Abstract
Previously we reported that feeders formed from human placental fibroblasts (hPFs) support derivation and long-term self-renewal of human embryonic stem cells (hESCs) under serum-free conditions. Here, we show, using antibody array and ELISA platforms, that hPFs secrete ~6-fold higher amounts of the CXC-type chemokine, GROα, than IMR 90, a human lung fibroblast line, which does not support hESC growth. Furthermore, immunocytochemistry and immunoblot approaches revealed that hESCs express CXCR, a GROα receptor. We used this information to develop defined culture medium for feeder-free propagation of hESCs in an undifferentiated state. Cells passaged as small aggregates and maintained in the GROα-containing medium had a normal karyotype, expressed pluripotency markers, and exhibited apical–basal polarity, i.e., had the defining features of pluripotent hESCs. They also differentiated into the three primary (embryonic) germ layers and formed teratomas in immunocompromised mice. hESCs cultured as single cells in the GROα-containing medium also had a normal karyotype, but they downregulated markers of pluripotency, lost apical–basal polarity, and expressed markers that are indicative of the early stages of neuronal differentiation—βIII tubulin, vimentin, radial glial protein, and nestin. These data support our hypothesis that establishing and maintaining cell polarity is essential for the long-term propagation of hESCs in an undifferentiated state and that disruption of cell–cell contacts can trigger adoption of a neuronal fate.
Keywords: hESC, Pluripotency, Polarization, Cytokine–Cell, cell interaction, CXCL1
1. Introduction
hESCs, pluripotent/totipotent cells derived from pre-implantation embryos, have an unlimited capacity for self-renewal, yet they retain the ability to differentiate into specialized cell types. They share certain properties with components of the inner cell mass (ICM) from which they arise, i.e., the expression of particular pluripotency markers such as Oct 3/4, Nanog, and Sox2 (Adjaye et al., 2005). hESCs also have distinct characteristics such as apical–basal polarity, a feature of many epithelial cells (Krtolica et al., 2007). Over the last decade there has been great interest in deriving and culturing these cells as they are a ready source of stem, progenitor, and differentiated cells for regenerative therapies, in vitro diagnostic technologies, developmental models, and drug discovery/screening efforts (Peura et al., 2007). However, there is a need to develop defined, reproducible methods for deriving and maintaining these cells under conditions that support their self-renewal/undifferentiated growth and genomic stability while minimizing exposure to potential pathogens.
Recent insights into the molecular mechanisms regulating hESC self-renewal have driven the evolution of protocols for deriving and culturing hESCs under well-defined conditions (reviewed by McDevitt and Palecek (2008)). A major goal of these studies has been identification of feeder-free methods and defined xeno-free medium with human rather than animal components. The current status of cell culture methods for hESC maintenance has been extensively reviewed (Hasegawa et al. 2010). In recent years, the most important improvements include the development of commercially available xeno-free and feeder-free defined culture media, such as, mTeSR2 (StemCell Technologies), StemPro (Invitrogen), SBX (AxCell), NutriStem (Stemgent), and VitroHES (Vitrolife). These systems are based on different combinations of factors, suggesting that the downstream consequences could include variations in hESC molecular signatures. Other efforts include the identification of extracellular matrix components that provide optimal cell culture substrates. Additionally, the method of passaging appears to be critical for the long-term propagation of hESCs as both enzymatic and chemical dissociation may carry a high risk of increasing chromosomal abnormalities that provide a survival advantage by favoring expansion of cells that carry specific mutations (Mitalipova et al., 2005; Hasegawa et al., 2010). Therefore, the formulation of optimal cell culturing systems remains an active area of investigation and understanding the influences of the in vitro microenvironment on hESC phenotype and function will enable rational and evidence-based selection process.
Here we describe a strategy for identifying novel factors that are critical for hESC propagation on human serum with a particular emphasis on the role that polarization plays in supporting hESCs growth in an undifferentiated state. This approach was based on our previous observation that feeders formed of fibro-blasts derived from early gestation human placentas (e.g., hPFs) support long-term propagation and derivation of hESCs under nearly xeno- and serum-free conditions (Genbacev et al. 2005). Subsequently, in work described in this report, we used an antibody array approach to assess hPF secretion of factors that could play a role in this phenomenon as compared to the IMR90 human fibroblast line, which does not support hESC self-renewal. The results identified the interleukin-8-related chemotactic cytokine, GROα (CXCL-1 or melanoma growth stimulatory activity), as a key factor. This observation enabled formulation of a novel hESC culture medium that included this cytokine. Interestingly, when the cells were cultured in this medium, the outcome depended on whether the hESCs were passaged as aggregates or had been dissociated into single hESCs. Retaining cell–cell contacts promoted self-renewal and their loss triggered assumption of a neuronal fate. Accordingly, we concluded that hESC responses to GROα in vitro are contextual, a principle that could apply to many other signals.
2. Material and methods
2.1. Cell culture
The H7 (WA07, WISC Bank), H9 (WA09, WISC Bank) and UCSF4 (NIH registry #0044) hESC lines were cultured in MEF-conditioned (MEF CM) or human placental fibroblast-conditioned (hPF CM) KSR medium on matrigel as previously described (Genbacev et al., 2005, Krtolica et al., 2007) or in mTeSR medium (Stemcell Technologies) on matrigel according to manufacturer’s instructions. The methods used for derivation and propagation of hPFs and for culturing of IMR90 cells were also described previously (Genbacev et al., 2005). In other experiments, the same hESC lines were cultured on human serum as previously described (Stojkovic et al., 2005) in KSR medium that contained 100 ng/ml hbFGF, 10 mM lactate, 0.5 ng/ml TGFβ, and 10 ng/mL GROα. Accutase (Millipore) digestion of hESC colonies was used to produce small clumps and single cell suspensions. Cell survival was assessed after accutase passaging of the single cell suspension and ranged between 10% and 40% depending on cell line. Uterine stromal cells and human placental cytotrophoblasts were isolated and cultured as previously described (Basu et al., 2008; Penna et al., 2008; Tanaka and Umesaki, 2008).
2.1.1. Lactate measurements
Lactate levels in the medium were measured using a Lactate Assay Kit according to the manufacturer’s instructions (BioVision).
2.2. Antibody arrays
hPFs and IMR90 cells were repeatedly washed in PBS, then incubated in KSR medium. After 24 h, CM was collected and the cell number was determined (and used for normalization purposes, see below). The CM was filtered (0.2 μm pore size) and frozen at −80 °C prior to analysis. Briefly, antibody arrays (Chemicon; Human cat #AA1001CH-8) were performed according to the manufacturer’s instructions using a modified detection method as we previously described (Coppe et al., 2008). Briefly, the CM was thawed and concentrated 2- to 3-fold (by volume) using a Centricon filter apparatus (3 kDa cut-off, 4 °C). CM samples equivalent to the fractional volumes produced by 2 × 105 cells were diluted to 1.2 ml with KSR medium and mixed with 300 μl blocking solution. Array membranes were pre-incubated with 1.5 ml blocking solution before overnight incubation at 4 °C with CM. Then they were washed 5 × with blocking buffer and incubated with a biotin-conjugated antibody cocktail for 1 h 45 min at room temperature. After 5 washes with blocking buffer, detection solution containing 0.265 μCi 35S-streptavidin (732 Ci/mmol; 0.1 mCi/ml) was added and the membranes were incubated (1 h 45 min, room temperature) before washing. Bound 35S-streptavidin was detected by using a phosphorimager. Signals were quantified as previously described (Coppe et al., 2008).
2.3. Immunoassay
The concentration of GROα in CMs was measured by an ELISA kit (Quantikine) as previously described (Coppe et al., 2006).
2.4. Immunoblot procedures
Cells were lysed in modified RIPA buffer (Krtolica et al., 2007). Lysates that contained equal amounts of protein were boiled, separated by SDS-PAGE, transferred to nitrocellulose (Schleicher & Schuell), and probed with an anti-CXCR2 (R&D) antibody diluted 1:500 as described (Krtolica and Ludlow, 1996). All secondary antibodies were from Jackson ImmunoResearch. Equal amounts of protein were loaded in each lane as confirmed by staining transfers with Ponceau S dye after transfer (data not shown).
2.5. Alkaline phosphatase assay
The assay was performed according to the manufacturer’s instructions (ATCC).
2.6. Embryoid body (EB) differentiation
hESC colonies were manually detached from cell culture dishes and placed in suspension culture in low attachment 6-well plates in differentiation medium (KSR medium [80% knockout DMEM, 20% serum-free knockout serum replacement supplemented with 1 mM MEM non-essential amino acids, 0.1 mM β-mercaptoethanol, and 1 mM L-glutamine] supplemented with 10% FCS in the absence of bFGF) as previously described (Krtolica et al., 2007). After 5 days, the EBs were moved to matrigel-coated dishes and allowed to differentiate for 2–3 weeks. The expression of markers of the three primary germ layers was detected by using the immunolocalization approach described previously and outlined briefly below (Krtolica et al., 2007).
2.7. Immunolocalization
To assess differentiation (endodermal, mesodermal, and ectodermal) or epithelial polarization, hESCs or EBs were fixed in 90% acetone or 4% paraformaldehyde, permeabilized in 0.5% Triton X-100, then incubated with primary antibodies that reacted with the following antigens at 5 μg/ml concentrations: SSEA-4, TRA-1–60, TRA-1–81, Nestin (Millipore), Nanog (R&D), Oct 3/4 (Santa Cruz Biotech.), Vimentin, SMA, βIII tubulin, alpha-fetoprotein (αFP; Sigma), E-cadherin (BD Biosciences), RGP (Developmental Studies Hybridoma Bank University of Iowa), Actin, ZO-1, or occludin (Invitrogen). All secondary antibodies were purchased from Jackson ImmunoResearch and used at a dilution of 1:100. Hoechst 33342 was obtained from Invitrogen.
2.8. Karyotyping
G-banding of hESC lines was performed at a certified Cytogenetics Laboratory, Children’s Hospital and Research Center, Oakland, CA.
2.9. Teratoma formation
Six to eight week old NOD.CB17/J female mice (Jackson Laboratories, West, Sacramento, CA) were injected subcutaneously with 4–5 million hESCs. 8–12 weeks after transplantation the mice were sacrificed and the tumors excised. They were fixed by overnight incubation in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin–eosin. The sections were examined with a light microscope to confirm lack of malignant phenotype and the presence of derivatives of the three germ layers.
2.10. Flow cytometry
hESC colonies were dissociated into single cells using accutase (Millipore) digestion. The cells were fixed and permeabilized using the fix and perm cell permeabilization reagent (Invitrogen) according to the manufacturer’s instructions. All antibodies, which were for intracellular staining, were added during the permeabilization step in accordance with the manufacturer’s protocol. They were: Alexa Fluour 488 Sox2, AlexaFluor 488 mouse IgG2a (isotype control), PE Nanog, PE Oct3/4, PE mouse IgG1, PerCP-Cy5.5 Oct3/4 (BD Pharmigen), and PerCP-Cy5.5 mouse IgG1 (isotype control; Becton Dickinson). Cells were analyzed using a FACSCalibur (BD Biosciences) and FlowJo (Tree Star, Inc., Ashland, OR) software. Isotype controls were Ig subtype matched to the corresponding antibodies.
2.11. Statistical analyses
The statistical significance of the data was evaluated using a two-tailed Student’s t-test, and an assumption of equal variance. In graphical representation, error bars correspond to standard error around the mean.
3. Results
3.1. hPF CM replaced MEF CM for long-term feeder-free culture of hESCs on matrigel or human serum substrates
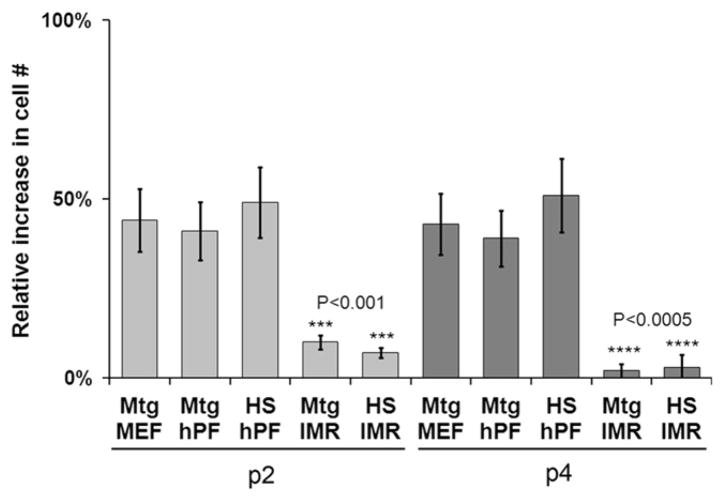
Previously we reported that hPFs can be used as feeders to derive and propagate hESC lines (Genbacev et al., 2005). In a continued effort to develop a defined animal product- and feeder-free hESC culture system, we hypothesized that hPFs are an important source of soluble factors that support hESC propagation. To test this theory, we substituted hPF CM for MEF CM, which is routinely used for the feeder-free culture of hESCs on substrates such as matrigel (Carpenter et al., 2004). To eliminate this animal-derived product, we coated the tissue culture wells with human serum, which supports hESC growth and self-renewal (Stojkovic et al., 2005). As a positive control, we cultured hESCs in MEF CM on matrigel substrates. As a negative control, we cultured hESCs in CM from IMR90 cells (human lung fibroblasts that do not support long-term self- renewal of hESCs) on matrigel or human serum substrates (Genbacev et al., 2005). Routinely, the cells (H7 and H9 lines) were passaged by manual dissection and their morphology was visually inspected on a daily basis. For quantification, accutase treatment was used to dissociate identical cultures into single cells, which were counted after passages (P) 2 and 4 (Fig. 1A). H7 hESCs cultured on human serum-coated wells in hPF CM proliferated at the same rate as the positive controls. H9 hESCs exhibited the same behavior (data not shown). Indeed, both lines continued to grow in an undifferentiated state for more than 14 passages. As expected, IMR90 human lung fibroblast CM did not support hESC growth on either matrigel or human serum substrates. These data confirmed our hypothesis that hPFs produce soluble factors that support hESC self-renewal.
Fig. 1.
hESC self-renewal under various feeder-free conditions. The H7 cell line was grown for up to 5 passages (P) on matrigel (Mtg)- or human serum (HS)-coated dishes in mouse embryo fibroblast (MEF), human placental fibroblast (hPF), or IMR90 (IMR) CM. Cell numbers at P2 and P4 were expressed as the percent relative increase/day. MEF and hPF CM performed equally well; IMR90 CM did not support hESC self-renewal. Error bars represent standard error of the mean of triplicate samples.
3.2. Growth factor supplemented KSR failed to support long-term hESC self-renewal
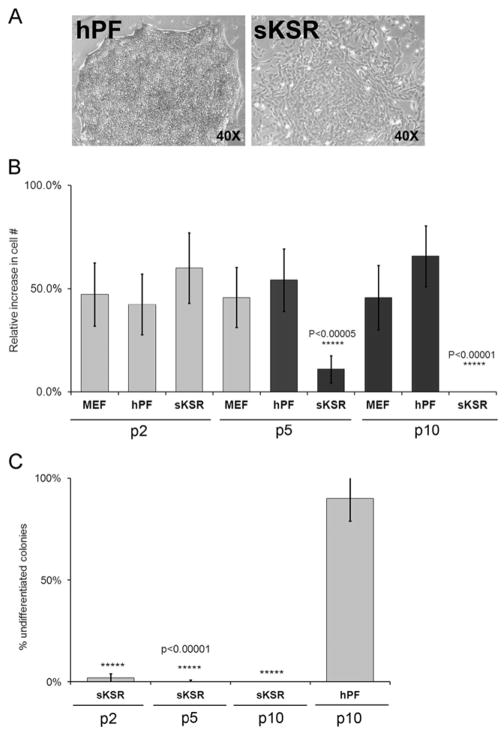
Next, we used our results and those of other groups to begin formulating a new hESC growth medium. Given the reported importance of TGFβ signaling to the maintenance of hESC pluripotency in vitro (Vallier et al., 2005), we added this growth factor, at 0.5 ng/ml, to standard KSR hESC medium that contained 100 ng/ml hbFGF (Levenstein et al., 2006) and designated formulation supplemented KSR (sKSR). By P2, the attachment rates of manually dissected hESC aggregates cultured in sKSR appeared lower than in control cultures that were maintained in hPF or MEF CM. As compared to cells maintained in hPF CM, colonies grown under the experimental conditions also exhibited morphological alterations (e.g., irregular shapes, loss of tight cell–cell contacts) and the cells began to differentiate, particularly in colony centers and at the edges (Fig. 2A). At P2, the number of hESCs in experimental and control cultures was not statistically different (Fig. 2B). By P5, attachment and growth of hESCs in sKSR was significantly reduced as compared to hESCs maintained under control conditions. By P10, only a few cells remained in the experimental cultures. Overall, cell numbers and the extent of differentiation positively correlated with these visual observations. Thereafter, the ability of sKSR medium to sustain hESC self-renewal significantly diminished. In parallel, the number of undifferentiated cells decreased as determined by morphological criteria (Noaksson et al., 2005; Fig. 2C). Therefore, we concluded that sKSR did not support long-term maintenance of hESC growth in an undifferentiated state.
Fig. 2.
Supplemented KSR could not replace MEF or hPF conditioned medium for long-term maintenance of hESCs in culture. (A) Phase contrast microscopy showed that hESCs grown in hPF CM on matrigel substrate had the typical morphology of undifferentiated colonies, which grew as compact aggregates of hundreds of cells with a uniform appearance (left panel). When hESCs were propagated in supplemented KSR (sKSR) containing TGFβ and hbFGF for 2 Ps, the colonies were smaller and did not have distinct border (right panel). (B) By P5, the proliferation rate of hESCs propagated in sKSR was significantly less than colonies that were maintained in MEF or hPF CM and by P10 these cultures were devoid of cells. (C) After every doubling, colonies propagated in sKSR contained fewer cells that had the morphological appearance of undifferentiated hESCs as compared to colonies that were maintained in hPF CM for 10 Ps. Error bars represent standard error of the mean of triplicate samples.
3.3. Comparative protein profiling of hPF and IMR90 CMs revealed candidate regulators of hESC self-renewal
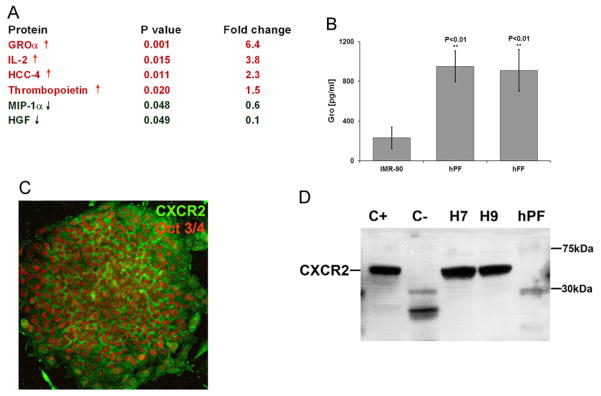
We hypothesized that the relevant factors will be found at higher levels in hPF CM, which supports long-term hESC growth in an undifferentiated state, than in IMR90 CM, which does not. To test this hypothesis we used a commercially available Human Cytokine Antibody Array System (RayBiotech; RayBio). In these experiments, the signal produced by the bound antibody was amplified by using 35S-labeled streptavidin for detection, a modification that we previously introduced, which improves the reproducibility and sensitivity of this approach (Coppe et al. 2008). Our analysis revealed 4 growth factors and cytokines that were significantly increased and 2 that were significantly decreased in hPF CM as compared to IMR90 CM (p<0.05; Fig. 3A). Among the upregulated proteins, the most striking differences were in the expression of GROα (6.4-fold), IL-2 (3.8- fold), HCC-4 (2.3-fold), and thrombopoietin (1.5 fold). The proteins that were significantly downregulated included MIP-1α (1.7-fold) and HGF (10-fold). Interestingly, the molecules identified in this screen regulate differentiation, migration, and cell growth.
Fig. 3.
hPF feeders secreted higher amounts of GROα than IMR90 cells, and hESCs expressed CXCR2, the GROα receptor. (A) hPF and IMR90 CMs were profiled using a protein array approach (see ‘Section Material and Methods’). GROα was the most highly differentially expressed analyte, elevated 6.4-fold in hPF as compared to IMR90 CM. (B) These results were confirmed by using an ELISA approach (p<0.01). CM from human foreskin fibroblasts (hFF), which also supports hESC self-renewal, contained GROα levels that were comparable to those of hPF CM. (C) Immunolocalization analyses showed that undifferentiated Oct 3/4-positive hESCs (the H7 line) expressed the GROα receptor, CXCR2, and antibody reactivity localized to the plasma membrane. (D) Immunoblot analyses of undifferentiated H7 and H9 cells with an antibody that specifically reacted with CXCR2 revealed a band of the expected molecular weight (~40 kDa). Extracts prepared from human placental cytotrophoblasts served as positive controls (C+) and human follicular phase uterine stromal cells served as negative controls (C−). hPFs, which secreted GROα, do not express CXCR2.
In designing functional studies, we considered the fact that IL-2 stimulates the growth, differentiation, and survival of antigen-selected cytotoxic T cells and HCC-4 is a chemoattractant for monocytes and lymphocytes. Accordingly, we focused on the mitogenic cytokine GROα (Sager et al., 1992). To estimate the biologically relevant concentration range for supporting hESC self-renewal, we used a GROα immunoassay kit to quantify levels of this chemokine in CM from cells that support hESC growth (hPFs and human foreskin fibroblasts [hFF]) and cells that do not (IMR90 fibroblasts; Fig. 3B). The GROα concentration was severalfold higher in hPF and hFF CM than in IMR90 CM; levels were undetectable in KSR medium that had not been exposed to cells (data not shown). Thus, these findings were consistent with the protein array data.
3.4. hESCs expressed the GROα receptor, CXCR2
To determine whether hESCs can respond to this chemokine, we characterized the expression of its receptor, CXCR2. Initially we used an immunolocalization approach to analyze H7 and H9 hESCs grown on matrigel in hPF CM. The results revealed strong cell-surface staining for CXCR2 that co-localized with Oct 3/4 expression as shown by the pattern of H7 immunoreactivity (Fig. 3C). To confirm these results, we studied receptor expression in the context of another pluripotency marker, Nanog, using three hESC lines: H7, H9, and UCSF4 (Suppl. Fig. 2). To further bolster these data, we prepared cell extracts from H7 and H9 hESCs that were cultured in MEF or hPF CM on matrigel- or human serum-coated wells for four days. Extracts prepared from placental cytotrophoblasts served as positive controls (Genbacev et al., 2001); follicular phase uterine stromal cells served as negative controls (Basu et al., 2008; Penna et al., 2008; Tanaka and Umesaki, 2008). Immunoblot analyses revealed a band of the expected estimated molecular weight in all the hESC samples and in the positive control cytotrophoblasts. Extracts prepared from the negative control uterine cells lacked a corresponding band (Fig. 3D) as did hPFs, suggesting that the latter cells, which secrete this chemokine, do not have the molecular machinery to generate an autocrine response.
3.5. A novel GROα-containing medium supported hESC self-renewal
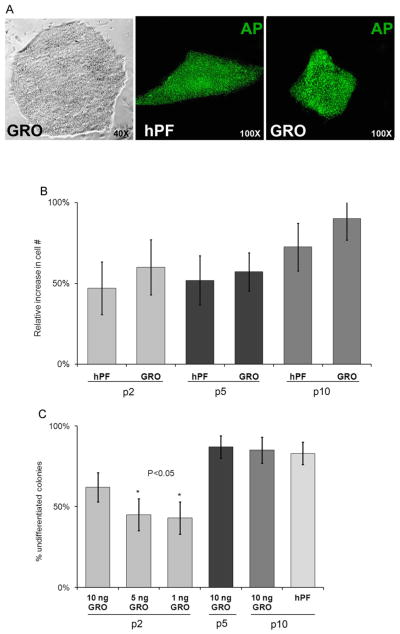
In preliminary experiments, we tested the ability of GROα to sustain hESC self-renewal on substrates formed from human serum. Specifically, we added GROα in concentrations that bracketed those detected in hPF CM (1, 5, or 10 ng/ml) to KSR containing 100 ng/ml hbFGF and 5 mM lactate. Because the activity of recombinant proteins may be different from that of their native counterparts, we also added higher levels of this chemokine (50 and 100 ng/ml). After four passages, colony morphology and cell numbers suggested that the optimal GROα concentration was 10 ng/ml (data not shown).
Accordingly, we performed a more detailed analysis. In these experiments, H7 cells were seeded in KSR containing 100 ng/ml hbFGF, 0.5 ng/ml TGFβ, 5 mM lactate, and 8 ng/ml GROα on human serum-coated 6-well plates and manually passaged every 5–6 days. As a control, parallel cultures were established in hPF CM. Experimental and control conditions were tested in triplicate in two separate experiments. Colony morphology, hESC expression of alkaline phosphatase (amarker of pluripotency), and cell numbers were assessed after P2, P5, and P10. At P10, hESCs cultured in GROα-containing medium grew as aggregates with normal colony morphology that showed no signs of differentiation, and their levels of alkaline phosphatase activity were comparable to control cultures (Fig. 4A). At every passage analyzed, the number of cells in cultures that were maintained in the experimental medium was equal to or greater than the number in the control wells (Fig. 4B). The same results were obtained when this experiment was repeated using H9 cells, which were also maintained through P10 under both conditions. Next, we asked whether GROα effects were dose- dependent. Specifically, we added 1, 5, or 10 ng/ml of this chemokine to the same medium and quantified, by using morphological criteria, differentiation of H7 hESCs at P2, P5, and P10 (Fig. 4C). As early as P2, it was evident that the highest concentration of GROα was most effective in preventing differentiation. At P5 and P10, colonies in medium that contained 10 ng/ml GROα exhibited the same morphology as control cells, which supported our earlier conclusion that this was the optimal chemokine concentration.
Fig. 4.
KSR containing serum replacement supplemented with TGFβ, hbFGF, lactate, and GROα supported hESC propagation on substrates formed from human serum. Growth of H7 (A–C) and H9 (not shown) hESCs in this new formulation was compared to cells that were propagated in hPF CM on matrigel substrates. (A) Phase contrast microscopy shows that hESCs grown in medium that contains GROα and lactate for 20Ps have the typical morphology of undifferentiated colonies. Additionally, there was no discernable difference in their alkaline phosphatase (AP) activity as compared to cells that were grown on hPF feeders, suggesting that they remained pluripotent under both conditions. (B) Scoring the relative increase in cell numbers at P2, P5, and P10 showed no difference in the proliferative rate of hESCs grown under the experimental and control conditions. (C) Colonies maintained in KSR with lactate that contained 10 ng/ml GROα tended to have fewer differentiated cells as compared to those that were grown in medium with lower levels of this chemokine, although this difference showed statistical significance only in comparison to P5 cells (p<0.05). By P10, the proportion of undifferentiated cells in colonies that were grown in the GROα formulation or the hPF/matrigel combination was not statistically different. Error bars represent standard error of the mean of triplicate samples.
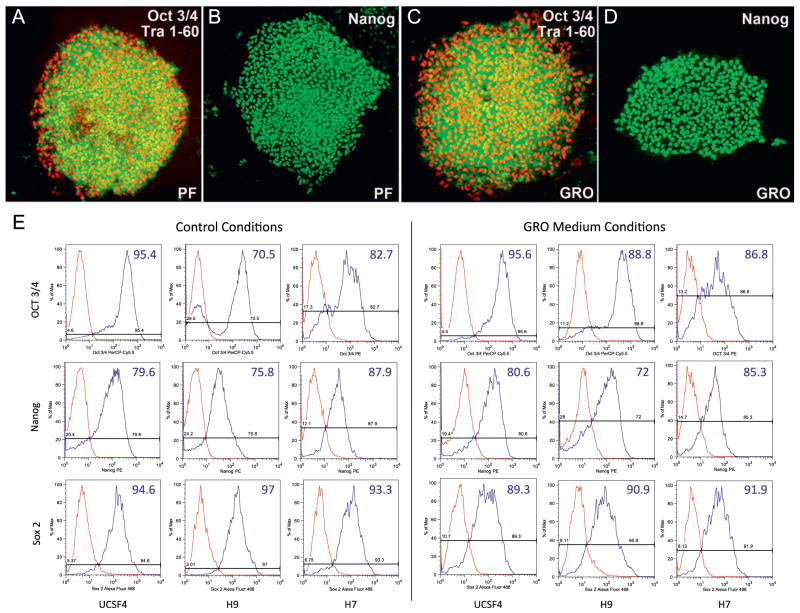
In additional experiments, we cultured hESCs (the H7 line) in the optimized GROα-containing KSR formulation and evaluated their expression of markers of pluripotency (Oct 3/4, Nanog, TRA 1–60, and TRA 1–81) at P3, P6, P12, and P20. As a positive control, hESCs were propagated for the same number of passages in hPF CM. In both culture conditions, at least 95% of the cells expressed the markers of interest (Fig. 5A–D; Tra 1–81 immunoreactivity not shown). To show that the GROα-containing KSR formulation supported maintenance of hESC lines other than H7, we cultured H7, H9, and UCSF4 for 10 passages in GROα-containing medium, hPF CM, and mTeSR, a commercially available medium for feeder-free hESC culture containing 100 ng/ml hbFGF, and assessed the quality of hESC colonies by measuring by flow cytometry expression of transcription factors associated with pluripotency—OCT3/4, NANOG, and SOX2 (Fig. 5E). As expected, all the cell lines expressed these markers at similar levels (>70% positive) across the conditions tested. Indeed, with respect to colony morphology, expression of markers of pluripotency, and cell proliferation, we did not observe any substantial difference between cells grown in standard hESC growth conditions and those maintained in our novel GROα-containing KSR formulation. Finally, we demonstrated that cells grown under these conditions on two different lots of human serum and frozen at passages 5, 10, or 20 can subsequently recover and successfully resume growth in an undifferentiated state (data not shown). Taken together, these results show that our novel medium and substrate combination supports hESC self-renewal.
Fig. 5.
hESCs grown in GROα-containing medium on human serum substrates continued to express markers of pluripotency. The H7 line was cultured to P15 under control (A, B; hPF CM on a matrigel substrate) or experimental conditions (C, D). In both cases, cells expressed markers of pluripotency including Oct 3/4, Tra 1–60 (A, C), and Nanog (B, D). The H7, H9, and UCSF4 cell lines, which were cultured under standard hESC conditions (mTeSR medium on a matrigel substrate) and experimental conditions for 10 passages expressed similar levels of pluripotency markers—Oct3/4, Nanog, and Sox2 (E). Flow cytometric analysis was performed at P6, P8, and P10 with no significant differences observed. Cells grown in hPF CM were previously examined by flow cytomery for the expression of pluripotency markers and no significant difference were observed between cells cultured in hPF CM and mTeSR (data not shown).
3.6. hESCs maintained in GROα-supplemented medium on human serum-coated substrates differentiated into the three primary germ layers
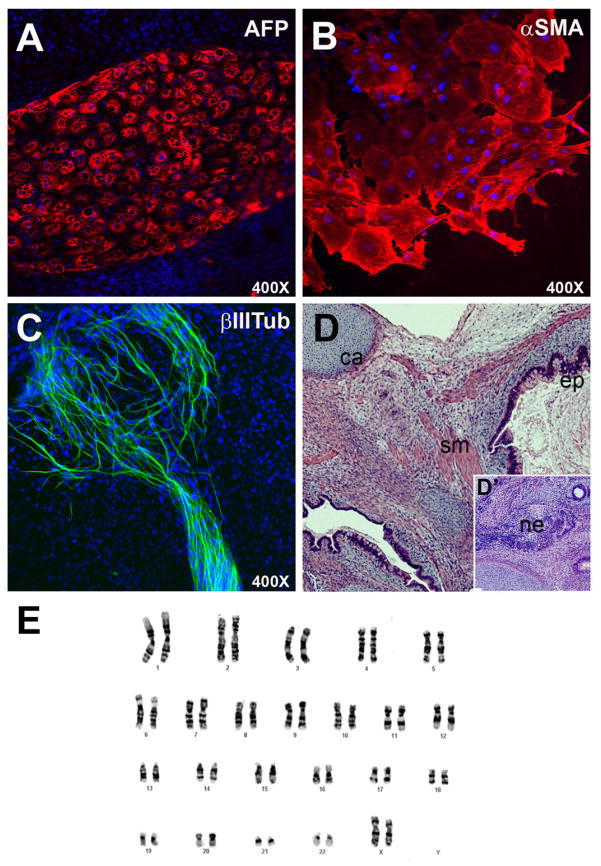
Next, we tested the differentiation capacity of hESCs grown under these conditions by forming embryoid bodies (EBs) at P20. These structures developed at the same rate in the experimental and control groups. Interestingly, the number of EBs that contained contracting heart muscle was higher in the GROα/lactate group (7/30 EBs) than in controls (3/30 EBs), a possible indication that H7 hESCs maintained in this medium differentiate more readily into cardiomyocytes (data not shown). Immunolocalization of stage-specific antigens showed expression of α-fetoprotein (Fig. 6A; AFP), α-smooth muscle actin (Fig. 6B, αSMA), and βIII tubulin (Fig. 6C, βIIITub), suggesting differentiation into endodermal, mesodermal, and ectodermal derivatives, respectively. H7 cells (P15) injected into immunocompromised mice formed teratomas, benign tumors that contain the differentiated progeny of the three germ layers, additional evidence that these culture conditions supported hESC self-renewal in a pluripotent state (Fig. 6D). Finally, G-banding at P20 showed a normal karyotype, which suggested that the cells were genetically stable (Fig. 6E).
Fig. 6.
hESCs cultured in GROα-containing medium differentiated into derivatives of the three primary germ layers in vitro, formed teratomas in mice, and exhibited a normal karyotype. The H7 line, which was cultured up to P10 under the experimental conditions, formed embryoid bodies that contained (A) endodermal (α-fetoprotein [AFP]-positive), (B) mesodermal (α-smooth muscle actin [α-SMA]-positive), and (C) ectodermal (βIII-tubulin [βIII-tub]-positive) derivatives. (D, D′) These cells also generated teratomas when they were injected into immunocompromised mice. Histopathological analysis of tissue sections of these tumors revealed multiple tissue types including bone, cartilage (ca), differentiated epithelia (ep), neuroectoderm (ne), smooth muscle cells (sm), and vascular elements. (E) G-banding confirmed that H7 cells grown up to P20 in GROα-containing medium had a normal karyotype (46, XX).
3.7. hESCs passaged as single cells in GROα-supplemented medium on human serum-coated substrates lost apical–basal polarity and initiated neuronal differentiation
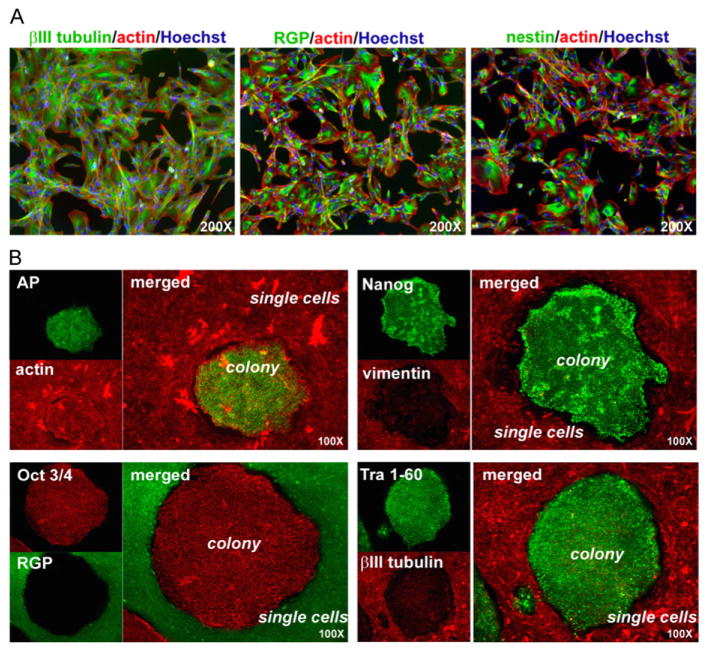
Previously we showed that pluripotent hESC colonies exhibit epithelial-like, apical–basal polarity (Krtolica et al. 2007). Here we asked whether hESCs (H7 line) grown under the experimental feeder-free conditions described above and dissociated into single-cell suspensions re-polarize. Controls were cultured under the same conditions and passaged as small clumps of 5–10 cells. After plating, single cells in experimental cultures, which exhibited clear morphological alterations as compared to the controls, did not reorganize into colonies, suggesting that they were unable to reestablish polarity. To test this hypothesis, we assessed the expression of antigens that play important functional roles in this specialized form of cell–cell adhesion. Immunolocalization experiments showed that control hESC colonies expressed ZO-1, occludin and E-cadherin at the sites of cell–cell contact, but hESCs that had been plated as single cells did not (data not shown). The loss of markers of apical–basal polarity was accompanied by downregulated/lack of expression of antigens that are associated with pluripotency (Oct 3/4, Nanog, SSEA-4, Tra 1–60, and Tra 1–81). (data not shown). However, immunolocalization experiments showed that the cells continued to express βIII tubulin, radial glial protein (RGP), and nestin (Fig. 7A), indicative of neuronal differentiation (Daadi et al., 2008). The differentiated hESCs, proliferated and maintained a stable phenotype for up to 4 weeks in culture (P8), when the experiment was terminated (data not shown). To confirm that these changes did not involve soluble factors, we cultured the differentiated cells with manually dissected clumps of polarized undifferentiated cells and immunostained the co-cultures with antibodies that recognized markers of pluripotency (alkaline phosphatase, Nanog, Oct 3/4, and TRA 1–60) or neuronal differentiation (vimentin, RGP, and βIII tubulin). The expression pattern of neither cell type changed (Fig. 7B). In summary, these results demonstrate that the disruption of cell–cell contacts can induce loss of hESC pluripotency and trigger neuronal differentiation.
Fig. 7.
The effects of GROα-containing medium on hESCs depended on cell–cell contact. (A) When plated as single cells in GROα-containing medium, hESCs expressed markers associated with neuronal commitment: βIII tubulin, RGP, and nestin. All cells were visualized with the nuclear dye Hoechst 33342 and/or rhodamine-conjugated phalloidin that binds specifically to actin filaments. (B) Undifferentiated hESCs and differentiated single cells co-cultured in the GROα-containing medium retained their original phenotypes. Cells were co-cultured for one passage. Undifferentiated hESC colonies expressed pluripotency markers (alkaline phosphatase, Nanog, Oct 3/4, and TRA-1–60) and differentiated cells stained for markers of early neural progenitors (vimentin, RGP, and βIII tubulin).
4. Discussion
In this study, we report the development of a cost-effective, feeder-, and nearly xeno-free culture system for hESC propagation. Specifically, we started with KSR medium containing serum replacement, which includes a small amount of bovine albumin. Since, as discussed below, we used substrates formed from human proteins and added only recombinant products to the medium, the serum supplement was the only source of non-human proteins in our culture system.
With regard to the culture substrate, maintaining the cells on human serum eliminated the need for feeder cells, thus providing a cost-effective alternative to complex mouse matrices (e.g., matrigel) or purified human ECM components (e.g., fibronectin). Serum, the fluid by-product of clotted blood, contains ~60–80 mg protein/ml and various other constituents such as salts, lipids, amino acids, and sugars. Mass-spectrometry-based analyses of albumin- and immunoglobulin-depleted serum detected about 650 different proteins in a very broad range of concentrations (Schenk et al., 2008). It is likely that coating tissue culture dishes with serum immobilizes, among other components, ECM constituents in this body fluid such as vitronectin, fibrinogen, perlecan, fibronectin, and collagens IV and XI (Adkins et al., 2002; Adkins et al., 2005). Serum is also rich in various ECM- and growth factor-binding proteoglycans such as heparan sulfate, which plays an important role in hESC self-renewal (Furue et al., 2008). Additionally, using substrates formed from human serum increases the likelihood that the native conformation, glycosylation, and hence, activity of the bound glycoprotein substrates are preserved.
With regard to the culture medium, many previous studies have focused on the role of protein constituents – namely, growth factors and cytokines – with evidence suggesting that other molecules such as lipids also play important roles (Pebay et al., 2005). Several groups independently reported that hbFGF is necessary and sufficient for feeder-free hESC growth in the absence of MEF-conditioned medium (Amit et al., 2004; Wang et al., 2005; Xu et al., 2005). Under these conditions, increasing the concentration of hbFGF ~25-fold to 100 ng/ml sustains hESC growth in a pluripotent state (Levenstein et al., 2006). The requirement for high concentrations of hbFGF was attributed to the low stability of hbFGF in culture medium and/or to the recruitment of additional signaling pathways. TGFβ/activin/nodal signaling also plays a significant role in regulating hESC pluripotency when the cells are cultured in the absence of feeders (James et al., 2006; Saha et al., 2008). Thus, our basal medium was KSR supplemented with bFGF and TGF-β.
In devising a new formulation for propagating hESCs under feeder-free conditions, we hypothesized that lactate, which was at significantly higher concentrations in CMs that supported hESC self-renewal, may have a beneficial effect. The evidence presented here supports this theory (see Supplementary Data). However, initial experiments showed that basal medium containing lactate failed to maintain hESC growth in an undifferentiated state for more than 2–3 passages. To identify other novel additives, we compared the secretomes of hPFs, which we use as hESC feeders (Genbacev et al., 2005), and IMR90 cells, which do not sustain hESC replication in an undifferentiated state. The most obvious difference was higher production of GROα by hPFs. Accordingly, in addition to lactate, we added to the basal medium a recombinant version of this protein. In other experiments, we demonstrated the ability of H7 and H9 cells, cultured under these novel conditions for 10 and 20 passages, to differentiate into derivatives of the three primary germ layers. However, we noticed an increased propensity of the H7 line to form contractile cardiomyocytes suggesting a potential bias that could be exploited in the directed differentiation of these cells. At P10 and P20 the hESCs expressed a normal karyotype and at P15 they formed teratomas. Taken together, the results show that this novel substrate and medium combination sustains the long-term growth and proliferation of hESCs in an undifferentiated state while preserving their pluripotency.
Adding GROα, a member of the CXC chemokine family, was key to formulating this new medium. Initially, this molecule was isolated based on its ability to stimulate proliferation of malignant melanoma cells in vitro (Anisowicz et al., 1987; Richmond and Thomas, 1988). However, GROα promotes the growth of many cell types (Haghnegahdar et al., 2000; Aust et al., 2001; Li et al., 2004). Its receptor, CXCR2, which belongs to the G-coupled seven-transmembrane- domain family, transmits signals via the mitogen-activated protein kinase and protein kinase C pathways (Stillie et al., 2009). Here we show that hESCs express this receptor, providing further rationale for addition of GROα to the hESC culture medium.
Because hESCs grow as polarized epithelia (Krtolica et al., 2007), we were interested in whether single cells, cultured under the novel conditions described here, could reestablish this highly specialized form of cell–cell adhesion. Interestingly, despite the high levels of hbFGF in our defined medium, single cells that were re-plated failed to form colonies. The same concentration of hbFGF (100 ng/ml) that we used sustained self-renewal of hESCs grown as single cells on matrigel for up to 164 population doublings, i.e., performed very similarly to MEF CM (Levenstein et al., 2006). This dichotomy is likely created by the presence of GROα (and perhaps other growth factors) in our novel culture medium and differences in the microenvironment provided by the serum vs. matrigel substrate (Ilic, 2006). One possible interpretation of this result is that dissociation of colonies into single cell suspensions destroys the tight and adherent junction complexes by removing the basement membrane components that hESCs synthesize (Evseenko et al., 2009). Consequently, when single cells are plated on substrates that are not enriched in basement membrane components, they may not be able to reestablish polarity, which is one of the essential characteristics of undifferentiated hESCs (Krtolica et al., 2007). Interestingly, it was recently demonstrated that two chemicals that upregulate E-cadherin expression significantly increase the reprogramming efficiency of induced pluripotent stem cells (iPSCs) (Chen et al., 2010). This finding provides additional evidence that pluripotency is associated with acquisition of a polarized phenotype. Taken together, these findings and our results suggest that minimizing the loss of cell–cell and cell–extracellular matrix contacts during passaging of hESC colonies reduces the risk of chromosomal abnormalities and renders the cells less dependent on extracellular matrix composition. This also explains why mechanical dissection of colonies into uniform sized clumps seems to be the best current practice for establishment and maintenance of hESCs (Hasegawa et al., 2010).
In our system single cells continued to proliferate, but changed their morphology and downregulated hESC-associated pluripotency markers. Coincidentally, they upregulated the expression of markers of neuronal progenitors. This finding may reflect the fact that hESCs appear to be closely related to components of the epiblast (Henderson et al., 2002) which, among other cell types, gives rise to progenitors that ultimately form central and peripheral nervous systems. The results of this study provide further evidence that various combinations of factors can support self-renewal of hESC. It is possible that specific culturing conditions may predispose hESC to more efficiently undergo directed differentiation towards specific lineages and that the choice of the optimal culturing conditions will depend on hESC application. Addressing these issues will involve an iterative process in which we use our increasing knowledge of the molecular determinants that enable self-replication of the true pluripotent hESC-like cell population in vivo as well as the signals that are involved in early lineage decisions to improve the culture conditions that are used to propagate pluripotent hESCs in vitro.
Supplemental data
MEF and hPF CMs contain high lactate levels
In addition to growth factors and cytokines, hESCs, like human embryos, may have specialized needs in terms of energy sources. To test this hypothesis, we measured the lactate concentration of KSR±MEFs or hPFs. The results are shown in Suppl. Fig. 1. In the absence of cells, KSR medium did not contain detectable lactate levels. The same medium conditioned by MEFs or hPFs contained 5–9 mM lactate, levels that are similar to those that are used for human embryo culture (Leese, 1988). Thus, we concluded that the inclusion of lactate in the formulation of a defined hESC medium might be beneficial.
Lactate measurements
Lactate levels in the medium were measured using a Lactate Assay Kit according to the manufacturer’s instructions (BioVision).
Acknowledgments
This work was supported by grants from the California Institute for Regenerative Medicine (RC1-00113 and RL1-00648); JPC and CP were supported by NIH grants R01-AG009909 and T32-000266. Partial funding was also provided by StemLifeLine, Inc.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.diff.2011.01.001.
References
- Adjaye J, Huntriss J, Herwig R, BenKahla A, Brink TC, Wierling C, Hultschig C, Groth D, Yaspo ML, Picton HM, Gosden RG, Lehrach H. Primary differentiation in the human blastocyst: comparative molecular portraits of inner cell mass and trophectoderm cells. Stem Cells. 2005;23:1514–1525. doi: 10.1634/stemcells.2005-0113. [DOI] [PubMed] [Google Scholar]
- Adkins JN, Monroe ME, Auberry KJ, Shen Y, Jacobs JM, Camp DG, 2nd, Vitzthum F, Rodland KD, Zangar RC, Smith RD, Pounds JG. A proteomic study of the HUPO plasma proteome project’s pilot samples using an accurate mass and time tag strategy. Proteomics. 2005;5:3454–3466. doi: 10.1002/pmic.200401333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- Anisowicz A, Bardwell L, Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci USA. 1987;84:7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust G, Steinert M, Boltze C, Kiessling S, Simchen C. GRO-alpha in normal and pathological thyroid tissues and its regulation in thyroid-derived cells. J Endocrinol. 2001;170:513–520. doi: 10.1677/joe.0.1700513. [DOI] [PubMed] [Google Scholar]
- Basu S, Pioli PA, Conejo-Garcia J, Wira CR, Sentman CL. Estradiol regulates MICA expression in human endometrial cells. Clin Immunol. 2008;129:325–332. doi: 10.1016/j.clim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MK, Rosler ES, Fisk GJ, Brandenberger R, Ares X, Miura T, Lucero M, Rao MS. Properties of four human embryonic stem cell lines maintained in a feeder-free culture system. Dev Dyn. 2004;229:243–258. doi: 10.1002/dvdy.10431. [DOI] [PubMed] [Google Scholar]
- Chen T, Yuan D, Wei B, Jiang J, Kang J, Ling K, Gu Y, Li J, Xiao L, Pei G. E-cadherin-mediated cell–cell contact is critical for induced pluripotent stem cell generation. Stem Cells. 2010 doi: 10.1002/stem.456. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Kauser K, Campisi J, Beausejour CM. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem. 2006;281:29568–29574. doi: 10.1074/jbc.M603307200. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS ONE. 2008;3:e1644. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evseenko D, Schenke-Layland K, Dravid G, Zhu Y, Hao QL, Scholes J, Wang XC, Maclellan WR, Crooks GM. Identification of the critical extracellular matrix proteins that promote human embryonic stem cell assembly. Stem Cells Dev. 2009;18:919–928. doi: 10.1089/scd.2008.0293. [DOI] [PubMed] [Google Scholar]
- Furue MK, Na J, Jackson JP, Okamoto T, Jones M, Baker D, Hata R, Moore HD, Sato JD, Andrews PW. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci USA. 2008;105:13409–13414. doi: 10.1073/pnas.0806136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Kaelin W, Fisher SJ. Human cytotrophoblast expression of the von Hippel–Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev Biol. 2001;233:526–536. doi: 10.1006/dbio.2001.0231. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Zdravkovic T, Brunette E, Powell S, Nath A, Caceres E, McMaster M, McDonagh S, Li Y, Mandalam R, Lebkowski J, Fisher SJ. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil Steril. 2005;83:1517–1529. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, Cardwell N, Luan J, Shattuck-Brandt R, Richmond A. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000;67:53–62. doi: 10.1002/jlb.67.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Pomeroy JE, Pera MF. Current technology for the derivation of pluripotent stem cell lines from human embryos. Cell Stem Cell. 2010;6:521–531. doi: 10.1016/j.stem.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, Andrews PW. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2002;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- Ilic D. Culture of human embryonic stem cells and the extracellular matrix microenvironment. Regen Med. 2006;1:95–101. doi: 10.2217/17460751.1.1.95. [DOI] [PubMed] [Google Scholar]
- James D, Noggle SA, Swigut T, Brivanlou AH. Contribution of human embryonic stem cells to mouse blastocysts. Dev Biol. 2006;295:90–102. doi: 10.1016/j.ydbio.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Genbacev O, Escobedo C, Zdravkovic T, Nordstrom A, Vabuena D, Nath A, Simon C, Mostov K, Fisher SJ. Disruption of apical–basal polarity of human embryonic stem cells enhances hematoendothelial differentiation. Stem Cells. 2007;25:2215–2223. doi: 10.1634/stemcells.2007-0230. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Ludlow JW. Hypoxia arrests ovarian carcinoma cell cycle progression, but invasion is unaffected. Cancer Res. 1996;56:1168–1173. [PubMed] [Google Scholar]
- Leese HJ. Human embryo culture: back to nature. J Assist Reprod Genet. 1988;15:466–468. doi: 10.1023/A:1022526219202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Varney ML, Singh RK. Constitutive expression of growth regulated oncogene (gro) in human colon carcinoma cells with different metastatic potential and its role in regulating their metastatic phenotype. Clin Exp Metastasis. 2004;21:571–579. doi: 10.1007/s10585-004-5458-3. [DOI] [PubMed] [Google Scholar]
- McDevitt TC, Palecek SP. Innovation in the culture and derivation of pluripotent human stem cells. Curr Opin Biotechnol. 2008;19:527–533. doi: 10.1016/j.copbio.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitalipova MM, Rao RR, Hoyer DM, Johnson JA, Meisner LF, Jones KL, Dalton S, Stice SL. Preserving the genetic integrity of human embryonic stem cells. Nat Biotechnol. 2005;23:19–20. doi: 10.1038/nbt0105-19. [DOI] [PubMed] [Google Scholar]
- Noaksson K, Zoric N, Zeng X, Rao MS, Hyllner J, Semb H, Kubista M, Sartipy P. Monitoring differentiation of human embryonic stem cells using real-time PCR. Stem Cells. 2005;23:1460–1467. doi: 10.1634/stemcells.2005-0093. [DOI] [PubMed] [Google Scholar]
- Pebay A, Wong RC, Pitson SM, Wolvetang EJ, Peh GS, Filipczyk A, Koh KL, Tellis I, Nguyen LT, Pera MF. Essential roles of sphingosine-1-phosphate and platelet-derived growth factor in the maintenance of human embryonic stem cells. Stem Cells. 2005;23:1541–1548. doi: 10.1634/stemcells.2004-0338. [DOI] [PubMed] [Google Scholar]
- Penna I, Du H, Ferriani R, Taylor HS. Calpain5 expression is decreased in endometriosis and regulated by HOXA10 in human endometrial cells. Mol Hum Reprod. 2008;14:613–618. doi: 10.1093/molehr/gan055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peura TT, Bosman A, Stojanov T. Derivation of human embryonic stem cell lines. Theriogenology. 2007;67:32–42. doi: 10.1016/j.theriogenology.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Richmond A, Thomas HG. Melanoma growth stimulatory activity: isolation from human melanoma tumors and characterization of tissue distribution. J Cell Biochem. 1988;36:185–198. doi: 10.1002/jcb.240360209. [DOI] [PubMed] [Google Scholar]
- Sager R, Anisowicz A, Pike MC, Beckmann P, Smith T. Structural, regulatory, and functional studies of the GRO gene and protein. Cytokines. 1992;4:96–116. [PubMed] [Google Scholar]
- Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Schoenhals GJ, de Souza G, Mann M. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008;1:41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Przyborski S, Stewart R, Armstrong L, Evans J, Zhang X, Stojkovic M. Human-serum matrix supports undifferentiated growth of human embryonic stem cells. Stem Cells. 2005;23:895–902. doi: 10.1634/stemcells.2004-0326. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Umesaki N. Leptin regulates the proliferation and apoptosis of human endometrial epithelial cells. Int J Mol Med. 2008;22:683–689. [PubMed] [Google Scholar]
- Wang G, Zhang H, Zhao Y, Li J, Cai J, Wang P, Meng S, Feng J, Miao C, Ding M, Li D, Deng H. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem Biophys Res Commun. 2005;330:934–942. doi: 10.1016/j.bbrc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O’Sullivan C, Delavan-Boorsma K, Mok M, Bronstein A, Carpenter MK. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]