O. 2010. In vitro and in vivo comparison of the anti-staphylococcal efficacy of generic products and the innovator of oxacillin. BMC Infect. Dis. 10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. United States Pharmacopeial Convention 2011. The United States pharmacopeia 34—the national formulary 29. United States Pharmacopeial Convention, Rockville, MD [Google Scholar]
- 16. Vesga O, Agudelo M, Salazar BE, Rodriguez CA, Zuluaga AF. 2010. Generic vancomycin products fail in vivo despite being pharmaceutical equivalents of the innovator. Antimicrob. Agents Chemother. 54:3271–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuluaga AF, et al. 2010. Determination of therapeutic equivalence of generic products of gentamicin in the neutropenic mouse thigh infection model. PLoS One 5:e10744. [DOI] [PMC free article] [PubMed] [Google Scholar]