Abstract
Among the 219 vancomycin-resistant Enterococcus faecium isolates collected in 20 Taiwanese hospitals from 2006 to 2010, all were susceptible to linezolid and daptomycin, and 98.6% were susceptible to tigecycline. There was a shift toward higher tigecycline MIC values (MIC90s) from 2006-2007 (0.06 μg/ml) to 2008–2010 (0.12 μg/ml). The MIC90s of daptomycin and linezolid remained stationary. Although pulsotypes among the isolates from the 20 hospitals varied, intrahospital spreading of several clones was identified in 13 hospitals.
TEXT
Enterococcus species were first isolated from a patient with endocarditis in the early 1900s (16) and have been recognized as an important cause of nosocomial urinary tract infection and bacteremia since the mid-1970s (4, 23). Penicillin, ampicillin, and aminoglycosides are the first-line drugs of choice for the treatment of enterococcal infection. Glycopeptides have been effective antibiotics for the treatment of infections due to beta-lactam-resistant enterococci since the 1980s (7). However, the rate of isolation of vancomycin-resistant enterococcus (VRE) strains is on the rise in North America (17), Europe (2), and Asia (4). In Taiwan, a nationwide surveillance study of antimicrobial resistance in patients admitted to intensive care units clearly demonstrated a significant rise in VRE among regional hospitals and medical centers during the period 2003 to 2009 (5). Specifically, at the National Taiwan University Hospital, rates of VRE among all enterococcus species causing health care-associated infection significantly increased, from 1.7% (0.007 per 100 inpatient-days) in 2000 to 25.1% (0.188 per 100 inpatient-days) in 2009 (14). Clonal dissemination and the presence of multiple clones of VRE have also been reported in hospitals in Taiwan (8, 9). Tigecycline, daptomycin, and linezolid are now the drugs of choice for the treatment of infections due to VRE. However, the in vitro susceptibilities of VRE species to these agents have been shown to vary with time and between countries. Herein, we report the trends in in vitro susceptibility of vancomycin-resistant E. faecium to tigecycline, daptomycin, and linezolid in Taiwan during the period 2006 to 2010.
Clinical isolates of VRE were collected as part of the Tigecycline In Vitro Surveillance in Taiwan (TIST) study, a nationwide, multicenter prospective surveillance study conducted in 20 medical centers and regional hospitals throughout Taiwan during the period January 2006 to December 2010 (5). Duplicate isolates from the same patients were excluded (one isolate per patient). Consecutive VRE isolates during a 3-month period per year from each participating hospital were collected, with a maximal number of isolates of 10 per hospital per year. These VRE isolates were identified, and vancomycin resistance was determined by the disk diffusion method at each hospital and reconfirmed by the central laboratory at the National Taiwan University Hospital (NTUH). MICs of three antimicrobial agents, namely, tigecycline, daptomycin, and linezolid, were determined using the broth microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (6). Susceptibility testing for the three agents was performed at the same central laboratory at NTUH using same type of media, Mueller-Hinton broth (BBL Microbiology Systems, Cockeysville, MD), and the same protocol every year. Susceptibilities to the three agents were classified based on U.S. Food and Drug Administration (FDA), the European Committee on Antimicrobial Susceptibility Testing (EUCAST-2012; www.eucast.org), and CLSI 2012 breakpoints (6).
In order to understand the genetic relatedness of vancomycin-resistant E. faecium isolates, particularly those with daptomycin MICs of 4 μg/ml, pulsed-field gel electrophoresis (PFGE) profiles of SmaI-digested genomic DNAs of these isolates were determined as previously described (9).
A total of 219 clinical isolates of vancomycin-resistant E. faecium were collected from 20 hospitals during the 5-year study period (Table 1). Numbers of clinical isolates obtained from 2006 to 2010 were 13 in 2006, 15 in 2007, 41 in 2008, 78 in 2009, and 72 in 2010 (5). Susceptibility of vancomycin-resistant E. faecium to tigecycline was 98.6% according to U.S. FDA and EUCAST-2012 breakpoints (22). The three VRE isolates that were less susceptible to tigecycline had tigecycline MICs of 0.5, 0.5, and 1 μg/ml. All isolates were susceptible to daptomycin and linezolid according to CLSI-2012 and EUCAST-2012 criteria (6). The MICs of daptomycin were 2 μg/ml, and the MICs of linezolid were 1 to 2 μg/ml for the three tigecycline-nonsusceptible isolates.
Table 1.
Pulsotypes of 219 isolates of vancomycin-resistant E. faecium from 20 hospitals in Taiwan from 2006 to 2010
| Hospital designation | No. of isolates | No. of pulsotypes identified | No. of pulsotypes with ≥2 isolates at hospital/no. of isolates within each of these pulsotypes |
|---|---|---|---|
| H01 | 10 | 7 | 3/2, 2, 2 |
| H02 | 31 | 16 | 4/5, 5, 3, 2 |
| H03 | 12 | 11 | 2/2, 2 |
| H04 | 5 | 4 | 1/2 |
| H05 | 4 | 3 | 1/2 |
| H06 | 30 | 17 | 6/4, 3, 3, 2, 2, 2 |
| H07 | 9 | 8 | 1/2 |
| H08 | 0 | 0 | 0 |
| H09 | 0 | 0 | 0 |
| H10 | 10 | 8 | 3/2, 2, 2 |
| H11 | 10 | 0 | |
| H12 | 18 | 14 | 3/2, 2, 2 |
| H13 | 11 | 7 | 2/3, 2 |
| H14 | 13 | 12 | 0 |
| H15 | 11 | 9 | 1/2 |
| H16 | 1 | 1 | 1/2 |
| H17 | 10 | 7 | 0 |
| H18 | 13 | 12 | 2/3, 2 |
| H19 | 11 | 11 | 0 |
| H20 | 10 | 8 | 0 |
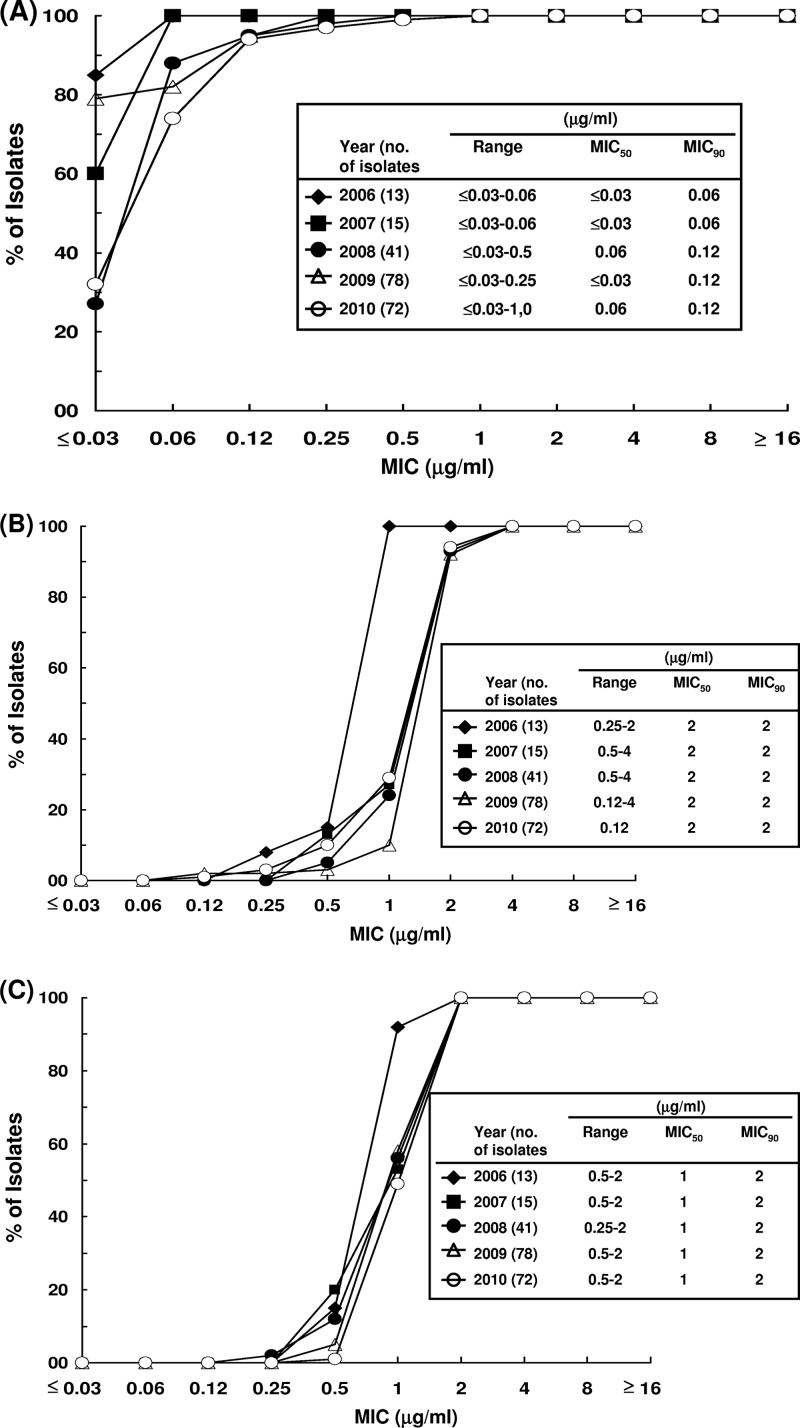
The distributions of MIC values of the three agents against the vancomycin-resistant E. faecium strains are shown in Table 2. MIC50 and MIC90 values of tigecycline, daptomycin, and linezolid against the clinical isolates were ≤0.03 μg/ml and 0.12 μg/ml (range, ≤0.03 to 1 μg/ml), 2 μg/ml and 2 μg/ml (range, 0.12 to 4 μg/ml), and 1 μg/ml and 2 μg/ml (range, 0.25 to 2 μg/ml), respectively. There was a shift toward higher tigecycline MIC values from 2006-2007 (MIC90 of 0.06 μg/ml) to 2008-2010 (MIC90 of 0.12 μg/ml) (Fig. 1A). The MIC values of daptomycin and linezolid against the isolates remained stationary during the study period (Fig. 1B and C).
Table 2.
Distribution of MICs of tigecycline, daptomycin, and linezolid for 219 vancomycin-resistant E. faecium isolates collected from 20 hospitals in Taiwan between 2006 and 2010
| Agent | No. (cumulative %) of isolates with indicated MIC (μg/ml) |
MIC (μg/ml) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ≥16 | 50% | 90% | |
| Tigecycline | 116 (53) | 65 (83) | 28 (95) | 7 (99) | 2 (99) | 1 (100) | 0 (100) | 0 (100) | 0 (100) | 0 (100) | ≤0.03 | 0.12 |
| Daptomycin | 0 (0) | 0 (0) | 2 (0.9) | 2 (2) | 11 (7) | 32 (21) | 158 (94) | 14 (100) | 0 (100) | 0 (100) | 2 | 2 |
| Linezolid | 0 (0) | 0 (0) | 0 (0) | 1 (0.5) | 14 (7) | 108 (56) | 96 (100) | 0 (100) | 0 (100) | 0 (100) | 1 | 2 |
Fig 1.
Distribution of MICs of vancomycin-resistant E. faecium to tigecycline (A), daptomycin (B), or linezolid (C) from 2006 to 2010.
A total of 14 vancomycin-resistant E. faecium isolates were susceptible to daptomycin, with MICs of 4 μg/ml (Table 3). Those isolates were also susceptible to tigecycline and linezolid. All of the isolates were susceptible to tigecycline at a concentration of 0.06 μg/ml, and 9 of the 14 clinical isolates were susceptible to linezolid at a concentration of 2 μg/ml.
Table 3.
Designations and MICs of 14 isolates of vancomycin-resistant E. faecium with daptomycin MICs of 4 μg/ml
| Yr of isolation | Hospital | Designation of isolate | MIC (μg/ml) |
Pulsotype | |
|---|---|---|---|---|---|
| Linezolid | Tigecycline | ||||
| 2010 | H01 | A | 1 | 0.06 | I |
| 2009 | H03 | B1 | 1 | 0.03 | II |
| 2009 | H03 | B2 | 2 | 0.03 | III |
| 2008 | H06 | C1 | 2 | 0.06 | IV |
| 2008 | H06 | C2 | 0.5 | 0.06 | V |
| 2009 | H06 | C3 | 2 | 0.03 | VI |
| 2010 | H06 | C4 | 2 | ≤0.03 | VII |
| 2010 | H10 | D | 2 | 0.06 | VIII |
| 2008 | H12 | E | 1 | 0.03 | IX |
| 2007 | H15 | F1 | 2 | 0.06 | X |
| 2009 | H15 | F2 | 2 | 0.03 | XI |
| 2009 | H15 | F3 | 1 | 0.03 | XI |
| 2010 | H15 | F4 | 2 | 0.03 | XII |
| 2009 | H20 | G | 2 | 0.03 | XIII |
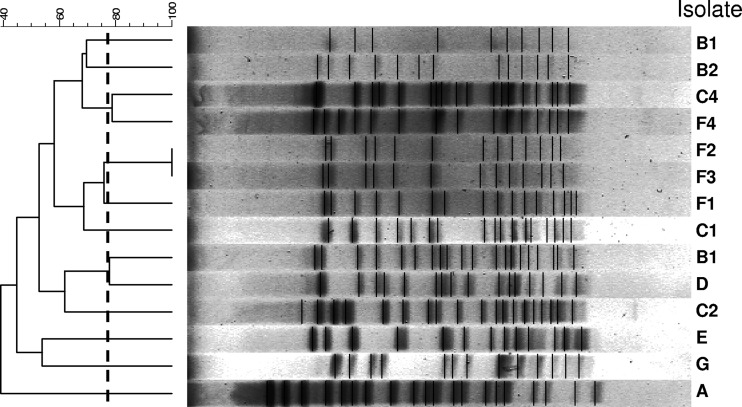
Even though the pulsotypes of the isolates varied among the 20 hospitals, 13 hospitals in Taiwan showed evidence of intrahospital spreading of VRE isolates with the same pulsotypes (Table 3). Thirteen pulsotypes were found among the 14 isolates of vancomycin-resistant E. faecium with daptomycin MICs of 4 μg/ml, indicating the absence of interhospital spreading of these strains among Taiwanese hospitals (Fig. 2).
Fig 2.
Pulsed-field gel electrophoresis profiles and dendrogram of 14 isolates of vancomycin-resistant E. faecium with daptomycin MICs of 4 μg/ml.
Although the incidence of infection due to VRE has risen in recent years in many countries (1, 15), the incidence of VRE in Taiwan remains relatively low (3 to 7%) (15), especially compared to the incidence of hospital-acquired VRE infection in the United States (28%).VRE species are recovered from colonized rather than infected patients in many institutions (24), but VRE infection is associated with higher recurrence and mortality (21). In addition, the treatment regimens for VRE infections are limited to tigecycline, daptomycin, and linezolid. However, only a few reports regarding the antimicrobial susceptibility of VRE isolates to these antibiotics in certain hospitals in Taiwan have been published (10, 11), and no nationwide surveillance studies of susceptibility of VRE species to the above-mentioned antimicrobial agents have been conducted.
In this study, we found that all clinical isolates of VRE were susceptible to daptomycin and linezolid and that almost all VRE isolates (98.6%) were susceptible to tigecycline. Studies conducted in Europe and the United States demonstrated similar findings (3, 18–20). Daptomycin-nonsusceptible enterococci may be an emerging clinical problem in other countries (12), but they were not identified in our study. In this study, the reason the VRE isolates with daptomycin MICs of 4 μg/ml were selected for molecular typing to elucidate the possible clonal spreads was because there are concerns about achieving adequate concentrations of daptomycin using standard doses (≤6 mg/kg of body weight) when the MICs of the isolates approach the upper end (4 μg/ml) of the susceptible range (13). Higher doses of daptomycin may be needed to treat infections caused by VRE isolates with higher MICs (3 to 4 μg/ml), although there was no significant difference in time to microbiological cure between MIC subgroups of ≤2 μg/ml versus >2 and ≤ 4μg/ml for VRE isolates causing bacteremia (13). We found that there was no marked increase in the trend of resistance to daptomycin and linezolid; however, we did find that there was an increase in resistance to tigecycline during the study period relative to the trend in resistance rates reported previously in Taiwan (10, 11).
In conclusion, tigecycline, daptomycin, and linezolid remain active against VRE isolates. Nonetheless, the emerging resistance of VRE to these agents should be monitored. Interhospital spread of VRE among Taiwanese hospitals were not evident; however, intrahospital dissemination of several VRE clones was found in several hospitals.
Footnotes
Published ahead of print 9 April 2012
REFERENCES
- 1. Ben RJ, et al. 1996. Clinical isolation of vancomycin-resistant Enterococcus faecalis in Taiwan. J. Formos. Med. Assoc. 95:946–949 [PubMed] [Google Scholar]
- 2. Bonten MJM, Willems R, Weinstein RA. 2001. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect. Dis. 1:314–325 [DOI] [PubMed] [Google Scholar]
- 3. Bourdon N, et al. 2011. Changing trends in vancomycin-resistant enterococci in French hospitals, 2001–08. J. Antimicrob. Chemother. 66:713–721 [DOI] [PubMed] [Google Scholar]
- 4. Cetinkaya Y, Falk P, Mayhall CG. 2000. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13:686–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen YH, et al. 2012. Trends in the susceptibility of clinically important resistant bacteria to tigecycline: results from the tigecycline in vitro surveillance in Taiwan study, 2006 to 2010. Antimicrob. Agents Chemother. 56:1452–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Grayson ML, et al. 1991. Increasing resistance to beta-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob. Agents Chemother. 35:2180–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsieh YC, Lee WS, Ou TY, Hsueh PR. 2010. Clonal spread of CC17 vancomycin-resistant Enterococcus faecium with multilocus sequence type 78 (ST78) and a novel ST444 in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 29:25–30 [DOI] [PubMed] [Google Scholar]
- 9. Hsueh PR, et al. 1999. Emergence of vancomycin-resistant enterococci at a university hospital in Taiwan: persistence of multiple species and multiple clones. Infect. Control Hosp. Epidemiol. 20:828–833 [DOI] [PubMed] [Google Scholar]
- 10. Hsueh PR, Chen WH, Teng LJ, Luh KT. 2005. Nosocomial infections due to methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci at a university hospital in Taiwan from 1991 to 2003: resistance trends, antibiotic usage and in vitro activities of newer antimicrobial agents. Int. J. Antimicrob. Agents 26:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang YT, Liao CH, Teng LJ, Hsueh PR. 2008. Comparative bactericidal activities of daptomycin, glycopeptides, linezolid and tigecycline against blood isolates of Gram-positive bacteria in Taiwan. Clin. Microbiol. Infect. 14:124–129 [DOI] [PubMed] [Google Scholar]
- 12. Kelesidis T, Humphries R, Uslan DZ, Pegues DA. 2011. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin. Infect. Dis. 52:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. King EA, McCoy D, Desai S, Nyirenda T, Bicking KK. 2011. Vancomycin-resistant enterococcal bacteraemia and daptomycin: are higher doses necessary? J. Antimicrob. Chemother. 66:2112–2118 [DOI] [PubMed] [Google Scholar]
- 14. Lai CC, et al. 2011. Correlation between antimicrobial consumption and resistance among Staphylococcus aureus and enterococci causing healthcare-associated infections at a university hospital in Taiwan from 2000 to 2009. Eur. J. Clin. Microbiol. Infect. Dis. 30:265–271 [DOI] [PubMed] [Google Scholar]
- 15. Lo WT, et al. 2011. Changing trends in antimicrobial resistance of major bacterial pathogens, 1985–2005: a study from a medical center in northern Taiwan. J. Microbiol. Immunol. Infect. 44:131–138 [DOI] [PubMed] [Google Scholar]
- 16. Murray BE. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray BE. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710–721 [DOI] [PubMed] [Google Scholar]
- 18. Rathe M, Kristensen L, Ellermann-Eriksen S, Thomsen MK, Schumacher H. 2010. Vancomycin-resistant Enterococcus spp.: validation of susceptibility testing and in vitro activity of vancomycin, linezolid, tigecycline and daptomycin. APMIS 118:66–73 [DOI] [PubMed] [Google Scholar]
- 19. Sader HS, Moet GJ, Farrell DJ, Jones RN. 2011. Antimicrobial susceptibility of daptomycin and comparator agents tested against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: trend analysis of a 6-year period in US medical centers (2005–2010). Diagn. Microbiol. Infect. Dis. 70:412–416 [DOI] [PubMed] [Google Scholar]
- 20. Sader HS, Streit JM, Fritsche TR, Jones RN. 2006. Antimicrobial susceptibility of gram-positive bacteria isolated from European medical centres: results of the Daptomycin Surveillance Programme (2002–2004). Clin. Microbiol. Infect. 12:844–852 [DOI] [PubMed] [Google Scholar]
- 21. Salgado CD, Farr BM. 2003. Outcomes associated with vancomycin-resistant enterococci: a meta-analysis. Infect. Control Hosp. Epidemiol. 24:690–698 [DOI] [PubMed] [Google Scholar]
- 22. Tseng SH, Lee CM, Lin TY, Chang SC, Chang FY. 2011. Emergence and spread of multi-drug resistant organisms: think globally and act locally. J. Microbiol. Immunol. Infect. 44:157–165 [DOI] [PubMed] [Google Scholar]
- 23. Uttley AH, Collins CH, Naidoo J, George RC. 1988. Vancomycin-resistant enterococci. Lancet i:57–58 [DOI] [PubMed] [Google Scholar]
- 24. Wang JL, Hsueh PR. 2009. Therapeutic options for infections due to vancomycin-resistant enterococci. Expert Opin. Pharmacother. 10:785–796 [DOI] [PubMed] [Google Scholar]