Abstract
The emergence of multidrug-resistant Salmonella isolates has created the need for new therapeutic agents. We evaluated the intracellular activity of four carbapenem compounds against clinical nontyphoid Salmonella (NTS) isolates in vitro and ex vivo. Subsequently, the efficacy of carbapenem treatment against selected Salmonella isolates in vivo was assessed using a murine peritonitis model. The MIC50 and MIC90 for doripenem, ertapenem, imipenem, and meropenem against 126 NTS isolates were found to be 0.062 and 0.062, 0.015 and 0.015, 0.5 and 1, and 0.031 and 0.031 μg/ml, respectively. The intracellular killing effect of ertapenem was sustained for 24 h and was superior to that of imipenem, meropenem, and doripenem; its effect was comparable to that of ceftriaxone. Ertapenem demonstrated an excellent pharmacokinetic profile with a percent time above the MIC of 75.5% and an area under the concentration-time curve/MIC ratio of 20,733. When peritoneal exudate cells were examined directly ex vivo from mice with Salmonella-induced peritonitis, cells from mice treated with ertapenem and ceftriaxone had intracellular and extracellular bacterial counts reduced 102- to 104-fold and exhibited killing effects similar to each other. The survival rates of mice inoculated with 1 × 105 and 106 CFU of a ceftriaxone-susceptible Salmonella isolate that were subsequently treated with ertapenem or ceftriaxone were 100% and 90%, respectively. When mice were inoculated with 5 × 104 and 105 CFU of a ceftriaxone-resistant and ciprofloxacin-resistant Salmonella isolate, mice treated with ertapenem had a higher survival rate than mice treated with ceftriaxone (70% versus 0% and 50% versus 0%, respectively; P < 0.001). Our results suggest that ertapenem is at least as effective as ceftriaxone in treating murine Salmonella infections and show that further clinical investigations on the potential use of ertapenem in treatment of human Salmonella infections are warranted.
INTRODUCTION
Salmonellosis is an important food-borne disease, and its most common clinical manifestation (gastroenteritis) often resolves without antibiotic treatment. However, antimicrobial therapy is indicated for invasive Salmonella infections such as bacteremia, vascular infections, and osteomyelitis. In recent years, antimicrobial resistance to fluoroquinolones and extended-spectrum cephalosporins among clinical Salmonella isolates has been a serious problem, particularly in Asia (18, 31). According to a case-control cohort study from Denmark, the 2-year mortality rate for people infected with drug-resistant Salmonella strains was 4.8 to 10.3 times higher than that for the general population (11). These data suggest that additional antimicrobial agents that are effective against drug-resistant Salmonella strains are critically needed.
The majority of antibiotics that demonstrate antimicrobial activity in vitro cannot cure human Salmonella infections because of their limited intracellular penetration (4). In addition to this limitation, antimicrobial resistance among Salmonella isolates is emerging (35). Both of these factors contribute to the increasing clinical challenge of treating antimicrobial-resistant Salmonella infections. Novel drugs with good extracellular and intracellular antibacterial activity are urgently needed to treat drug-resistant Salmonella infections. According to previous in vitro and in vivo studies, tigecycline has been shown to achieve high intracellular concentrations and exhibits a promising survival outcome in infected mice compared with traditional ceftriaxone therapy (20, 32). However, the low serum levels of tigecycline reached by currently recommended dosages might pose a clinical concern for treating Salmonella bacteremia in humans (23).
Carbapenems have been reported to be active against Salmonella species in vitro (3). However, clinical data regarding the use of carbapenems in the treatment of invasive Salmonella infections are limited. In one published case, imipenem salvage therapy cured an infant with Salmonella meningitis who had relapsed after 1 month of cefotaxime treatment (17). Another patient with relapsing spinal osteomyelitis caused by ciprofloxacin-resistant, extended-spectrum-beta-lactamase (ESBL)-producing Salmonella was also cured with imipenem treatment after 7 weeks of cefepime therapy (16). Additionally, high cellular-to-extracellular-concentration ratios have also been reported for meropenem (10). Given these results, we believe that the potential use of carbapenems for treating invasive Salmonella infections warrants further study. Using ceftriaxone as a comparator, we evaluated the antimicrobial efficacy of ertapenem, imipenem, meropenem, and doripenem against nontyphoid Salmonella NTS isolates in vitro. Furthermore, we assessed the in vivo efficacy of these four carbapenems in the treatment of Salmonella peritonitis in mice.
MATERIALS AND METHODS
Bacterial isolates.
Overall, 126 clinical Salmonella isolates were collected: 77 were obtained from patients at the Chi Mei Foundation Hospital, and 49 were obtained from patients at the National Cheng Kung University Hospital, Tainan, Taiwan. Isolates were obtained from pus, stool, blood, or other body fluids and included Salmonella serogroups A, B, C1, C2, D, E, and G. All isolates were stored at −80°C in Protect bacterial preservers (Technical Service Consultant Limited, Heywood, Lancashire, England). One ceftriaxone- and ciprofloxacin-susceptible bacteremic isolate, Salmonella enterica serotype Typhimurium (S129-42), was randomly selected from the 126 isolates and used throughout the study. Another isolate, S. enterica serotype Choleraesuis (S1-9210131), obtained from the National Cheng Kung University Hospital, was used for in vivo murine experiments. S1-9210131 is resistant to ciprofloxacin (MIC, 32 μg/ml) and ceftriaxone (MIC, 12 μg/ml) (16).
Macrophage cell line.
The RAW 264.7 murine macrophage cell line was obtained from the American Type Culture Collection (ATCC). RAW 264.7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS). Medium supplements were purchased from Gibco, Australia.
MICs.
An agar dilution method utilizing Mueller-Hinton agar was used to determine the MICs according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI) (8). Unless otherwise indicated, all antibiotics were purchased from Sigma (St. Louis, MO). The antibiotic concentrations used to determine MICs were as follows: ampicillin, 2 to 256 μg/ml; trimethoprim-sulfamethoxazole, 0.25/1.75 to 16/304 μg/ml; chloramphenicol, 1 to 256 μg/ml; ceftriaxone, 1 to 128 μg/ml; ciprofloxacin, 0.125 to 128 μg/ml (Bayer AG, Frankfurt, Germany); meropenem, 0.015 to 128 μg/ml; imipenem, 0.015 to 128 μg/ml; ertapenem, 0.015 to 128 μg/ml (Merck, Rahway, New Jersey); and doripenem, 0.015 to 128 μg/ml (Shionogi, Osaka, Japan). The antibiotic-containing plates were prepared within 24 h of use and stored at 4°C. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used in each assay as controls.
Intracellular antibiotic antibacterial activity.
RAW 264.7 cells were seeded in 24-well plates in DMEM with 10% FBS at a concentration of 1 × 106 cells per well and allowed to adhere for 2 h. The medium was then removed from the wells, and the cells were washed with phosphate-buffered saline (PBS). An inoculum of 1 × 106 CFU of S129-42 was added to each well. After 1 h, the culture plates were washed three times with PBS and incubated in medium containing 100 μg/ml gentamicin for 30 min at 37°C to kill extracellular bacteria. The plates were then washed three additional times with PBS. The MIC of gentamicin for S129-42 was 1 μg/ml. According to previously published data from studies with humans, the maximum serum concentrations (Cmax) for parenteral administration of 0.4 g ciprofloxacin, 1 g ceftriaxone, 1 g ertapenem, 0.5 to 1 g imipenem, 0.5 g doripenem, and 0.5 to 1 g meropenem are 4.0 (19), 128.7 (6, 36), 154.9 (22), 48 to 60 (2, 13, 27, 37), 32.6 (7), and 26 to 30 μg/ml (14, 26, 37), respectively. Based on these data, the following concentrations were added to the culture plates 4 h later (28): ciprofloxacin, 3 μg/ml; ceftriaxone, 100 μg/ml; ertapenem, 150 μg/ml; imipenem, 50 μg/ml; doripenem, 30 μg/ml; and meropenem, 30 μg/ml. Additionally, two lower concentrations of each drug, 1/2 and 1/10 of the above concentrations, were tested. At selected intervals, the bacterial loads in the wells were assessed. At each time point, cells were washed with ice-cold PBS, and bacteria were released from the cells using a lysis buffer (1% Triton X-100, 20 mM Tris, 0.2 M NaCl, 2 mM EDTA). The lysates containing released bacteria were serially diluted (1:10 in PBS), plated on LB agar plates, and cultured overnight (24). The limit of detection for this plate counting method was 100 CFU/ml.
Pharmacokinetic studies.
Because of the poor intracellular killing effect of imipenem and ciprofloxacin, we excluded these two antimicrobial agents from animal studies. For animal studies, female BALB/c mice 6 to 8 weeks of age and weighing between 18 and 20 g were obtained from the Animal Center of the National Science Council, Taipei, Taiwan. In preliminary studies, the 50% lethal dose (LD50) of S129-42 for healthy female BALB/c mice was less than 30 CFU by intraperitoneal infection (data not shown), indicating that female BALB/c mice are susceptible to Salmonella infection. To determine the serum concentrations of ceftriaxone, ertapenem, doripenem, and meropenem in these mice, each drug was subcutaneously administered to healthy mice as a single dose as previously described: 100 mg/kg of body weight for ceftriaxone (1) and 50 mg/kg for ertapenem (34), doripenem (12), and meropenem (15). At 0.5, 1, 2, 4, 6, and 8 h postinjection, blood samples from six anesthetized mice per treatment group were collected by cardiac puncture. Antibiotic concentrations were determined using the paper disk diffusion bioassay method; the E. coli control strains ATCC 25933 and ATCC 25922 were used as controls (21). All serum samples were assayed in triplicate. The lower limits of detection for the antibiotics were as follows: ceftriaxone, 0.1 μg/ml; ertapenem, 0.05 μg/ml; doripenem, 0.1 μg/ml; and meropenem, 0.02 μg/ml.
Ex vivo analysis of intracellular antibacterial activity in murine peritoneal exudate cells.
The intracellular antibacterial activity of each drug was examined in a murine peritonitis model in which healthy mice were infected intraperitoneally with 8.5 × 105 CFU of S129-42. Beginning 6 h later, 50-mg/kg doses of ertapenem, doripenem, or meropenem were administered every 6 h and 100 mg/kg doses of ceftriaxone were administered every 12 h, both subcutaneously, for a total of 72 h. The mice were sacrificed after the last scheduled dosing. The peritoneum of each animal was washed with 5 ml of normal saline, and the extracellular bacterial load in peritoneal fluids was assessed using a plate counting method on LB agar. Peritoneal exudate cells were washed with sterile PBS, and 100 μg/ml of gentamicin was added to kill extracellular bacteria. The cells were washed again with ice-cold PBS, and bacteria were released using a cell lysis buffer. The lysates containing released bacteria were serially diluted (1:10 in PBS), plated on LB agar, and cultured overnight for bacterial counting.
In vivo studies.
Two murine experiments were designed to investigate the therapeutic efficacy of ertapenem, doripenem, meropenem, and ceftriaxone for the treatment of Salmonella peritonitis. S129-42 and the ceftriaxone- and ciprofloxacin-resistant isolate S1-9210131 were tested at an inoculum of approximately 105 CFU. All animal experiments complied with the relevant guidelines of the Republic of China and the Chi Mei Foundation Medical Center Animal Use Policy.
Bacterial suspensions were diluted in fresh Mueller-Hinton broth. A 0.1-ml volume of the bacterial suspension was injected intraperitoneally into individual mice from each of the four antibiotic groups, and each group contained 10 mice. Ertapenem, doripenem, and meropenem were subcutaneously injected at a dose of 50 mg/kg every 6 h for 1 week. Ceftriaxone was given every 12 h at a dose of 100 mg/kg every 12 h for 1 week. The number of surviving mice was recorded at 12-h intervals for 2 weeks.
Statistical methods.
Data analysis was performed using SPSS 10.0 for Windows (SPSS Inc., Chicago, IL). To compare the effects between different treatment groups, two-way and one-way within-repeated-subjects analysis of variance (ANOVA) tests were applied. The log-rank test was applied to compare the survival rates of different groups. P values of less than 0.05 were considered statistically significant.
RESULTS
Bioassays and pharmacodynamic parameters.
The values for Cmax and the area under the curve from 0 to 24 h (AUC0–24) are listed in Table 1 for mice treated with ceftriaxone (100 mg/kg every 12 h), meropenem (50 mg/kg every 6 h), doripenem (50 mg/kg every 6 h), or ertapenem (50 mg/kg every 6 h). Our pharmacokinetic data are comparable to previously published human data. In mice, the time above MIC (T>MIC) for ceftriaxone was 60.8% against S129-42 and 17.4% against S1-9210131 (Table 2). At a dose of 50 mg/kg every 6 h, the T>MIC of ertapenem for the two Salmonella strains was >75.5% and the AUC/MIC ratio was >20,733. Both parameters for ertapenem were higher than those for meropenem or doripenem; these values were far above the recommended pharmacodynamic targets of carbapenem treatment for Gram-negative bacterial infections (25, 29).
Table 1.
Pharmacokinetic data for selected antibiotic dosages in mice and humans
| Drug (reference[s]) | Published human data |
Mice (present study) |
||||
|---|---|---|---|---|---|---|
| Dose (g) | Cmax (μg/ml)a | AUC0-24 (μg · h/ml)a | Dosage | Cmax (μg/ml)a | AUC0-24 (μg · h/ml)a | |
| Ceftriaxone (5, 36) | 1 g | 128.7 ± 14.8 | 971.1 ± 120.6 | 100 mg/kg q12h | 270 ± 50 | 941.2 ± 138.2 |
| Meropenem (26) | 0.5 g | 21.1-30 | 27.2-32 | 50 mg/kg q6h | 26.9 ± 4.7 | 45.1 ± 9.3 |
| Doripenem (7) | 0.5 g | 32.6 ± 4.4 | 71.8 ± 4.4 | 50 mg/kg q6h | 29.1 ± 3.7 | 66 ± 5.6 |
| Ertapenem (22) | 1 g | 154.9 ± 22 | 572.1 ± 68.8 | 50 mg/kg q6h | 146.0 ± 36.4 | 451.8 ± 52.3 |
Data are means ± standard deviations.
Table 2.
Pharmacokinetic profiles of ceftriaxone, ertapenem, meropenem, and doripenem against murine infections with Salmonella isolates S129-42 and S1-9210131
| Drug and dosage | S129-42 |
S1-9210131 |
||||||
|---|---|---|---|---|---|---|---|---|
| MIC (μg/ml) | Cmax/MIC | AUC/MIC | T> MIC | MIC (μg/ml) | Cmax/MIC | AUC/MIC | T> MIC | |
| Ceftriaxone, 100 mg/kg q12h | 0.062 | 4,354 | 7,070 | 60.8 | 12 | 22.5 | 36.5 | 17.4 |
| Meropenem, 50 mg/kg q6h | 0.031 | 868 | 1,454 | 50. | 0.031 | 868 | 1,454 | 50.1 |
| Doripenem, 50 mg/kg q6h | 0.062 | 469 | 1,064 | 36.1 | 0.25 | 116 | 264 | 25.0 |
| Ertapenem, 50 mg/kg q6h | <0.015 | >6,339 | >20,733 | >75.5 | <0.015 | >6,339 | >20,733 | >75.5 |
Antibacterial activity of carbapenems in vitro.
The in vitro susceptibility data for nine drugs tested against the 126 NTS isolates are listed in Table 3. The susceptibility rates for ceftriaxone and ciprofloxacin were 88.1 and 88.9%, respectively. According to the latest CLSI criteria, all NTS isolates were susceptible to the carbapenem compounds ertapenem, imipenem, meropenem, and doripenem (8).
Table 3.
MICs against 126 clinical NTS isolates and study isolates S129-42 and S1-9210131a
| Drugb | Nontyphoid Salmonella isolates |
MIC (μg/ml) |
|||
|---|---|---|---|---|---|
| MIC50 (μg/ml) | MIC90 (μg/ml) | % susceptiblea | S129-42 | S1-9210131 | |
| AMP | 32 | >512 | 48.8 | >256 | >256 |
| CHL | 8 | >256 | 53.2 | 256 | 64 |
| CIP | <0.125 | 2 | 88.9 | <0.125 | >32 |
| CRO | <1 | 4 | 88.1 | 0.062 | 12 |
| SXT | 0.25/4.75 | >32/608 | 69.8 | 0.5 | >16 |
| DOR | 0.062 | 0.062 | 100 | 0.062 | 0.062 |
| EPT | <0.015 | <0.015 | 100 | <0.015 | <0.015 |
| IMI | 0.5 | 1 | 100 | 0.5 | 0.25 |
| MEM | 0.031 | 0.031 | 100 | 0.031 | 0.031 |
Susceptibility was determined by the updated breakpoints recommended in the M100-S21 CLSI document (8).
AMP, ampicillin; CRO, ceftriaxone; CIP, ciprofloxacin; CHL, chloramphenicol; SXT, trimethoprim-sulfamethoxazole; EPT, ertapenem; MEM, meropenem; IMI, imipenem; and DOR, doripenem.
Antibacterial activity of intracellular antibiotics.
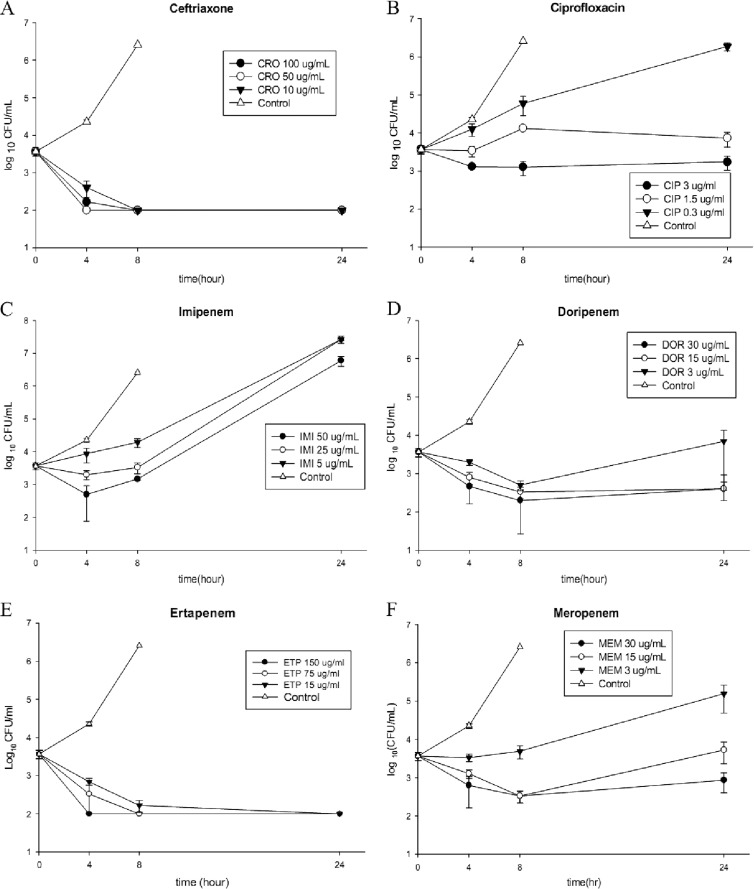
RAW 264.7 cells were incubated with S129-42 at an initial inoculum of 1 × 106 CFU/ml. One hour later, the intracellular bacterial load was determined to be 103 to 104 CFU/ml. The intracellular bacterial load had increased to 104 to 105 CFU/ml at 4 h and to 106 to 107 CFU/ml by 8 h postinoculation (Fig. 1). After 8 h, the RAW 264.7 cells were lysed because of the increasing intracellular bacterial loads; consequently, no intracellular counts were available at the 24-h postinoculation time point. When infected RAW 264.7 cells were incubated with ceftriaxone at concentrations of 100, 50, or 10 μg/ml, the intracellular bacterial counts declined markedly, and the antibacterial effect lasted for 24 h (Fig. 1A). When infected RAW 264.7 cells were incubated with ciprofloxacin at concentrations of 3, 1.5, or 0.3 μg/ml, the intracellular bacterial counts at 24 h postinoculation showed a minimal decrease only at the highest concentration, 3 μg/ml (Fig. 1B). Imipenem and ciprofloxacin were excluded from further animal studies because of their poor intracellular inhibitory effects. Of the four carbapenems tested, only ertapenem exhibited sustained intracellular antibacterial activity at levels similar to those seen with ceftriaxone; this effect was prominent even at the lowest concentration of 15 μg/ml (Fig. 1E).
Fig 1.
Intracellular antibacterial activities of ceftriaxone (A), ciprofloxacin (B), ertapenem (C), doripenem (D), imipenem (E), and meropenem (F) within RAW 264.7 macrophage cells infected with S129-42 at an inoculum of 1 × 106 CFU/ml. The drug concentrations were as follows: ciprofloxacin, 3 μg/ml; ceftriaxone, 100 μg/ml; ertapenem, 150 μg/ml; imipenem, 50 μg/ml; doripenem, 30 μg/ml; and meropenem, 30 μg/ml. Additionally, 1/2 and 1/10 doses of the original concentrations were tested. All experiments were performed in triplicate. Intracellular bacterial loads are shown as means ± standard deviations.
Intracellular antibacterial activity of murine peritoneal exudate cells ex vivo.
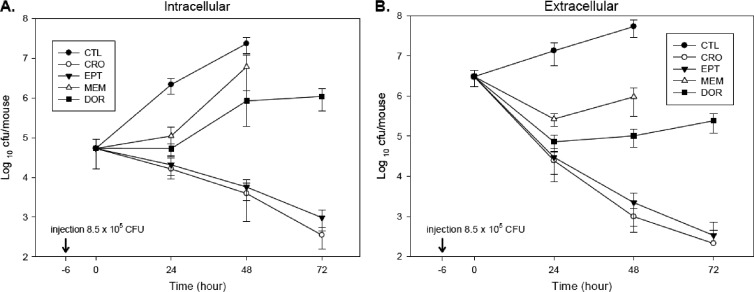
In the control group (infected mice without antimicrobial therapy), the extracellular and intracellular bacterial counts both increased from 106 to 107 and 104 to 105 CFU/ml to 107 to 108 CFU/ml at 48 h postinoculation. The intracellular colony counts decreased to 102 to 103 CFU/ml at 72 h postinoculation in the ertapenem and ceftriaxone groups. In contrast, the bacterial counts in the meropenem and doripenem groups increased at 48 and 72 h postinoculation (Fig. 2A). No significant differences in antibacterial activity against intracellular and extracellular Salmonella were observed between peritoneal exudate cells isolated at 72 h postinoculation from ertapenem- and ceftriaxone-treated mice (P = 0.86, by repeated ANOVA test) (Fig. 2).
Fig 2.
Ex vivo intracellular (A) and extracellular (B) antibacterial activity of ceftriaxone, ertapenem, meropenem, and doripenem in a murine peritonitis model. At 6 h after intraperitoneal inoculation of 8.5 × 105 CFU of S129-42, mice were treated subcutaneously with ceftriaxone (100 mg/kg every 12 h), ertapenem (50 mg/kg every 12 h), meropenem (50 mg/kg every 12 h), doripenem (50 mg/kg every 12 h), or saline (control). Intracellular and extracellular counts were evaluated over a period of 72 h.
Survival rates of mice with Salmonella-induced peritonitis. (i) Experiment 1.
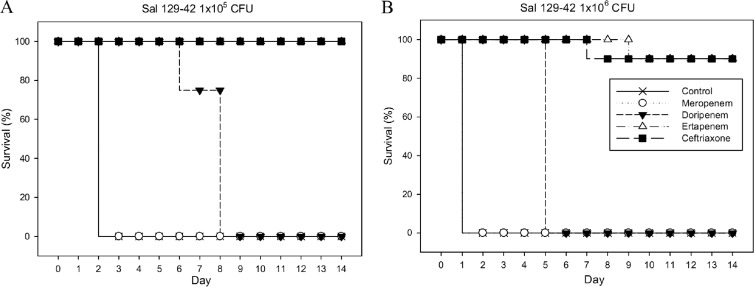
Animals that received either no therapy or meropenem therapy did not survive beyond 2 days after intraperitoneal inoculation with 1 × 105 CFU of S129-42. Animals treated with doripenem did not survive beyond 8 days postinoculation. However, all mice treated with either ertapenem or ceftriaxone survived at least 14 days postinoculation (Fig. 3A). When the inoculum was increased 10-fold to 1 × 106 CFU, the survival rate was 90% at 14 days postinoculation in the ertapenem- and ceftriaxone-treated groups (Fig. 3B).
Fig 3.
Survival rates of mice (10 per group) that were intraperitoneally infected with 1 × 105 (A) or 1 × 106 (B) CFU of S129-42 and treated with ceftriaxone (100 mg/kg every 12 h), ertapenem (50 mg/kg every 12 h), meropenem (50 mg/kg every 12 h), doripenem (50 mg/kg every 12 h), or saline (control) for 1 week.
The mean survival time of mice in the doripenem-treated group was longer than that of the meropenem-treated group (7.4 versus 2 days; P < 0.001 by log-rank test). However, the survival rate of mice treated with ertapenem or ceftriaxone at 14 days postinoculation was significantly higher than that of the two aforementioned groups (100% versus 0%; P < 0.001 by log-rank test). Therefore, the therapeutic effect of ertapenem was similar to that of ceftriaxone and superior to that of either doripenem or meropenem for the treatment of infection with the ceftriaxone-susceptible Salmonella isolate S129-42 (Fig. 3).
(ii) Experiment 2.
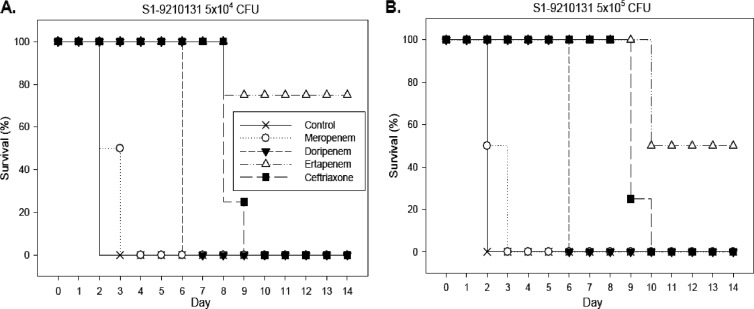
When mice were infected with an initial inoculum of 5 × 104 CFU of the ceftriaxone- and ciprofloxacin-resistant strain S1-9210131, only 70% of the mice treated with ertapenem survived until day 14 (Fig. 4A). Infecting mice with a 10-fold-concentrated inoculum (5 × 105 CFU) reduced day 14 survival to 50% for the group treated with ertapenem (Fig. 4B). Without therapy or with meropenem, doripenem, or ceftriaxone therapy, no animals survived for more than 10 days irrespective of the inoculum size.
Fig 4.
Survival rates of mice (10 per group) intraperitoneally infected with 5 × 104 (A) or 5 × 105 (B) CFU of the ceftriaxone- and ciprofloxacin-resistant strain S1-9210131. Following infection, animals were treated with ceftriaxone (100 mg/kg every 12 h), ertapenem (50 mg/kg every 12 h), meropenem (50 mg/kg every 12 h), doripenem (50 mg/kg every 12 h), or saline (control) for 1 week.
DISCUSSION
Although antimicrobial therapy has been suggested to be indicated only for invasive Salmonella infections, not for the more common Salmonella gastroenteritis, resistance to expanded-spectrum cephalosporins is emerging among human NTS isolates in Taiwan (5, 35). In a surveillance study, 1.5% of the 3,592 NTS isolates collected between 1999 and 2003 were found to be resistant to ceftriaxone, mainly because of CMY-2 and ESBLs (31). However, ceftriaxone resistance in S. enterica serotype Choleraesuis and serogroup B Salmonella isolates was shown to have increased by up to 10% in 2009; furthermore, ceftriaxone resistance has also emerged in serotypes other than S. enterica serotype Choleraesuis (30). These findings are even more concerning in light of the reported concurrent resistance to fluoroquinolones in S. enterica serotype Choleraesuis isolates (16). The emergence of antimicrobial resistance to nalidixic acid and ceftriaxone in invasive NTS isolates is also problematic in the United States (9). Collectively, these findings justify the use of carbapenems (the drugs of choice for treating ESBL-producing Enterobacteriaceae) in the treatment of human Salmonella infections.
In this study, we demonstrated that four carbapenems (ertapenem, imipenem, meropenem, and doripenem) are active against nontyphoid Salmonella isolates in vitro. The MICs of ertapenem for NTS isolates were lower than those of all other carbapenems tested; consistent with this, the Cmax/MIC and AUC/MIC values for ertapenem were higher than those for the other three carbapenems. Both in vitro in a macrophage cell line and ex vivo in murine peritoneal exudate cells, ertapenem showed the best intracellular bactericidal activity among the tested carbapenems, and this activity was similar to that of ceftriaxone. Furthermore, in ex vivo experiments, ertapenem achieved these results at levels achievable in serum using the currently recommended dose. In a murine peritonitis model based on infection with a ceftriaxone- and carbapenem-susceptible Salmonella isolate, ceftriaxone and ertapenem exhibited therapeutic efficacy superior to that of meropenem or doripenem. Not surprisingly, in mice infected with a ceftriaxone-resistant and carbapenem-susceptible Salmonella isolate, ertapenem therapy led to a better outcome than ceftriaxone, meropenem, or doripenem. Our data demonstrate that ertapenem may be a potential alternative for treating human salmonellosis in an era of emerging antimicrobial resistance. However, in light of the species differences between mice and humans, the extrapolation of results from animal studies to clinical situations should be made cautiously. Additional clinical trials involving ertapenem therapy for the treatment of invasive Salmonella infections are warranted.
The in vitro antibacterial activity of different drugs in macrophage cell lines may be related to the different drug concentration/MIC ratios, though other variables can also affect the intracellular antibacterial activity. In the case of S129-42, the drug concentration/MIC values are low for ciprofloxacin (3/<0.125 = >24) and imipenem (50/0.5 = 100), moderate for doripenem (30/0.062 = 484) and meropenem (30/0.031 = 968), and high for ceftriaxone (100/0.062 = 1,613) and ertapenem (150/<0.015 = >1,000). The latter two drugs exhibited sustained intracellular antibacterial activity for at least 24 h. However, whether using higher doses of imipenem, meropenem, doripenem, or ciprofloxacin will increase their antibacterial activity against intracellular Salmonella bacteria requires further study.
Antimicrobial dosages used in a murine model can result in pharmacokinetic profiles similar to previously published data obtained with humans, thus justifying the rationale of drug doses tested in mice. The minimal pharmacokinetic bactericidal target of β-lactam agents for Gram-negative organisms is >40% for the T>MIC (29), whereas an AUC/MIC (AUIC) ratio of >125 is associated with preventing the development of antimicrobial resistance (33). In the present study, where we used the pharmacological profile of ceftriaxone as a reference, only ertapenem therapy resulted in a higher T>MIC in mice. This finding is consistent with the similarity in therapeutic efficacy of ertapenem and ceftriaxone observed in mice infected with the ceftriaxone-susceptible Salmonella isolate. Although meropenem has been shown to exhibit excellent intracellular penetration with a high cellular-to-extracellular-concentration ratio of 3 to 12, in addition to its immunomodulatory activity (10), our results show that its intracellular antibacterial activity against Salmonella isolates and its therapeutic efficacy in the treatment of murine peritonitis are inferior to those of ertapenem. However, a higher dose of meropenem, such as 100 mg/kg, may be able to achieve a higher T>MIC in mice and improve the survival rate of mice with Salmonella peritonitis.
In conclusion, ertapenem is as effective as ceftriaxone in treating mice with Salmonella infections, suggesting that it has potential use as a treatment in humans infected with antimicrobial-resistant Salmonella.
ACKNOWLEDGMENTS
We thank Chien-Shun Chiou for his assistance in serotyping of two Salmonella isolates and the staff of the Research Laboratory of Infectious Diseases at the Chi-Mei Medical Center for their assistance with the statistical analysis.
This work was supported in part by grants from the Chi-Mei Medical Center Research Foundation (CMFHR9901), National Science Council, Taiwan (NSC 96-2628-B-006-004-MY3), Department of Health, Executive Yuan, Taiwan (DOH100-TD-B-111-002), and Merck Sharp & Dohme Corp.
We declare no competing interests.
Footnotes
Published ahead of print 2 April 2012
REFERENCES
- 1. Barry B, et al. 1996. Efficacy of single-dose ceftriaxone in experimental otitis media induced by penicillin- and cephalosporin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 40:1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buckley MM, Barradell RN, Goa KL. 1992. Imipenem/cilastatin. A reappraisal of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs 44:408–444 [DOI] [PubMed] [Google Scholar]
- 3. Capoor MR, et al. 2009. Minimum inhibitory concentration of carbapenems and tigecycline against Salmonella spp. J. Med. Microbiol. 58:337–341 [DOI] [PubMed] [Google Scholar]
- 4. Chang HR, Vladoianu IR, Pechere JC. 1990. Effects of ampicillin, ceftriaxone, chloramphenicol, pefloxacin and trimethoprim-sulphamethoxazole on Salmonella typhi within human monocyte-derived macrophages. J. Antimicrob. Chemother. 26:689–694 [DOI] [PubMed] [Google Scholar]
- 5. Chen PL, et al. 2007. Extraintestinal focal infections in adults with Salmonella enterica serotype Choleraesuis bacteremia. J. Microbiol. Immunol. Infect. 40:240–247 [PubMed] [Google Scholar]
- 6. Chiu LM, Menhinick AM, Johnson PW, Amsden GW. 2002. Pharmacokinetics of intravenous azithromycin and ceftriaxone when administered alone and concurrently to healthy volunteers. J. Antimicrob. Chemother. 50:1075–1079 [DOI] [PubMed] [Google Scholar]
- 7. Cirillo I, et al. 2009. Pharmacokinetics, safety, and tolerability of doripenem after 0.5-, 1-, and 4-hour infusions in healthy volunteers. J. Clin. Pharmacol. 49:798–806 [DOI] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. CLSI document M100–S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Crump JA, et al. 2011. Antimicrobial resistance among invasive nontyphoidal Salmonella enterica isolates in the United States: National Antimicrobial Resistance Monitoring System, 1996 to 2007. Antimicrob. Agents Chemother. 55:1148–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuffini AM, et al. 1993. The entry of meropenem into human macrophages and its immunomodulating activity. J. Antimicrob. Chemother. 32:695–703 [DOI] [PubMed] [Google Scholar]
- 11. Helms M, Vastrup P, Gerner-Smidt P, Molbak K. 2002. Excess mortality associated with antimicrobial drug-resistant Salmonella Typhimurium. Emerg. Infect. Dis. 8:490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilliard JJ, et al. 2011. Comparative effects of carbapenems on bacterial load and host immune response in a Klebsiella pneumoniae murine pneumonia model. Antimicrob. Agents Chemother. 55:836–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jaruratanasirikul S, Raungsri N, Punyo J, Sriwiriyajan S. 2005. Pharmacokinetics of imipenem in healthy volunteers following administration by 2 h or 0.5 h infusion. J. Antimicrob. Chemother. 56:1163–1165 [DOI] [PubMed] [Google Scholar]
- 14. Jaruratanasirikul S, Sriwiriyajan S, Punyo J. 2005. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob. Agents Chemother. 49:1337–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim A, Banevicius MA, Nicolau DP. 2008. In vivo pharmacodynamic profiling of doripenem against Pseudomonas aeruginosa by simulating human exposures. Antimicrob. Agents Chemother. 52:2497–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ko WC, et al. 2005. A new therapeutic challenge for old pathogens: community-acquired invasive infections caused by ceftriaxone- and ciprofloxacin-resistant Salmonella enterica serotype Choleraesuis. Clin. Infect. Dis. 40:315–318 [DOI] [PubMed] [Google Scholar]
- 17. Koc E, Turkyilmaz C, Atalay Y, Sen E. 1997. Imipenem for treatment of relapsing Salmonella meningitis in a newborn infant. Acta Paediatr. Jpn. 39:624–625 [DOI] [PubMed] [Google Scholar]
- 18. Lee HY, et al. 2009. High rate of reduced susceptibility to ciprofloxacin and ceftriaxone among nontyphoid Salmonella clinical isolates in Asia. Antimicrob. Agents Chemother. 53:2696–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lettieri JT, Rogge MC, Kaiser L, Echols RM, Heller AH. 1992. Pharmacokinetic profiles of ciprofloxacin after single intravenous and oral doses. Antimicrob. Agents Chemother. 36:993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu CY, Huang YT, Liao CH, Hsueh PR. 2008. In vitro activities of tigecycline against clinical isolates of Aeromonas, Vibrio, and Salmonella species in Taiwan. Antimicrob. Agents Chemother. 52:2677–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lund ME, Blazevic DJ, Matsen JM. 1973. Rapid gentamicin bioassay using a multiple-antibiotic-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 4:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Majumdar AK, et al. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob. Agents Chemother. 46:3506–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. 2005. Pharmacokinetic/pharmacodynamic profile for tigecycline-a new glycylcycline antimicrobial agent. Diagn. Microbiol. Infect. Dis. 52:165–171 [DOI] [PubMed] [Google Scholar]
- 24. Menashe O, Kaganskaya E, Baasov T, Yaron S. 2008. Aminoglycosides affect intracellular Salmonella enterica serovars Typhimurium and Virchow. Antimicrob. Agents Chemother. 52:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moczygemba LR, Frei CR, Burgess DS. 2004. Pharmacodynamic modeling of carbapenems and fluoroquinolones against bacteria that produce extended-spectrum beta-lactamases. Clin. Ther. 26:1800–1807 [DOI] [PubMed] [Google Scholar]
- 26. Moon YS, Chung KC, Gill MA. 1997. Pharmacokinetics of meropenem in animals, healthy volunteers, and patients. Clin. Infect. Dis. 24(Suppl. 2):S249–S255 [DOI] [PubMed] [Google Scholar]
- 27. Norrby SR. 1995. Carbapenems. Med. Clin. North Am. 79:745–759 [DOI] [PubMed] [Google Scholar]
- 28. Plaisance KI, Drusano GL, Forrest A, Weir MR, Standiford HC. 1990. Effect of renal function on the bioavailability of ciprofloxacin. Antimicrob. Agents Chemother. 34:1031–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santos Filho L, Kuti JL, Nicolau DP. 2007. Employing pharmacokinetic and pharmacodynamic principles to optimize antimicrobial treatment in the face of emerging resistance. Braz. J. Microbiol. 37:183–193 [Google Scholar]
- 30. Su LH, et al. 2011. Increasing ceftriaxone resistance in Salmonellae, Taiwan. Emerg. Infect. Dis. 17:1086–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Su LH, et al. 2005. Increasing ceftriaxone resistance in Salmonella isolates from a university hospital in Taiwan. J. Antimicrob. Chemother. 55:846–852 [DOI] [PubMed] [Google Scholar]
- 32. Tang HJ, et al. 2011. Tigecycline against clinical nontyphoid Salmonella isolates: comparisons of in vitro and in vivo intracellular killing with ceftriaxone. Antimicrob. Agents Chemother. 55:2755–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van den Bogaard AE, Bruinsma N, Stobberingh EE. 2000. The effect of banning avoparcin on VRE carriage in The Netherlands. J. Antimicrob. Chemother. 46:146–148 [DOI] [PubMed] [Google Scholar]
- 34. Xuan D, et al. 2002. Pharmacodynamic assessment of ertapenem (MK-0826) against Streptococcus pneumoniae in a murine neutropenic thigh infection model. Antimicrob. Agents Chemother. 46:2990–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan JJ, et al. 2003. Emergence of ceftriaxone-resistant Salmonella isolates and rapid spread of plasmid-encoded CMY-2-like cephalosporinase, Taiwan. Emerg. Infect. Dis. 9:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhanel GG, Branham RC. 1988. Third-generation cephalosporins. Can. J. Hosp. Pharm. 41:183–194 [Google Scholar]
- 37. Zhanel GG, Simor AE, Vercaigne L, Mandell L. 1998. Imipenem and meropenem: comparison of in vitro activity, pharmacokinetics, clinical trials and adverse effects. Can. J. Infect. Dis. 9:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]