Abstract
Objectives
A safe and effective topical prevention strategy will likely require sustained delivery of potent antiviral drugs and a delivery system that simultaneously maximizes drug distribution and overcomes the behavioural challenges related to adherence. Activity against HIV and herpes simplex virus (HSV) would be advantageous, given the epidemiological link between the two pathogens. We hypothesize that tenofovir disoproxil fumarate (tenofovir DF), a prodrug of tenofovir, may be more potent than tenofovir and ideal for sustained intravaginal ring (IVR) delivery.
Methods
The anti-HIV and anti-HSV activity of tenofovir and tenofovir DF were assessed in cell and explant models. Cumulative tenofovir DF release and stability from polyether urethane (PEU), ethylene-co-vinyl acetate (EVA) and silicone IVRs were compared, and the activity and safety of drug released were evaluated in cervical explants and in a polarized dual-chamber model.
Results
Tenofovir DF inhibited HIV and HSV at ∼100-fold lower concentrations than tenofovir and retained activity in the presence of semen. PEU rings delivered >1 mg/day of tenofovir DF for 30 days. Pre-treatment of cervical explants with 10 μg/mL tenofovir DF or eluants from PEU minirings resulted in >90% inhibition of HIV and reduced HSV-2 yields by 2.5 log. Tenofovir DF and eluants did not prevent cell growth or polarization, or have any deleterious effects on an epithelial barrier.
Conclusions
The findings support the development of a PEU tenofovir DF ring, which may provide potent and sustained protection against HIV and HSV.
Keywords: microbicides, PrEP, NRTIs
Introduction
The HIV epidemic is fuelled by the synergistic relationship between HIV and genital herpes, one of the most prevalent sexually transmitted infections worldwide. Thus, the development and implementation of strategies to prevent both infections are a public health imperative. Globally, the predominant mode of transmission of HIV and herpes simplex virus (HSV) is heterosexual intercourse, with women disproportionately impacted. This reflects the increased risk of male-to-female transmission as well as social disparities that limit access to effective prevention modalities, such as condoms. Therefore, the development of female-controlled prophylactic products is crucial. The recent success of the CAPRISA 004 placebo-controlled trial, in which there was a 39% reduction in HIV and an unanticipated 51% reduction in HSV-2 acquisition among women who were instructed to apply 1% tenofovir gel intravaginally before and after intercourse, illustrates the potential for delivering safe and effective vaginal prevention products.1 The protection against HSV-2 may reflect the high levels of drug achieved within the genital tract epithelium following vaginal application, which is consistent with in vitro studies demonstrating that tenofovir inhibits HSV-2 replication at concentrations ranging from 100 to 500 μg/mL.2,3
Additional trials are in progress to confirm the results of CAPRISA 004, extend the studies to younger adolescent and pregnant women, evaluate the efficacy of alternative dosing regimens, and examine whether the time interval between gel application and coitus impacts pharmacokinetics.4 Daily treatment with tenofovir gel, one of the arms in the Vaginal and Oral Interventions to Control the Epidemic study, was recently discontinued due to lack of efficacy following a Data and Safety Monitoring Board review.5 While adherence data, drug levels and potential mechanisms that may have contributed to the disappointing results are not yet available, the findings underscore the need to develop more potent drugs and formulations, such as intravaginal rings (IVRs) that may overcome some of the difficulties associated with adherence to daily or coitally dependent dosing.
The importance of adherence is highlighted by subgroup analyses from CAPRISA 004. Tenofovir gel was 54% effective among participants who reported high gel use (≥80% of sex acts), but only 28% protective among those who reported gel application in <50% of sex acts.1 We suggest that IVR formulations will overcome some of the adherence barriers associated with coitally dependent or daily dosing of gels. The high acceptability of vaginal ring hormonal products, coupled with the ability of IVR to provide controlled and prolonged release of antiretroviral drugs, supports their development for HIV and HSV prevention.6,7
IVRs are composed of either thermosets like silicones (Estring® and Femring®) or thermoplastics, e.g. poly(ethylene-co-vinyl acetate) (EVA) (NuvaRing®).8,9 The hydrophobicity of silicone and EVA limits the number of antiretrovirals (ARVs) that can be delivered from them. Thermoplastic polyether urethane (PEU) elastomers are linear segmented copolymers with an amorphous ‘soft’ segment and a crystalline ‘hard’ segment. The morphology and properties of PEUs are influenced by the chemical composition of the component monomers, leading to higher drug solubility for many ARVs in comparison with silicone or EVA.10 Therefore, we evaluated a representative polymer from each class, silicones, EVA and PEU, to identify a suitable matrix to deliver milligram quantities of tenofovir disoproxil fumarate (tenofovir DF) per day.
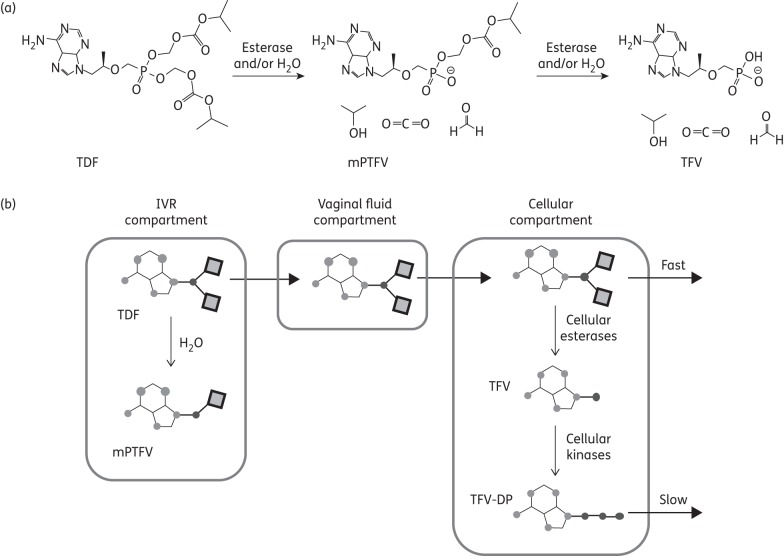
We hypothesize that tenofovir DF may provide increased protection against HIV and HSV, and may be amenable to IVR formulation. Tenofovir DF, a salt of tenofovir disoproxil with fumaric acid designed to improve solid-state stability, is a weak base prodrug of tenofovir (Figure 1a) that is susceptible to hydrolysis by water and cellular esterases.11 It replaced tenofovir for the treatment of HIV because of its greater oral bioavailability, but tenofovir DF has other distinct advantages that suggest it may be a superior microbicide. Tenofovir DF permeates cells more rapidly than tenofovir due to its increased hydrophobicity, resulting in increased intracellular accumulation of tenofovir diphosphate (TFV-DP), the active metabolite (Figure 1b).12 TFV-DP levels are ∼1000-fold higher in tenofovir DF-treated cells compared with tenofovir-treated cells and the tissue permeability of tenofovir DF is ≥10-fold higher than that of tenofovir.13 This suggests that less drug would need to be delivered, which could facilitate IVR formulation and reduce costs.
Figure 1.
(a) Tenofovir disoproxil hydrolysis to form mPTFV with the loss of one molecule each of isopropanol, formaldehyde and carbon dioxide. Further breakdown of the mPTFV yields tenofovir. (b) Proposed pharmacokinetic model for vaginal delivery of tenofovir DF from an IVR. Tenofovir DF is released from the IVR compartment because of a large concentration gradient into vaginal fluid, where the drug may hydrolyse to form mPTFV and tenofovir. Tenofovir DF more readily diffuses into the cellular compartment due to its increased hydrophobicity and therefore partitions into the cell membrane. In cells, tenofovir DF is converted into TFV-DP after several enzyme-catalysed reactions. Ultimately, the drug partitions into the bloodstream and is eliminated. TDF, tenofovir DF; TFV, tenofovir; mPTFV, monoprotected tenofovir.
The current study was designed to evaluate the antiviral activity and potential safety of tenofovir DF for topical vaginal prevention of HIV and HSV, and to determine if the drug could be stably formulated for sustained 30 day intravaginal delivery of daily low milligrams of tenofovir DF.
Materials and methods
Cells and viruses
CaSki (CRL 1550), HEC-1-A (HTB-112) and Vero (CCL-81) cells were obtained from the American Tissue Culture Collection. TZM-bl (8129) cells were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Jurkat-Tat-CCR5 (JT-CCR5) cells were provided by Quentin Sattentau (Sir William Dunn School of Pathology, University of Oxford, Oxford, UK). All cells were cultured as previously described.14,15 The laboratory-adapted HIV-1BaL (R5-utilizing strain) was grown as previously described.14,15 HSV-2 (G)16 was grown on Vero cells. HSV-2 (4674), a clinical isolate obtained from the clinical virology laboratory at Montefiore Hospital, Bronx, NY, USA, was propagated on human keratinocytes (HaCaT) (provided by David Johnson, Oregon Health & Sciences University, Portland, OR, USA). HSV stocks were stored at −80°C.
Drugs and reagents
Nonoxynol-9 (N-9) and aciclovir were purchased from Sigma (St Louis, MO, USA) and Bedford Laboratories (Bedford, OH, USA), respectively. Tenofovir and tenofovir DF were provided by Gilead (Foster City, CA, USA). Human semen was purchased from Lee Biosolutions, Inc. (St Louis, MO, USA). Semen was clarified by centrifugation at 1500 rpm for 10 min and the supernatant [seminal plasma (SP)] divided into aliquots; whole semen and SP were stored at −80°C. Polymers for IVR fabrication included PEU (Tecoflex® EG-85A; Lubrizol, Wickliffe, OH, USA), EVA (Elvax® 360; Dupont, Wilmington, DE, USA) and silicone (DDU-4351; NuSil Technology, Carpinteria, CA, USA).
Formulation of tenofovir DF in IVRs
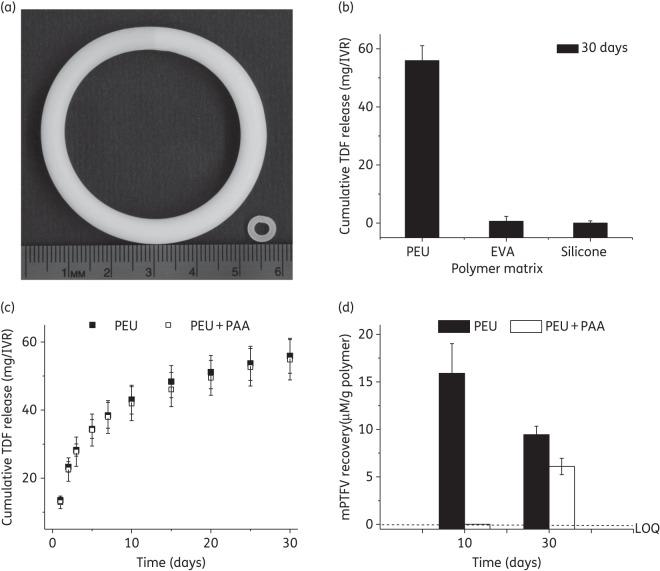
Tenofovir DF was formulated in PEU and EVA by hot-melt extrusion (HME) at 145 and 110°C, respectively, using a twin-screw extruder (Haake Minilab, Thermo Scientific, Newington, NH, USA) at a screw speed of 50 rpm17,18 and in three-part silicone by Pt-catalysed condensation polymerization. The sample was pelletized after the first extrusion using a Randcastle pelletizer (Randcastle Extrusion Systems Inc., Cedar Grove, NJ, USA) to ensure homogeneous drug distribution and re-extruded through a 4.7 mm (human-sized devices) or 2.1 mm (minirings) aluminium die. For human-sized IVRs, 15.5 cm segments of uniform diameter were cut and ends joined using induction welding (PlasticWelds Systems, Newfane, NY, USA) to prepare 55 × 5.3 mm rings (Figure 3a). IVRs were scaled to produce minirings that could be placed in Transwell® inserts. Segments of ∼17 mm were cut and the ends butt-welded to form 6.7 × 1.4 mm minirings (Figure 3a).7
Figure 3.
(a) Photograph showing 10 wt% tenofovir DF-loaded human-sized PEU IVR (55 × 5.3 mm) and miniring (6.7 × 1.4 mm). (b) 30 day cumulative tenofovir DF release in vitro from different polymers (PEU, EVA and silicone) under simulated conditions. (c) In vitro daily and cumulative tenofovir DF release from PEU segments with and without 0.5 wt% PAA (pH modifier). (d) mPTFV recovery from PEU segments with and without 0.5 wt% PAA subjected to in vitro release studies for 30 days in simulated vaginal fluid. mPTFV levels were calculated from tenofovir DF calibration curves assuming similar molar absorption coefficients at 260 nm. The concentration of mPTFV was below the limit of quantification (LOQtenofovir DF = 0.06 μg/mL) on day 10 in PEU segments with PAA. Results are means ± SD from three independent experiments. TDF, tenofovir DF; PAA, poly(acrylic acid).
In vitro elution study
Drug-loaded segments of ∼15 mm were end-capped with high-density polyethylene sheets using two-component Loctite® plastic glue, weighed and subjected to 30 day release studies under sink conditions in simulated vaginal fluid (25 mM acetate buffer, pH 4.2)18 at 37°C and 80 rpm shaking.
Analysis of tenofovir DF content in IVRs
Tenofovir DF content was determined by liquid-phase extraction and HPLC analysis. Approximately 50 mg of tenofovir DF-loaded polymer was dissolved in dichloromethane (DCM) using 19-norethindrone stock in DCM as an internal standard (IS, 1 mL, 3.35 mM). The polymer was precipitated with acetonitrile (1 : 9), and the solution filtered using 0.2 μm syringe filters and analysed by HPLC. For determining extraction efficiency, 50 mg of polymer pellets was spiked with tenofovir DF solution (1 mL, 1.5 mM) and IS, and treated as above. Tenofovir DF content was determined using an Agilent 1200 Series HPLC on an Eclipse XDB-C18 column (4.6 × 150 mm, 5 μm) at 25°C, with a flow rate of 0.7 mL/min and an acetonitrile/water gradient (0–3 min: 0%–50% acetonitrile; 3–5 min: 50%–75% acetonitrile; 5–7 min: 75%–90% acetonitrile; and 7–10 min: 90%–0% acetonitrile). Data collected at 260 nm (tenofovir DF) and 245 nm (IS) were computed using ChemStation32 software, and concentrations were derived from a calibration curve generated using methanol standards (0.01–1 mM). For in vitro elution samples, mobile phases included 50 mM phosphate buffer (pH 6.0) for the separation of tenofovir.
HIV assays
JT-CCR5 (1 × 105 cells/well) cells were exposed to drug 24 h prior to challenge with HIV-1BaL [103 50% tissue culture infective dose (TCID50)] in the absence or presence of 25% (v/v) SP in complete medium for 2 h. The plates were centrifuged at 1500 rpm for 5 min, and the cells were washed three times with serum-free medium and cultured for 5–7 days before determining HIV-1 p24 levels in culture supernatants by ELISA.15 We used SP rather than semen, so that it could be removed by centrifugation during the above washing steps. TZM-bl cells were exposed to HIV-1BaL (103 TCID50) in the presence of serially diluted miniring eluants. The virus and drug were left in culture for 48 h at 37°C, and then luciferase activity in the lysates was determined.14 Cytotoxicity was assessed by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay.14
HSV plaque assays
CaSki cells were plated in 24-well dishes and exposed to serial dilutions (0.1–1000 μg/mL) of drugs for 12–16 h at 37°C or DMEM alone, prior to challenge with 150 or 1500 pfu/well of HSV-2 (4674) in duplicate; the virus was added in DMEM or DMEM containing pooled human semen [final semen concentration 25% (v/v)]. The plaques were counted 48 h post-infection by immunostaining.19
Infection of explant cultures
Cervical tissue was collected from pre-menopausal women undergoing therapeutic hysterectomies at the Albert Einstein–Weiler Hospital (Bronx, NY, USA). Ectocervical tissue was cut into explants14,15,20 prior to 24 h exposure to unformulated drugs (tenofovir DF and aciclovir), PEU minirings containing 5 wt% or 10 wt% tenofovir DF, or eluants resulting from 24 h of incubation of minirings in 1 mL of complete medium. The treated explants were challenged with HIV-1BaL (105 TCID50) or HSV-2 (G) (106 pfu/explant) for 2 h, washed three times and cultured for 5–14 days in the presence or absence of drug. HIV infection was assessed by detecting provirus integration by Real-Time Quantitative (RTQ)-PCR;20 HSV yields in the culture supernatants were determined by plaque assay.
Epithelial integrity dual-chamber safety assay
HEC-1-A cells were grown in Transwell® inserts and allowed to polarize, effectively creating a barrier to HIV.21 Unformulated drugs or miniring eluants were added apically for 18 h, and removed by washing before the addition of HIV-1BaL and JT-CCR5 cells to the apical and baso-lateral chambers, respectively. The epithelial integrity was monitored by measuring transepithelial electrical resistance (TER) and determining the extent of viral replication in the baso-lateral chambers by measuring p24 levels. Alternatively, HEC-1-A cells were grown in Transwell® inserts in the absence or presence of drug and the time and extent of polarization compared by monitoring TER. Mitomycin C treatment (100 μg/mL) was used as a control to prevent cell division and polarization.
Results
Tenofovir DF has potent antiviral activity that is retained in the presence of SP
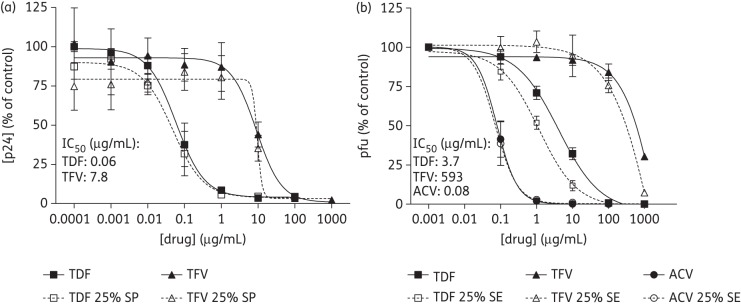
JT-CCR5 cells were exposed to increasing concentrations of tenofovir DF or tenofovir for 24 h prior to challenge. Tenofovir DF inhibited HIV infection at concentrations ∼100-fold less than required for tenofovir, resulting in an IC50 of 0.06 μg/mL (0.09 μM) compared with 7.8 μg/mL (27.2 μM) for tenofovir. Both drugs retained activity when HIV was introduced in 25% SP (Figure 2a). Parallel studies with HSV-2 were conducted including aciclovir as a positive control. Consistent with its greater cell permeability, tenofovir DF was >100-fold more potent than tenofovir, with an interpolated IC50 of 3.7 μg/mL (5.8 μM) compared with 593 μg/mL (2.1 mM), respectively. Aciclovir was the most effective drug against HSV-2, with an IC50 of ∼0.08 μg/mL (0.03 μM). Importantly, the antiviral activity of tenofovir DF was fully preserved if HSV was introduced in 25% semen (Figure 2b).
Figure 2.
Antiviral activity of tenofovir and tenofovir DF in vitro. (a) JT-CCR5 cells were pre-treated with indicated concentrations of tenofovir or tenofovir DF for 24 h prior to challenge with 103 TCID50 of HIV-1BaL in the presence or absence of 25% SP. Following 2 h of incubation at 37°C, cells were washed to remove drug and inocula, and cultured. Infection was monitored by determining the p24 level in culture supernatants 5 days post-infection. Results are means ± SEM from two experiments conducted in triplicate. (b) CaSki cells were pre-treated with the indicated doses of tenofovir, tenofovir DF or aciclovir for 12–16 h and then challenged with HSV-2 (4674) in the presence or absence of 25% semen (SE). Following 1 h of incubation, the inoculum was removed and cells were overlaid with medium. Plaques were counted 48 h post-infection. Results are means ± SEM obtained from at least two experiments conducted in duplicate. TDF, tenofovir DF; TFV, tenofovir; ACV, aciclovir.
Development of IVR for delivery of tenofovir DF
Drug release from any polymer is influenced by the solubility of the drug in the polymer matrix. Tenofovir DF has an octanol/water partition coefficient (logP) value of 1.2,22 a low number for effective drug solubility and release from most hydrophobic polymers. Therefore, we screened tenofovir DF at a 10 wt% loading in the three elastomeric polymers: PEU, EVA and silicone. We have shown previously that end-capped elastomeric segments can be utilized to model drug release rate from IVRs.7,17 Figure 3(a) shows a human-sized tenofovir DF–PEU IVR (55 × 5.3 mm) made by HME of the PEU–tenofovir DF mixture. Average tenofovir DF release from PEU IVRs (1.5 ± 0.2 mg/day) was much higher in comparison with EVA (0.3 mg/day) or silicone IVRs (0.1 mg/day) (Figure 3b). Based on these data we selected PEU for further evaluation.
There are two challenges with tenofovir DF formulation: (i) the hydrolysis of the compound to the monoprotected derivative of tenofovir (mPTFV) during processing and storage; and (ii) the hydrolysis of the compound in the device while it is exposed to vaginal fluid for the 21–30 day duration of drug elution.23 Consistent with the literature, over 10 days at 37°C, 13.6% of tenofovir DF degraded into mPTFV within the ring and a considerable fraction of the released material was the monoprotected drug in simulated vaginal fluid. Hydrolysis is pH dependent, with the lowest rate at pH 2–4.23 Therefore, a high molecular weight weak acid [poly(acrylic acid) (PAA) (Mn = 247 000 Da)] was added to the matrix. The addition of PAA (0.5 wt%) did not alter the drug release profile (Figure 3c) and prevented the hydrolysis of tenofovir DF; no mPTFV was detected up to 20 days in the release media and only 5.7% of mPTFV within the IVR was detected by day 30 (Figure 3d). Furthermore, the tenofovir DF–PAA formulation was stable for 6 months under accelerated stability conditions (40°C/75% relative humidity), with 96.7% ± 3.9% of the drug recovered.
Tenofovir DF released from PEU minirings retains antiviral activity
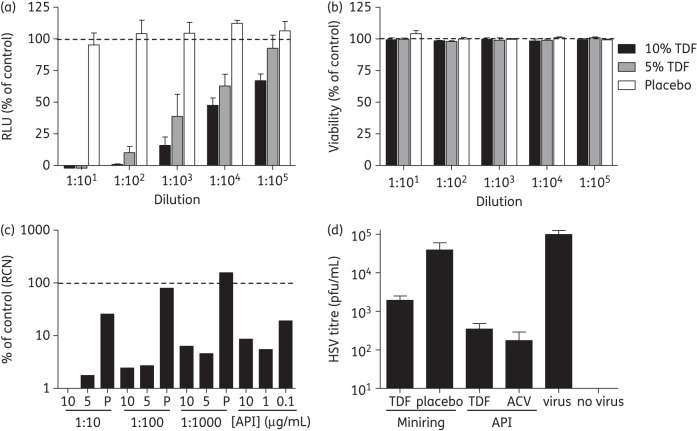
Minirings (PEU with 0.5 wt% PAA) loaded with 5 and 10 wt% tenofovir DF or placebo were incubated in 1 mL of complete RPMI for 24 h, and the resulting samples (eluants) were serially diluted and evaluated for antiviral activity and cytotoxicity. Eluants from 5 and 10 wt% tenofovir DF-loaded minirings containing 69 μg/mL (108 μM) and 130 μg/mL (204 μM) tenofovir DF, respectively, but not placebo samples, exhibited potent anti-HIV activity relative to the control (medium alone) (Figure 4a) and no cytotoxicity (Figure 4b).
Figure 4.
Antiviral activity of tenofovir DF released from ring formulation. (a) TZM-bl cells were infected with 103 TCID50 of HIV-1BaL in the presence or absence of diluted eluants from minirings (10 wt% and 5 wt% tenofovir DF or placebo). Virus and eluant were left in culture for 48 h at 37°C, and infectivity was monitored by a luciferase assay. (b) Cell viability was determined by MTS assay following exposure to eluant for 48 h. Results are means ± SEM obtained from two experiments conducted in triplicate. (c) Ectocervical explants were exposed to miniring eluants [10 wt% (10), 5 wt% (5) or placebo (P)] or the indicated concentrations of tenofovir DF for 24 h prior to viral challenge with 105 TCID50 HIV-1BaL. Virus and drug were removed by extensive washing after 2 h and explants were cultured for 14 days. 50% of the culture medium was replaced every 2–3 days and HIV provirus integration was assessed by RTQ-PCR. LTR relative copy numbers are expressed as the percentage of control explants challenged in the absence of inhibitor and are representative of three experiments, where each condition was tested in triplicate. (d) Ectocervical explant cultures were exposed to minirings (5 wt% tenofovir DF and placebo) or 1 mg/mL tenofovir DF or aciclovir for 24 h prior to challenge with 106 pfu/explant HSV-2 (G); input virus and drug were removed as above. Supernatants were collected 5 days post-infection and virus yields determined by plaque assays on Vero cells. Results are presented as virus yields and are means ± SEM obtained from at least three experiments conducted in triplicate. RLUs, relative luciferase units; TDF, tenofovir DF; ACV, aciclovir; LTR, long terminal repeat; API, active pharmaceutical ingredient; RCN, relative copy number.
Explant cultures provide a more stringent model of potential efficacy, because the ability of drugs to permeate tissue and protect multiple cell types may be important factors. Ectocervical explants were exposed to HIV-1BaL for 2 h in the presence of diluted tenofovir DF ring eluants, a range of unformulated tenofovir DF concentrations or control medium. HIV-1BaL was selected because R5 isolates predominate following sexual transmission.24 Eluants from tenofovir DF minirings were extremely potent, with even a 1 : 1000 dilution yielding >90% inhibition of HIV infection of explants (Figure 4c). In addition, 10 wt% and 5 wt% tenofovir DF eluants diluted 1 : 10 afforded complete protection and a 98% reduction in HIV provirus detection, respectively. Unformulated tenofovir DF inhibited infection with an IC90 <1 μg/mL, which is consistent with a previous report where 1 μg/mL tenofovir inhibited >90% of HIV infection of colorectal explants.25 Modest inhibition was also observed with placebo eluants at a dilution of 1 : 10 only, which may reflect weak antiviral activity of excipients eluted from the biomedical thermoplastic; no drug contamination was detected in placebo eluants by LC–MS analysis (data not shown).
Tenofovir DF eluants also protected ectocervical explants from HSV-2 infection. Explants were exposed to minirings (loaded with 5 wt% tenofovir DF or placebo rings), 1 mg/mL tenofovir DF or aciclovir, or medium for 24 h prior to challenge with HSV-2 (106 pfu/explant); this high inoculum is needed to infect explant tissue.14 The tenofovir DF ring released enough drug to reduce HSV yields by almost 2 log and tenofovir DF was almost as effective as aciclovir in this model (Figure 4d). Residual drug in culture supernatants could potentially block HSV infection during plaque assays. To address this, Vero cells were infected with HSV in the presence of supernatants from explants exposed to drug but not challenged, with no inhibition observed (data not shown).
Tenofovir DF does not disrupt epithelial cell tight junctions
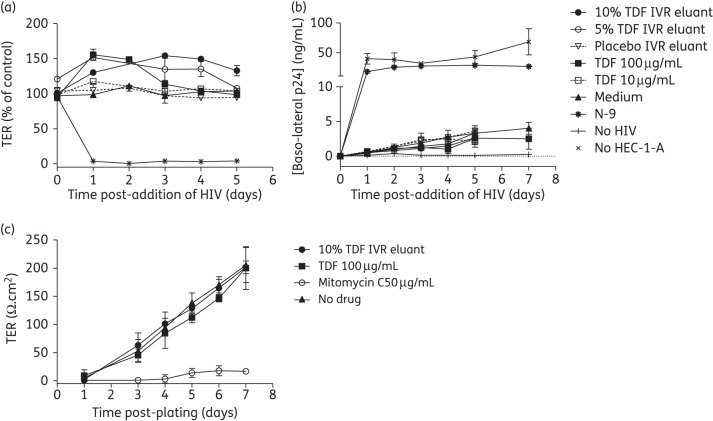
Microbicides may inadvertently increase HIV acquisition by disrupting the epithelium, which serves as a barrier to HIV.21 To evaluate this, polarized HEC-1-A cells were exposed apically to a single dose of tenofovir DF miniring eluants, known concentrations of tenofovir DF, 0.1% (v/v) N-9 or culture medium for 18 h. Exposure to eluants or tenofovir DF had little or no impact on TER, whereas N-9 completely disrupted tight junctions, with TER reaching background levels (Figure 5a). Addition of HIV and JT-CCR5 cells to the apical and baso-lateral chambers, respectively, enabled the assessment of the relative imperviousness of the epithelium to HIV. There was no increase in HIV replication in baso-lateral compartments following exposure to eluants or tenofovir DF compared with medium alone. In contrast, an increase in HIV migration, resulting in baso-lateral p24 levels similar to what was obtained when no cells were seeded, was observed in response to N-9 (Figure 5b).
Figure 5.
Exposure to tenofovir DF has no deleterious effect on the epithelial barrier. HEC-1-A cells were cultured in Transwell® inserts (0.5–1 × 105 cells/insert) and TER was monitored daily. After the TER reached a plateau (4–5 days), cells were exposed to the indicated microbicides (0.1% v/v N-9 and 10–100 µg/mL tenofovir DF) for 18 h. After removal of microbicides by washing three times, HIV-1BaL (40 ng p24) and JT-CCR5 cells (1 × 105/well) were added to the upper and lower chambers, respectively. (a) The TER was monitored 24 h after drug removal and then daily. (b) Supernatants were collected from the baso-lateral chambers at indicated times post-apical addition of HIV and tested for p24 content by ELISA. Results are means ± SD of two independent experiments, where each condition was tested in duplicate. (c) Alternatively, HEC-1-A cells were cultured in Transwell® inserts in the presence of tenofovir DF (unformulated or miniring eluant), culture medium or after treatment with mitomycin C (toxic control) and TER monitored daily. Results are means ± SD of three independent experiments, where each condition was tested in duplicate except miniring eluant and mitomycin C (two experiments). TDF, tenofovir DF.
Tenofovir DF and tenofovir are weak inhibitors of mammalian DNA polymerases, but at high concentrations may exhibit cytostatic effects.26 To begin to assess this, HEC-1-A cells were cultured in the absence or presence of tenofovir DF (drug or miniring eluant) and TER monitored daily. Continuous exposure to tenofovir DF had little effect on the ability of cells to polarize (Figure 5c). In contrast, cells seeded following 1 h treatment with mitomycin C failed to polarize, with TER values remaining close to background levels. These findings further support the safety of sustained tenofovir DF delivery.
Discussion
The results of these studies indicate that tenofovir DF is an excellent candidate for HIV and HSV topical IVR formulated prevention. The higher potency of tenofovir DF compared with tenofovir will likely reduce the vaginal drug concentration needed to achieve protection and reduce costs. Moreover, the increased activity of tenofovir DF and exceptionally long cellular half-life may mitigate adherence issues with intermittent ring removal. Importantly, tenofovir DF retains activity in the presence of semen.
The increased in vitro activity of tenofovir DF against HSV-2 is particularly noteworthy, as the relatively high concentrations of tenofovir needed to prevent HSV infection likely contribute to the lack of efficacy observed with oral tenofovir DF.27 Consistent with this are the drug levels observed in a study of daily tenofovir gel versus daily oral tenofovir DF therapy. Daily vaginal gel application led to >100-times higher concentrations of tenofovir or its active metabolite, TFV-DP, in cervicovaginal lavage (CVL) and vaginal tissue, respectively, although levels in keratinocytes or epithelial cells, the targets for HSV infection, were not measured.28 Unlike aciclovir, which must be phosphorylated by the viral thymidine kinase (TK) before being subsequently phosphorylated to aciclovir triphosphate by cellular kinases, tenofovir is directly phosphorylated by cellular kinases to TFV-DP. Thus, tenofovir DF may afford greater protection than aciclovir against HSV acquisition because the cells and tissue would be preloaded with active drug. In addition, TFV-DP directly blocks DNA polymerase and thus retains activity against most aciclovir-resistant strains, which carry mutations in viral TK.3
The optimal concentration of tenofovir DF that needs to be delivered from an IVR to confer protection against HIV and HSV is unknown. Results from the CAPRISA 004 trial indicate that concentrations >1 μg/mL in CVL were associated with a significant reduction in HIV incidence compared with placebo [2.4 versus 9.1 per 100 person-years; incidence rate ratio (IRR) = 0.26, 95% CI 0.05–0.80, P = 0.01].29 These data, combined with in vitro dose responses, suggest that delivery of 0.1–1 mg/day of tenofovir DF from an IVR will likely lead to substantially higher intracellular levels of TFV-DP than intermittent gel dosing. Both silicone and EVA have been used as IVR matrices for the delivery of hydrophobic compounds with logP values >3.30,31 However, drugs like tenofovir DF that are relatively hydrophilic do not have appreciable solubility in EVA or silicone and therefore the drug release is low.9 Earlier work in our laboratory has shown the capability to deliver both hydrophilic and hydrophobic compounds using PEU elastomers.7,32,33 Therefore, to achieve a target release of 0.1–1 mg/day of tenofovir DF we compared the two commonly utilized IVR elastomers, silicone and EVA, with the more contemporary PEUs. We were able to deliver 1.5 ± 0.2 mg/day of tenofovir DF from a 10 wt% PEU IVR, reflecting the increased polarity of PEU and resulting increased solubility of tenofovir DF in the elastomer matrix compared with the other elastomers. Furthermore, we were able to improve drug stability of tenofovir DF in the matrix by the addition of a highmolecularweight weak acid (PAA) to the PEU IVR. This study also demonstrates the advantages of using PEUs for IVR formulations for moderately hydrophilic drugs like tenofovir DF.
While adherence to an IVR is likely to be greater than with daily or coitally dependent gels, some women may remove the IVR intermittently (e.g. before sex) or a ring could be accidentally expelled. The remarkably prolonged intracellular half-life of TFV-DP, which is 12–15 h in activated lymphocytes and 33–50 h in resting lymphocytes, suggests that a sufficient tissue reservoir will be attained to overcome these concerns.12
Importantly, we showed that eluants from minirings had no adverse impact on the integrity of polarized HEC-1-A cells, which form a multilayered epithelial barrier that mimics the vaginal and ectocervical stratified squamous epithelium. This model detected unanticipated toxicities of cellulose sulphate, which may have contributed to the negative outcome of the clinical trial.21 Moreover, we extended the model to examine the impact of tenofovir DF on the growth and polarization of cells. This is particularly important because TFV-DP is a weak inhibitor of mammalian DNA polymerases.26 This could be a safety concern for adolescents, in whom the natural maturation of the cervix involves replacement of columnar epithelium (cervical ectopy) with the more protective stratified squamous epithelium.34 We observed little or no deleterious impact of tenofovir DF, further supporting the safety of sustained tenofovir DF IVR delivery.
Reverse transcriptase inhibitors, which block the productive viral life cycle, may have little impact on transinfection by Langerhans cells or other dendritic cells, which may transport viral particles from the mucosa to draining lymph nodes, where productive infection of CD4+ T cells occurs.35 This critical step in the expansion of HIV infection could be overcome if the biodistribution of drug released from the IVR reaches the draining lymph nodes or by developing dual-segment IVRs7 to deliver a combination of tenofovir DF with a second drug that blocks viral uptake by Langerhans or other dendritic cells. In ongoing work, we have observed additive or synergistic activity when tenofovir DF is combined with maraviroc and other entry inhibitors. In addition, it will be important to ascertain whether IVR-released drug is distributed to the rectal mucosa, an important site of HIV transmission. In a recent macaque study, vaginal application of tenofovir gel resulted in protective levels within the rectal compartment, suggesting that a vaginally delivered product may protect against infection following vaginal or anal intercourse.36
In summary, the delivery of tenofovir DF from a PEU IVR is feasible and could prove more effective than tenofovir gel formulation at preventing both HIV and HSV infection. Advantages include the ability to deliver drug for up to 30 days, coital independence, lack of leakage and the ability to combine the delivery of more than one drug in a single delivery system. Macaque studies are planned to evaluate drug distribution, pharmacokinetics and pharmacodynamics.
Funding
This work was supported by the National Institutes of Health (grant nos. U19AI076980 and R33AI079763) and the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center (grant no. NIH AI-51519).
Transparency declarations
None to declare.
Author contributions
P. F. K. and B. C. H. designed and supervised the study, and wrote the paper, P. M. M. M., R. R. and T. J. S. conducted experiments, analysed data and wrote the paper; R. S. T., N. M. T. and A. M. H. conducted experiments.
Disclaimer
This work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Acknowledgements
We are grateful to Drs Erika Banks, Patricia Dramitinos and Janice Falls (Department of Obstetrics and Gynecology, Weiler Hospital at the Albert Einstein College of Medicine) for providing cervical tissue from hysterectomy surgeries and to Ms Felicia Juliano-Mascia (Department of Anatomical Pathology, Montefiore Medical Center, Weiler Division) for tissue collection. We thank Gilead for providing tenofovir and tenofovir DF.
References
- 1.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini J, Holy A, Jindrich J, et al. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–8. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrei G, Lisco A, Vanpouille C, et al. Topical tenofovir, a microbicide effective against HIV, inhibits herpes simplex virus-2 replication. Cell Host Microbe. 2011;10:379–89. doi: 10.1016/j.chom.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herold BC, Mesquita PM, Madan RP, et al. Female genital tract secretions and semen impact the development of microbicides for the prevention of HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:325–33. doi: 10.1111/j.1600-0897.2010.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MTN. Microbicide Trials Network Statement on Decision to Discontinue Use of Tenofovir Gel in VOICE, a Major HIV Prevention Study in Women. http://www.mtnstopshiv.org/sites/default/files/attachments/MTNStatementNov17DSMB_final.pdf. (17 February 2012, date last accessed) [Google Scholar]
- 6.Smith DJ, Wakasiaka S, Hoang TD, et al. An evaluation of intravaginal rings as a potential HIV prevention device in urban Kenya: behaviors and attitudes that might influence uptake within a high-risk population. J Womens Health (Larchmt) 2008;17:1025–34. doi: 10.1089/jwh.2007.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson TJ, Gupta KM, Fabian J, et al. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur J Pharm Sci. 2010;39:203–12. doi: 10.1016/j.ejps.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Malcolm RK, Edwards KL, Kiser P, et al. Advances in microbicide vaginal rings. Antiviral Res. 2010;88(Suppl 1):S30–9. doi: 10.1016/j.antiviral.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Kiser PF, Johnson TJ, Clark JT. State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev. 2012;14:62–77. [PubMed] [Google Scholar]
- 10.Martin DJ, Meijs GF, Gunatillake PA, et al. The influence of composition ratio on the morphology of biomedical polyurethanes. J Appl Polym Sci. 1999;71:937–52. [Google Scholar]
- 11.Fardis M, Oliyai R. Prodrugs: Challenges and Rewards. 2008. Case study: tenofovir disoproxil fumarate: an oral prodrug of tenofovir; pp. 1347–57. Borchardt RT and Middaugh CR, ed. New York, USA: Springer, [Google Scholar]
- 12.Robbins BL, Srinivas RV, Kim C, et al. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl) adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42:612–7. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naesens L, Bischofberger N, Augustijns P, et al. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob Agents Chemother. 1998;42:1568–73. doi: 10.1128/aac.42.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madan RP, Mesquita PMM, Cheshenko N, et al. Molecular umbrellas: a novel class of candidate topical microbicides to prevent human immunodeficiency virus and herpes simplex virus infections. J Virol. 2007;81:7636–46. doi: 10.1128/JVI.02851-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesquita PM, Wilson SS, Manlow P, et al. Candidate microbicide PPCM blocks human immunodeficiency virus type 1 infection in cell and tissue cultures and prevents genital herpes in a murine model. J Virol. 2008;82:6576–84. doi: 10.1128/JVI.00335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–64. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 17.Gupta KM, Pearce SM, Poursaid AE, et al. Polyurethane intravaginal ring for controlled delivery of dapivirine, a nonnucleoside reverse transcriptase inhibitor of HIV-1. J Pharm Sci. 2008;97:4228–39. doi: 10.1002/jps.21331. [DOI] [PubMed] [Google Scholar]
- 18.Clark MR, Johnson TJ, McCabe RT, et al. A hot-melt extruded intravaginal ring for the sustained delivery of the antiretroviral microbicide UC781. J Pharm Sci. 2012;101:576–87. doi: 10.1002/jps.22781. [DOI] [PubMed] [Google Scholar]
- 19.Herold BC, Siston A, Bremer J, et al. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob Agents Chemother. 1997;41:2776–80. doi: 10.1128/aac.41.12.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhead P, Hayes P, Watts PS, et al. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–86. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mesquita PM, Cheshenko N, Wilson SS, et al. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: model of microbicide safety. J Infect Dis. 2009;200:599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sangster J. Octanol–Water Partition Coefficients of Simple Organic Compounds. NIST; 1989. Gaithersburg, MD, USA: [Google Scholar]
- 23.Yuan LC, Dahl TC, Oliyai R. Degradation kinetics of oxycarbonyloxymethyl prodrugs of phosphonates in solution. Pharm Res. 2001;18:234–7. doi: 10.1023/a:1011044804823. [DOI] [PubMed] [Google Scholar]
- 24.Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–81. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher PS, Elliott J, Grivel JC, et al. Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS. 2006;20:1237–45. doi: 10.1097/01.aids.0000232230.96134.80. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Hanes J, Johnson KA. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry. 2003;42:14711–9. doi: 10.1021/bi035596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan DH, Kaul R, Raboud JM, et al. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS. 2011;25:207–10. doi: 10.1097/QAD.0b013e328341ddf7. [DOI] [PubMed] [Google Scholar]
- 28.Hendrix C, Minnis A, Guddera V, et al. MTN-001: a phase 2 cross-over study of daily oral and vaginal TFV in healthy, sexually active women results in significantly different product acceptability and vaginal tissue drug concentrations. Abstracts of the Eighteenth Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2011; Alexandria, VA, USA: Foundation for Retrovirology and Human Health; Paper # 35LB. [Google Scholar]
- 29.Karim SS, Kashuba AD, Werner L, et al. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–81. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:416–23. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar NN. The combined contraceptive vaginal device (NuvaRing): a comprehensive review. Eur J Contracept Reprod Health Care. 2005;10:73–8. doi: 10.1080/13625180500131683. [DOI] [PubMed] [Google Scholar]
- 32.Kaur M, Gupta K, Poursaid A, et al. Engineering a degradable polyurethane intravaginal ring for sustained delivery of dapivirine. Drug Deliv Transl Res. 2011;1:223–37. doi: 10.1007/s13346-011-0027-1. [DOI] [PubMed] [Google Scholar]
- 33.Johnson TJ, Albright TH, Clark MR, et al. Long-term, time-independent release of the antiretroviral agent tenofovir in daily milligram quantities from an intravaginal ring. Abstracts of the Thirty-eighth Annual Meeting and Exposition of the Controlled Release Society, National Harbor, MD, 2011; Abstract no. 184. Controlled Release Society, St Paul, MN, USA. [Google Scholar]
- 34.Moscicki AB, Ma Y, Holland C, et al. Cervical ectopy in adolescent girls with and without human immunodeficiency virus infection. J Infect Dis. 2001;183:865–70. doi: 10.1086/319261. [DOI] [PubMed] [Google Scholar]
- 35.Ballweber L, Robinson B, Kreger A, et al. Vaginal Langerhans cells non-productively transporting HIV-1 mediate infection of T cells. J Virol. 2011;85:13443–7. doi: 10.1128/JVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuttall J, Kashuba A, Wang R, et al. The pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques. Antimicrob Agents Chemother. 2012;56:103–9. doi: 10.1128/AAC.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]