Abstract
Utilization of heme as an iron source by Bradyrhizobium japonicum involves induction of the outer membrane heme receptor gene hmuR and other genes within the heme utilization locus. Here, we discovered the hmuP gene located upstream of hmuR and transcribed divergently from it along with hmuTUV. hmuP encodes a small protein that accumulated under iron limitation and is transcriptionally controlled by the global iron-responsive regulator Irr, as were all genes within the heme utilization locus. Cross-linking and immunoprecipitation experiments showed that Irr occupies the hmuR-hmuP promoter in vivo. An hmuP mutant did not grow on heme as an iron source, but retained the ability to use ferric chloride. Correspondingly, induction of hmuR mRNA under iron limitation was severely diminished in an hmuP strain, but other genes within the Irr regulon were unaffected. HmuP occupied the hmuR-hmuP promoter, and thus it plays a direct regulatory role in gene expression. HmuP was not required for Irr occupancy, nor was ectopic expression of hmuP from an Irr-independent promoter sufficient to induce the hmuR gene. Thus, both HmuP and Irr occupancy are necessary for hmuR induction. We suggest that HmuP is a coactivator of Irr-dependent expression of hmuR.
INTRODUCTION
Iron is essential for most organisms and is required for many cellular processes. Iron can be a limiting nutrient in aerobic environments because the oxidized (ferric, Fe3+) form is extremely insoluble at physiological pH. Bacteria occupy diverse niches and have adopted a variety of mechanisms for acquiring iron from their environment to fulfill nutritional needs. Numerous bacterial species are able to use heme as an iron source (9, 31). Heme is an iron-containing tetrapyrrole that serves as the prosthetic group or active moiety of heme proteins. Over 70% of iron in mammals is incorporated into heme, mostly as hemoglobin (22), and therefore bacterial pathogens of animals potentially have a large source of iron.
Gram-negative bacteria have an outer membrane receptor that binds heme, heme proteins, or hemophores and additional accessory proteins to transduce energy from the cytoplasmic membrane to the receptor for translocation of heme into the periplasm. Heme is then shuttled to a periplasmic protein and ultimately delivered into the cytoplasm via an ABC transport system (9, 31). Gram-positive bacteria have surface-exposed receptors that shuttle heme through the cell wall to an ABC transporter that delivers it into the cytoplasm (4). Once in the cytoplasm, iron must be released either by cleavage of the tetrapyrrole ring or by dechelation (4, 17).
The Gram-negative bacterium Bradyrhizobium japonicum is a model organism for studying iron homeostasis and heme regulation in the Alphaproteobacteria, a large and diverse taxonomic group that includes pathogens, symbionts, and bacteria that occupy many other ecological niches (20). B. japonicum lives as a free-living organism or as the endosymbiont of soybean, where it fixes atmospheric nitrogen to ammonia within cells of a specialized organ called a root nodule. Heme utilization systems have been characterized in B. japonicum and other rhizobia (2, 6, 7, 18, 21, 32), and they are generally similar to those found in other Gram-negative bacteria. However, genetic regulation of heme utilization and iron homeostasis in general differs substantially in the Alphaproteobacteria from that in Escherichia coli or other well-studied model organisms. Whereas Fur is the primary global regulator of the iron stimulon in numerous bacterial species, this role is carried out by Irr, RirA, or both in the Alphaproteobacteria (3, 5, 6, 11, 23, 27, 28, 30, 34).
In B. japonicum, heme utilization genes are regulated by Irr (18, 23). An Irr binding site called the iron control element (ICE) is found upstream of the two clusters of divergent genes that comprise the heme utilization gene locus. Promoter activity of the heme receptor gene hmuR is diminished in an irr mutant or by mutation of ICE (18, 23). Microarray analysis indicates that heme utilization genes are negatively regulated by RirA in Sinorhizobium meliloti (6). B. japonicum does not contain RirA, whereas S. meliloti contains both Irr and RirA.
hmuP (hemP) is a small gene first identified incidentally many years ago in the sequencing of the heme transport gene cluster of Yersinia enterocolitica (26), and homologs have since been found in many proteobacteria. However, clues into the function of hmuP have emerged only recently in a screen for genes that affect expression of the outer membrane heme receptor gene shmR in S. meliloti (2). Expression of shmR in response to iron limitation is severely diminished in an hmuP mutant. A regulatory function for hmuP is interesting because it is found in bacteria known or suspected to have global regulators of iron homeostasis that also control heme transport. This suggests multiple levels of control of heme utilization gene expression.
In the present study, we identified an hmuP homolog in B. japonicum and demonstrate that HmuP is a direct regulator of heme utilization gene expression and that it occupies the hmuR promoter to mediate, along with Irr, iron-dependent gene expression.
MATERIALS AND METHODS
Strains and media.
Bradyrhizobium japonicum strains USDA110 and LO are the parent strains used in this study. LODTM5 is a mutant derived from strain LO that contains a transposon Tn5 inserted within the irr gene (10). B. japonicum strains were routinely grown at 29°C in glycerol-salts-yeast (GSY) medium as previously described (8). For low-iron conditions, modified GSY was used, which contains 0.5 g per liter yeast extract instead of 1 g per liter, with no exogenous iron added. The actual concentration of iron in unsupplemented medium was 0.3 μM, as determined by atomic absorption using a Perkin-Elmer model 1100B atomic absorption spectrometer. High-metal medium was supplemented with 12 μM FeCl3.
Construction of hmuP mutant strain.
The B. japonicum hmuP open reading frame and the flanking sequence, 700 bp upstream and 576 bp downstream was cloned with PCR primers hmuP forward (ATAGATCTAGACAAGCCGCGAATGTTGATAACGGT) and hmuP reverse (TATCTTCTAGAGTTCTGCAGCGACATCACGAACAT) into the XbaI site of pBluescript SK+. An in-frame deletion removing most of the open reading frame was constructed by inverse PCR using the primers delta hmuP forward (ATAGACATATGTAACCTGCAATGACATTTTGCCGC) and delta hmuP reverse (TATCTCATATGCATTGCCCGTGTGATGCGCCTTCG), and the inverse PCR product was digested with NdeI and ligated. The deletion was confirmed by DNA sequencing. The XbaI fragment containing the hmuP in-frame deletion was subcloned into the XbaI site of pLO1 (16) and introduced into B. japonicum strain USDA110 by conjugation. Single recombinants were selected based on kanamycin resistance and then screened for sensitivity on growth with 5% (wt/vol) sucrose due to the sacB gene harbored on the plasmid. Double recombinants arising from a selected single recombinant were selected based on sucrose resistance and then screened for kanamycin sensitivity. The hmuP deletion in the double recombinants was confirmed by the size and sequence of PCR products using the flanking primers TTGATGTCCTTGGTGCGGAACGAGACCA (forward) and CATTGCAGGTTACATATATCTCATATGCAT (reverse).
Western blot analysis.
Two-hundred-milliliter cultures were grown to mid-log phase under high-iron (12 mM FeCl3) or low-iron (no iron added) conditions, collected by centrifugation, washed twice, and resuspended in 0.75 ml phosphate-buffered saline (10 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl [pH 7.4]) with 10 mM phenylmethylsulfonyl fluoride and 1× protease inhibitor cocktail (Sigma). Cells were disrupted by passing them twice through a French pressure cell at 18,000 lb/in2 and clarified by centrifugation at 18,000 × g for 30 min. Protein concentration in cell extracts was determined by the bicinchoninic acid (BCA) method. Thirty micrograms of total protein was resolved in 15% SDS-PAGE (for Irr and GroEL) or 18% SDS-PAGE Tris-Tricine (for HmuP) and analyzed by Western blot analysis as described previously (33). Antibodies to Irr were raised as described previously (10). HmuP polyclonal antibody was raised against pure His-tagged HmuP by Rockland Immunochemicals, Inc. (Gilbertsville, PA).
The HmuP protein was overexpressed and purified as described previously (21). The B. japonicum hmuP open reading frame was isolated by PCR using genomic DNA from B. japonicum strain USDA110 as the template and the primers NdeI hmuP forward (ATAGACATATGTCAGCAACATCAGGAGA) and BamHI hmuP reverse (TCTATGGATCCTTACTTCGTCAGGATCAGCT) and cloned into the SmaI site of pBluescript SK+. The NdeI/BamHI fragment containing the hmuP open reading frame was subcloned into the NdeI/BamHI sites of pET14b.
Constitutive expression of hmuP in B. japonicum.
The hmuP open reading frame was amplified by PCR using B. japonicum genomic DNA as the template and primers NdeI-hmuP forward (ATAGACATATGTCAGCAACATCAGGAGACAGC) and NheI-hmuP reverse (CTATGCTAGCGTTACTTCGTCAGGATCAGCTTGT) and cloned into the SmaI site of pBluescript SK+. The NdeI-NheI fragment containing the hmuP open reading frame was subcloned into the NdeI-NheI sites of pSRKK vector (14) that contains a lacIq-lac promoter-operator complex for Irr-independent expression. The new construct pSRKK-hmuP and pSRKK were introduced into B. japonicum. Cells were grown in modified GSY medium supplemented with 50 μg/ml kanamycin under high-iron (12 μM FeCl3) or low-iron (no iron added) conditions. Overexpression of HmuP was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 16 h. Cells were harvested at mid-log phase (optical density at 540 nm [OD540] of 0.4 to 0.6).
Growth disk assays.
A total of 106 cells of the parent strain or hmuP mutant were quickly vortexed into 5 ml soft agar (0.8%) at 50°C containing 25 μM EDDHA (ethylenediamine-di-o-hydroxyphenylacetic acid) as a metal chelator and then poured onto solid medium. After solidification of the agar medium, Whatman paper disks (6-mm diameter) were placed at the center of the plate and spotted with 6 μl of 10 mM FeCl3 or hemin hydrochloride or with 6 μl of metal-free water as a negative control. Growth around the disk was assessed visually.
Analysis of RNA by real-time qPCR.
Expression levels of selected genes were determined by quantitative PCR (qPCR) with iQ SYBR green supermix (Bio-Rad) using the iCycler thermal cycler (Bio-Rad). RNA was isolated from B. japonicum cells using a hot phenol extraction method as previously described (34). cDNA was synthesized from 2 μg total RNA using iScript cDNA synthesis kit (Bio-Rad). qPCRs were carried out as previously described (12). Data were normalized to gapA and are expressed as average of three triplicates, and the standard deviation is represented by the error bars.
Quantitative in vivo cross-linking and immunoprecipitation.
Two hundred-milliliter cultures were grown to mid-log phase (OD540, 0.4 to 0.6) in high-iron (12 μM FeCl3) or low-iron (no iron added; 0.3 μM Fe as determined by atomic absorption) medium. Cross-linking and immunoprecipitation using antibodies against Irr or HmuP were performed as described elsewhere (25). Immunoprecipitated DNA of selected promoter regions was quantified using qPCR as described above, using 1 μl coimmunoprecipitated DNA template. The data were normalized to input DNA.
RESULTS
Identification of the hmuP gene in B. japonicum.
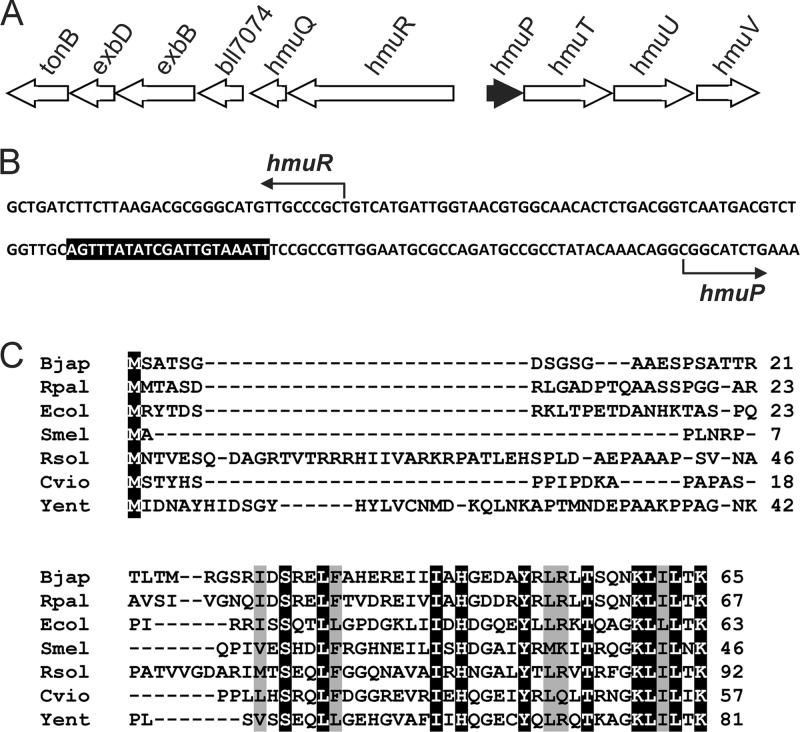
No hmuP gene homolog was identified in the annotated genomes of B. japonicum 110 or other Bradyrhizobium species, but a putative homolog is found in Rhodopseudomonas palustris, which is closely related to B. japonicum. An hmuP gene homolog was identified using R. palustris CGA009 HmuP to search the translated nucleotide database of the B. japonicum 110 genome using the tblastn program (1). This open reading frame encoded a predicted protein with 80% similarity (60% identity) to R. palustris HmuP, with less similarity to the proteins in more taxonomically distant species (Fig. 1). The small size of the hmuP open reading frame was probably the reason why it was not annotated originally.
Fig 1.
Localization of hmuP within the heme utilization heme locus and comparison of B. japonicum HmuP with homologs in other bacteria. (A) The hmuP gene was found upstream of the divergent genes hmuR and hmuT in the same orientation as hmuT. (B) Partial nucleotide sequence of the hmuR-hmuP intergenic region. The sequence shown corresponds to the coding strand of hmuP and the template strand of hmuR. The bent arrows show the transcription start sites of hmuR and hmuP as determined by Nienaber et al. (18). The shaded region denotes the ICE. (C) Alignment of HmuP homologs in B. japonicum (Bjap), were compared with those of Rhodopseudomonas palustris (Rpal; YP_782068.1), E. coli (Ecol; YdiE), Sinorhizobium meliloti (Smel; NP_386533.1), Ralstonia solanacearum (Rsol; NP_520087.1), Chromobacterium violaceum (Cvio; NP_903565.1), and Yersinia entercolitica (Yent; HEMP_YEREN). Identical amino acids are shaded in black with white letters, and conserved residues are shaded in gray.
The B. japonicum hmuP gene was found within the heme utilization gene locus characterized previously by Nienaber et al. (18) (Fig. 1). hmuP homologs are usually clustered with genes involved in heme utilization, but the exact arrangements and groupings of genes vary between organisms The B. japonicum heme utilization locus is organized in two clusters of genes divergent to each other. The hmuP gene was found immediately upstream of hmuT and is downstream of the transcription start site determined previously for that gene; thus hmuP and hmuT are cotranscribed. Moreover, there are no gaps between hmuT, hmuU, and hmuV open reading frames, thus hmuPTUV is very likely an operon. The second group within the cluster encodes HmuR, the heme receptor, the heme-degrading enzyme HmuQ, the accessory transport proteins ExbB, ExbD, and TonB, as well as a gene of unknown function. These genes contain small gaps between them, are coregulated as described below, and thus are probably organized in operon.
The hmuP gene encodes a protein that accumulates in an iron-dependent manner.
Putative HmuP proteins are small, ranging in size from 46 to 81 amino acids, have low homology to each other throughout, except at the C terminus, and have very few absolutely conserved residues (Fig. 1B). Moreover, HmuP/HemP proteins have not been detected previously in cells. Thus, we wanted to confirm that hmuP is a protein-encoding gene and establish its expression pattern in cells. Antibodies were raised against purified recombinant protein and used to detect HmuP by Western blot analysis in cells grown in low-iron or high-iron medium (Fig. 2A). A cross-reactive band about 7.6 kDa in size was observed in cells grown under iron limitation, which is in good agreement with the predicted size of 7,074 Da. The band was undetectable in wild-type cells grown in the presence of iron or in an hmuP mutant under either growth condition. The hmuP strain was constructed by making an in-frame deletion of most of the open reading frame, as described in Materials and Methods. hmuP mRNA was measured in the same cells by real-time qPCR (Fig. 2B). Transcript levels were high in the parent strain only under iron limitation, which correlated with protein accumulation. The findings show that hmuP is an iron-regulated gene, resulting in high HmuP levels under limitation of the metal.
Fig 2.
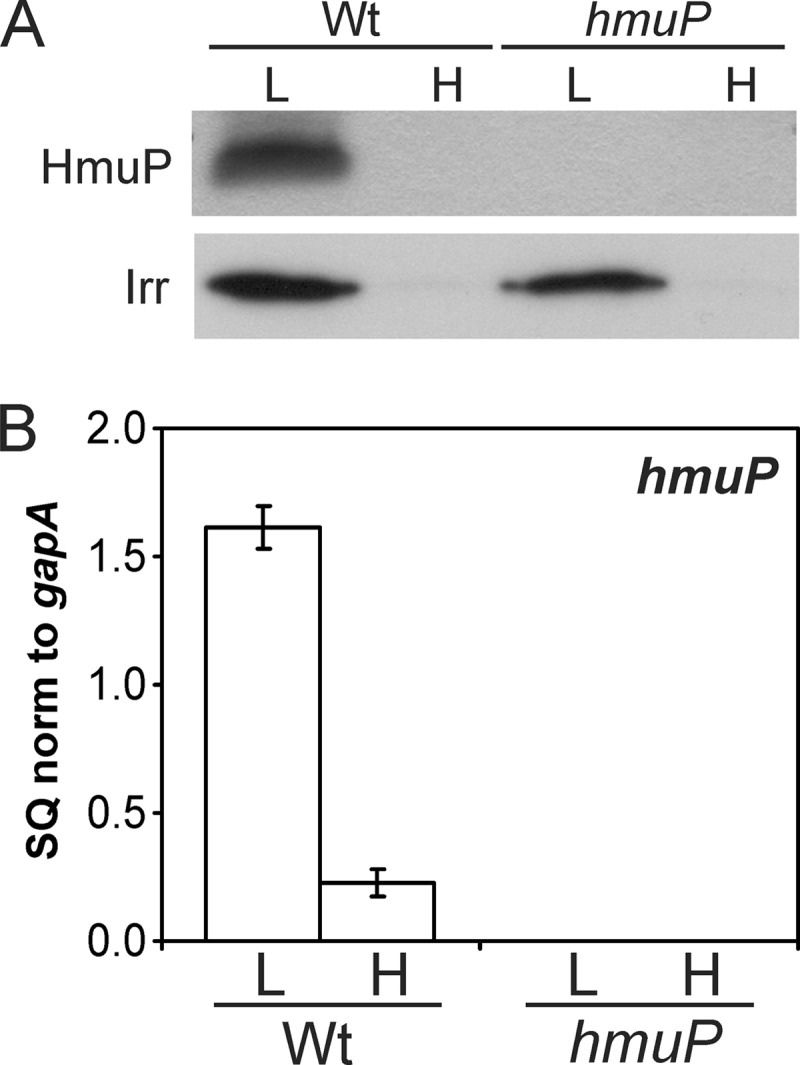
Iron-dependent expression of the hmuP gene. (A) HmuP or Irr protein in cells of the wild-type (Wt) or hmuP mutant was detected by Western blot analysis in cells grown in low-iron (L) or high-iron (H) medium. Thirty micrograms of protein was loaded per lane. (B) Analysis of hmuP mRNA by quantitative real-time PCR (qPCR) in wild-type or hmuP mutant cells grown in low- or high-iron medium. The data are expressed as the relative starting quantity (SQ) of hmuP mRNA normalized to the housekeeping gene gapA and are presented as the average of triplicate samples ± the standard deviation.
hmuP is required for growth on heme as an iron source.
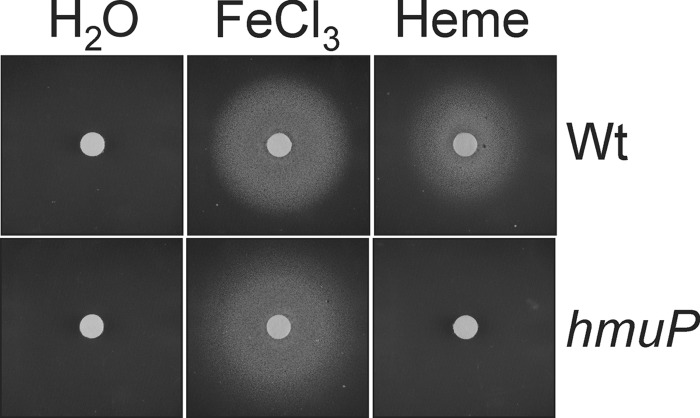
B. japonicum can use heme as an iron source (18, 19). To determine whether hmuP is required for this, we tested the ability of an hmuP mutant to grow on heme on plates using a disk assay (Fig. 3). Cells were spread on plates containing EDDHA to chelate iron. Heme (hemin hydrochloride) was spotted onto the disk, and growth around it was monitored. There was no growth of the wild-type or hmuP strains around the disk spotted with water, and both strains grew when the disk was spotted with ferric chloride (FeCl3) as the iron source. The parent strain also grew on heme as an iron source, but the hmuP strain showed no growth around the disk. The hmuP gene was introduced into the hmuP mutant in trans on pSRKK and found to restore growth on heme to a level comparable to that observed in parent strain harboring the empty vector (see Fig. S1A in the supplemental material). We conclude that hmuP is necessary for utilization of heme as an iron source.
Fig 3.
Growth on heme as an iron source requires hmuP. Cells of the wild-type or hmuP mutant were spread onto solid medium depleted of iron with EDDHA. Paper disks were placed on the agar and spotted with 6 μl of a 10 mM solution of FeCl3 or heme or with 6 μl of water. A halo of growth around the disk is noted.
The hmuP gene is regulated by Irr.
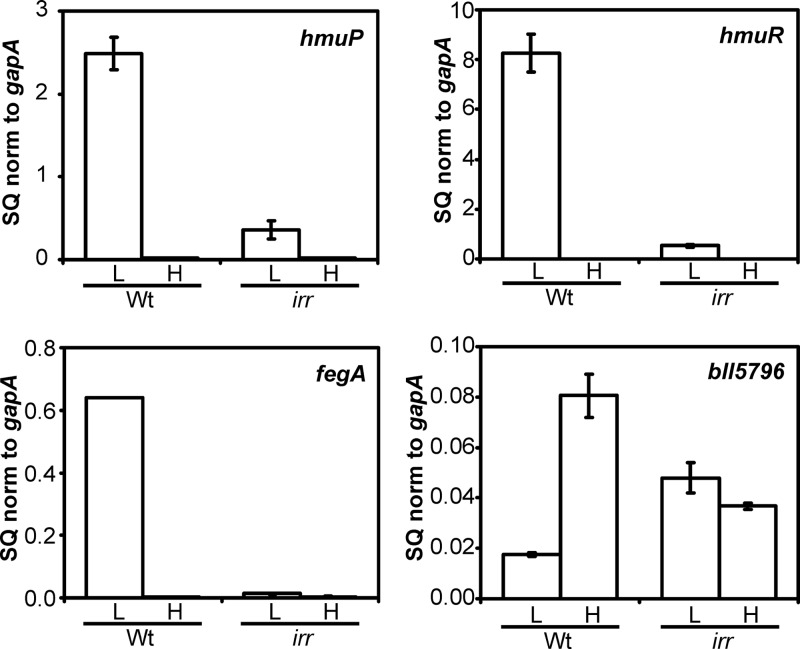
The hmuR-hmuP promoter region (originally identified as the hmuR-hmuT promoter region) contains an ICE, Irr binding element (18) (Fig. 1), and an hmuR-lacZ promoter shows diminished activity in an irr mutant (18, 23). However, hmuPTUV expression in an irr mutant has not been addressed. We examined hmuP RNA accumulation in an irr mutant strain by qPCR and found the transcript levels to be severely diminished under low-iron conditions compared to those of the parent strain (Fig. 4). Thus, Irr is required for normal iron-dependent expression of hmuP. We also examined mRNA levels of the downstream genes hmuTUV and found them to be similarly affected in the irr strain (see Fig. S2 in the supplemental material).
Fig 4.
Iron-dependent expression of mRNA of genes within the heme utilization locus are affected in an irr mutant. hmuP, hmuR, fegA, and bll5796 mRNAs were analyzed by quantitative real-time PCR (qPCR) in wild-type or irr mutant cells grown in low-iron (L) or high-iron (H) medium. The data are expressed as the relative starting quantity (SQ) The mRNAs were normalized to the housekeeping gene gapA and are presented as the average of triplicate samples ± the standard deviation. Additional genes within the heme utilization locus are shown in Fig. S2 in the supplemental material.
Previous studies show that a hmuR-lacZ fusion has diminished activity in an irr mutant, but direct measurement of transcripts in primer extension analysis did not detect differences between the wild-type and mutant strains (18). We measured hmuR mRNA by qPCR in the parent and irr strains and found that hmuR mRNA levels were reduced over 90% in the irr mutant compared to those in the parent strain grown under iron limitation (Fig. 4). Similar results were found for the genes downstream of hmuR (see Fig. S2). The results demonstrate that all 10 genes within the heme transport locus are strongly regulated by iron in an Irr-dependent manner.
Irr occupies the hmuR-hmuP promoter in vivo.
An iron control element (ICE) is found in the common promoter region of the divergent hmuR and hmuP genes (Fig. 1), and it has been shown to bind Irr in a yeast one-hybrid assay (18, 23). Here, in vivo occupancy of the hmuR-hmuP promoter was assessed by cross-linking/immunoprecipitation analysis (Fig. 5). Cells were grown to mid-log phase, followed by cross-linking of protein to DNA. DNA that coprecipitated with anti-Irr antibodies in cell extracts was analyzed by qPCR using primers that amplify the promoter region. The hmuR-hmuP promoter was occupied by Irr under iron limitation, the condition in which Irr accumulates in cells and the heme utilization genes are induced. Similar results were observed for the fegA (bll4920) and bll5796 genes, which were used as controls because Irr was shown previously to occupy their promoters (25, 34). The fegA gene encodes the ferrichrome receptor, and bll5796 encodes an iron-dependent fumarase.
Fig 5.
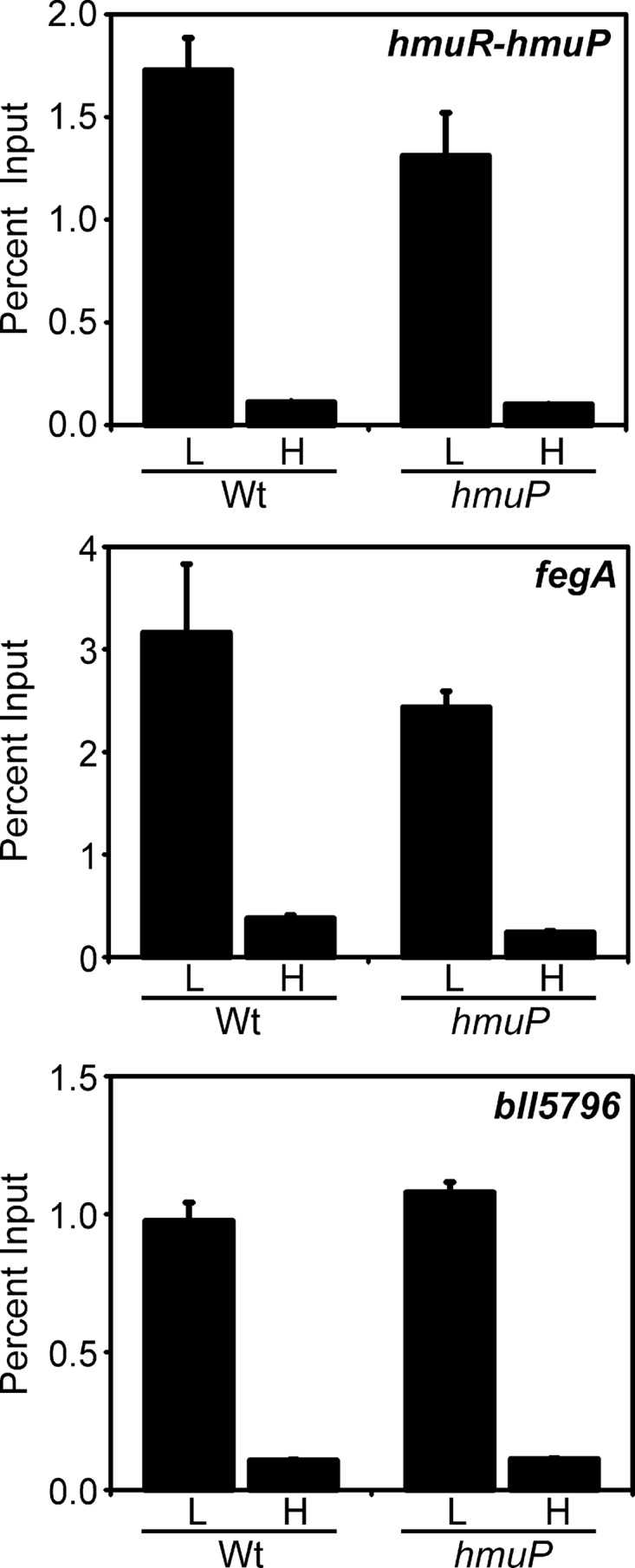
In vivo occupancy of the hmuR-hmuP promoter by Irr in wild-type and hmuP mutant cells. Cells were grown in low-iron (L) or high-iron (H) medium, cross-linked, and immunoprecipitated with anti-Irr antibodies as described in the text. The precipitated DNA was analyzed by qPCR using primers that amplify promoter DNA from hmuR-hmuP, fegA, or bll5796. The data are expressed as the relative starting quantity (SQ) of immunoprecipitated DNA normalized to input DNA.
Heme utilization genes are aberrantly expressed in an hmuP mutant.
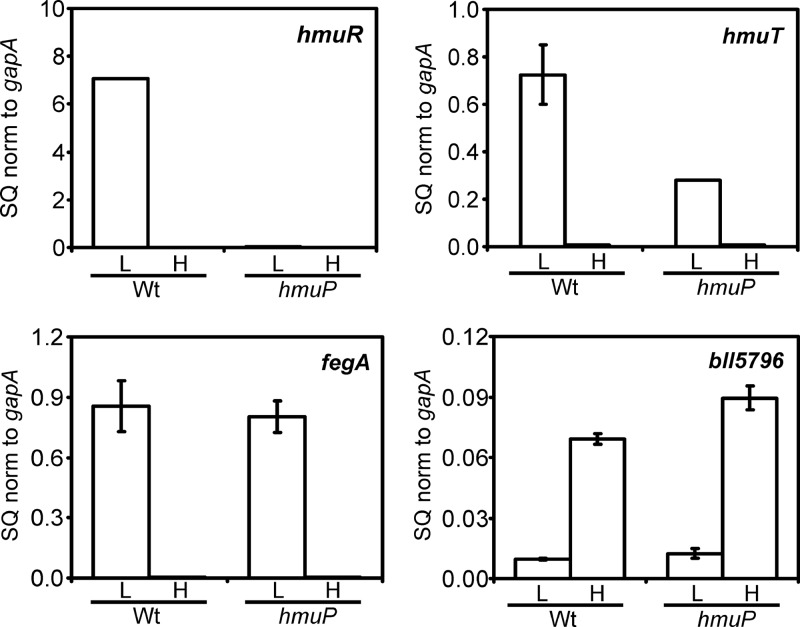
The requirement of hmuP for heme utilization led us to ask whether genes within the heme transport locus were aberrantly expressed in an hmuP mutant. hmuR encodes the outer membrane heme receptor and is necessary for heme transport (18). hmuR mRNA was analyzed by qPCR in cells of the parent and hmuP strains grown in low- or high-iron medium (Fig. 6). hmuR mRNA was strongly induced under iron limitation in the wild type, but this induction was almost completely abrogated in the hmuP mutant. Induction of hmuR mRNA was restored in the hmuP strain by introduction of the hmuP gene in trans (see Fig. S1B in the supplemental material). We also measured transcripts of the five genes downstream of hmuR, namely, hmuQ, bll7074, exbB, exbD, and tonB (see Fig. S3 in the supplemental material). These genes were regulated similarly to hmuR, showing strong induction in the parent strain under iron limitation that was severely diminished in the hmuP strain. The findings show that induction of the hmuR gene cluster (hmuR-hmuQ-bll7074-exbB-exbD-tonB) in response to iron limitation is dependent on both Irr and HmuP.
Fig 6.
Iron-dependent expression of mRNA of genes within the heme utilization locus is affected in an hmuP mutant. hmuR, hmuT, fegA, and bll5796 mRNAs were analyzed by quantitative real-time PCR (qPCR) in wild-type (Wt) or hmuP mutant cells grown in low-iron (L) or high-iron (H) medium. The data are expressed as the relative starting quantity (SQ) The mRNAs were normalized to the housekeeping gene gapA, and the results are presented as the average of triplicate samples ± the standard deviation. Additional genes within the heme utilization locus are shown in Fig. S3 in the supplemental material.
Transcripts of the heme transport genes hmuT, hmuU, and hmuV, which are transcribed divergently from divergent from hmuR, were also measured (Fig. 6; see Fig. S3 in the supplemental material). The strong induction of those genes under iron limitation in the wild type was diminished by about half in the hmuP mutant, but considerable iron-dependent regulation remained. Introduction of hmuP in trans did not restore full hmuT expression (see Fig. S1B in the supplemental material), suggesting that the in-frame deletion affected downstream genes. However, the transconjugant strain grew well on heme (see Fig. S1A) despite the diminished hmuT level, which agrees with a previous study showing that the hmuTUV genes are not required for heme-dependent growth (18). In that prior work, all cell constructs that were able to grow on heme contained an intact hmuR-hmuT intergenic region either on a plasmid or in the chromosome; hence they presumably contained a functional hmuP gene. We conclude that low expression of the hmuR gene cluster is responsible for the lack of growth of the hmuP mutant on heme as an iron source.
HmuP does not globally control the Irr regulon.
B. japonicum has a large iron stimulon, with many genes upregulated or downregulated in response to iron limitation (34). Irr is the major iron-responsive regulator in B. japonicum, as determined by the fact that most genes strongly regulated by iron are under the control of Irr. In order to address whether HmuP is a general regulator of iron-responsive genes, we examined the expression of two additional genes that are either positively or negatively affected by iron limitation. The fegA gene is induced in iron-limited wild-type cells, whereas bll5796 is repressed under that condition (25) (Fig. 4). The expression patterns of those genes were unaffected in the hmuP mutant (Fig. 6). The data show that HmuP controls iron-dependent expression of the heme utilization genes but is not a general regulator of the Irr regulon.
Expression of hmuP is not sufficient for the expression of hmuR.
Irr is required for the expression of hmuP, which is, in turn, necessary for hmuR expression. Thus, we wanted to ask whether the role of Irr in hmuR expression was only to activate the hmuP gene. We attempted to introduce a plasmid-borne hmuP gene under the control of an Irr-independent promoter into an irr strain, but that mutant is refractory to the introduction of plasmids in general for unknown reasons. Instead, the hmuP gene was expressed from the lac promoter on the broad-host-range vector pSRKK in the parent strain grown under high- or low-iron conditions (Fig. 7). HmuP protein levels were much higher in the strain harboring the ectopic hmuP gene regardless of the iron status compared with the levels found in the strain harboring the empty vector. However, high HmuP accumulation did not induce hmuR mRNA in iron-replete cells or a further increase in the transcript level in iron-limited cells. We suggest that hmuP expression is necessary but not sufficient to induce hmuR expression and that Irr has a role in addition to the induction of hmuP.
Fig 7.
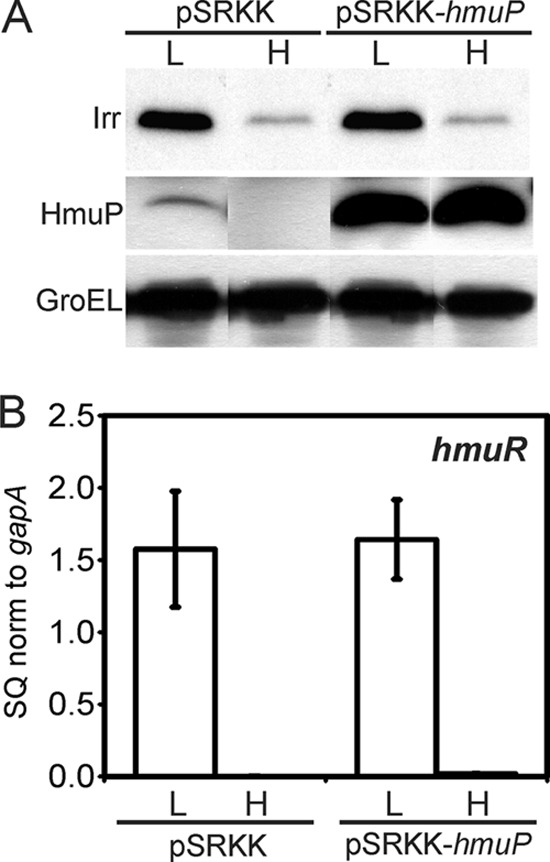
Overexpression of hmuP is not sufficient to induce hmuR expression. Wild-type cells harboring empty vector (pSRKK) or vector with the hmuP gene (pSRKK-hmuP) were grown in low-iron (L) or high-iron (H) medium. (A) Irr, HmuP, and GroEL were detected in whole cells by Western blot analysis. Thirty micrograms of protein was loaded per lane. (B) hmuR mRNA was analyzed by quantitative real-time PCR (qPCR). The data are expressed as the relative starting quantity (SQ). The mRNAs were normalized to the housekeeping gene gapA, and the results are presented as the average of triplicate samples ± the standard deviation.
HmuP occupies the hmuR-hmuP promoter.
The conclusion that HmuP does not generally affect Irr-responsive genes indicates that it does not control Irr levels, which was confirmed by the normal accumulation of Irr in an hmuP mutant (Fig. 2B) or in an hmuP overexpression strain (Fig. 7A). Thus, HmuP likely controls hmuR expression in a targeted manner. Therefore, we determined whether HmuP occupies the hmuR-hmuP promoter using cross-linking/immunoprecipitation followed by qPCR as described above, except that anti-HmuP antibody was used in the immunoprecipitation step (Fig. 8). High occupancy was observed in iron-limited cells, which correlates with hmuR accumulation in cells. Only background occupancy of the fegA or bll5796 gene promoter was observed, as determined by the similar level found in the hmuP-negative control, consistent with the finding that HmuP does not control those genes (Fig. 6).
Fig 8.
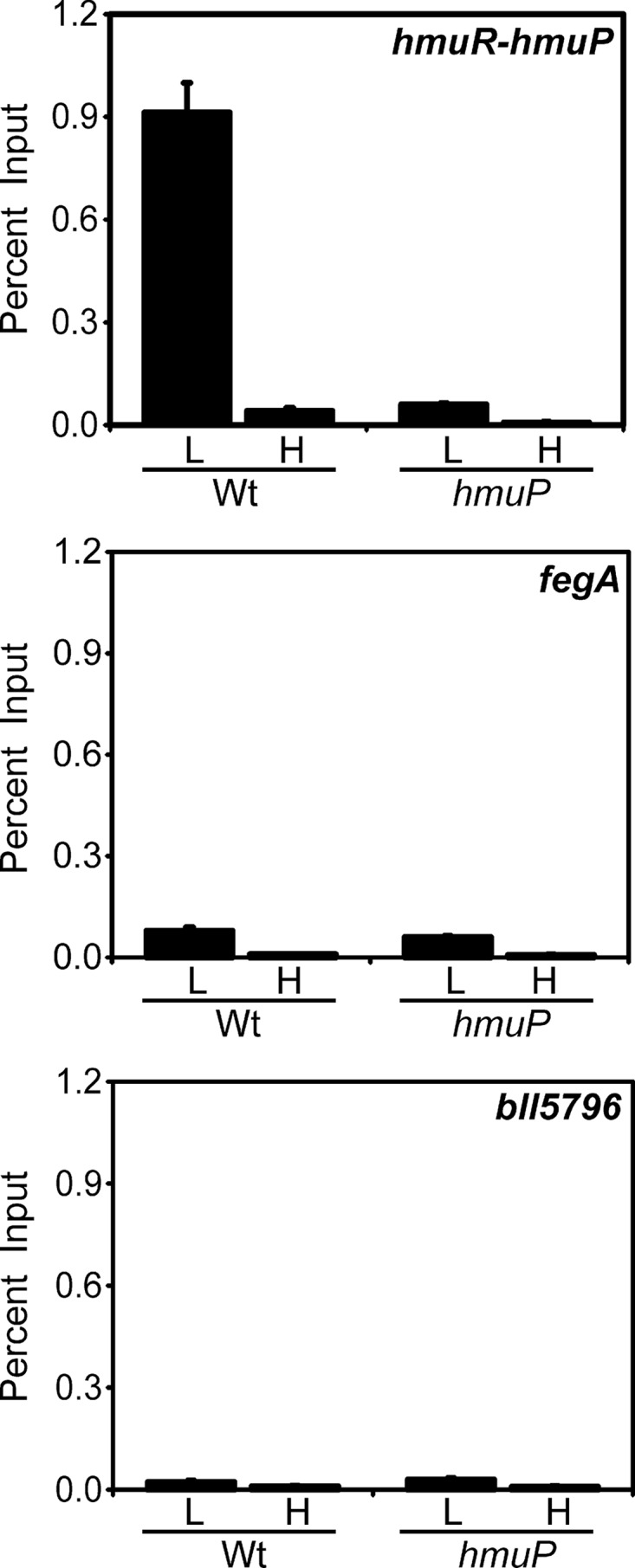
In vivo occupancy of the hmuR-hmuP promoter by HmuP. Cells of the wild-type (Wt) or hmuP mutant were grown in low-iron (L) or high-iron (H) medium, cross-linked, and immunoprecipitated with anti-HmuP antibodies as described in the text. The precipitated DNA was analyzed by qPCR using primers that amplify promoter DNA from hmuR-hmuP, fegA, or bll5796. The data are expressed as the relative starting quantity (SQ) of immunoprecipitated DNA normalized to input DNA.
To determine whether HmuP was needed for Irr occupancy of the hmuR-hmuP promoter, we carried out the cross-linking/immunoprecipitation experiment in the hmuP mutant using anti-Irr antibodies in the immunoprecipitation step (Fig. 5). Irr occupancy in iron-limited cells of the hmuP strain was similar to that found in the parent strain, showing that HmuP was not required to recruit Irr to the hmuR-hmuP promoter. A similar result was found for the promoter of fegA, a gene not regulated by HmuP. Collectively, the data show that HmuP is a direct coactivator of the hmuR gene and that hmuR expression requires occupancy of its promoter by both Irr and HmuP.
DISCUSSION
In the present study, we identified the hmuP gene in B. japonicum and showed it to be essential for growth when heme is the sole iron source. The failure to find hmuP previously from genome sequencing was probably due to its small size. The HmuP protein accumulates specifically under iron limitation and is needed for expression of the hmuR gene cluster, thereby explaining the growth phenotype of the hmuP mutant on heme. hmuP and the other nine genes in the heme utilization locus are regulated by Irr through binding to the hmuR-hmuP promoter.
Irr is the major global regulator of iron-dependent gene expression in B. japonicum. The present study represents the first example of a subset of genes within the Irr regulon that requires an additional level of control for activation under iron limitation. Thus, Irr is necessary but not sufficient to positively affect at least some genes under its control. As a negative regulator, Irr inhibits transcription in vitro (24), and thus promoter occupancy of target genes is likely to be sufficient for repression. Irr has also been shown to positively control its own transcription through an antirepressor mechanism (13). In that case, Irr relieves repression by the Mur protein by preventing Mur binding to the promoter. Irr is not needed for upregulation of irr mRNA in a mur mutant. Control of heme utilization genes is clearly different from this because both Irr and HmuP are positive effectors.
Heme utilization genes in Bordetella species are regulated by Fur and also by an extracytoplasmic function (ECF) sigma factor–anti-sigma factor system that serves to sense and respond to the extracellular heme status (15, 29). Thus, like B. japonicum, heme utilization gene expression in Bordetella is controlled by both a global iron regulator and by an additional mechanism specific to those genes. However, unlike Bordetella, iron limitation is sufficient to strongly induce the B. japonicum heme utilization gene cluster in the absence of exogenous heme. Thus, it is unlikely that HmuP has a role that is functionally equivalent to the ECF system of Bordetella.
Although the precise mechanism of control by HmuP remains unknown, its activity involves occupancy of the hmuR promoter along with Irr. This finding implicates HmuP as a direct coactivator of gene expression. In contrast, expression of the S. meliloti shmR gene under iron limitation likely involves both loss of function of the repressor RirA and the presence of an active HmuP protein. Similarly, although HmuP has not been studied in the numerous other bacteria that contain an hmuP homolog, heme utilization genes are negatively regulated by Fur in those organisms. Thus, if HmuP plays a similar role in those bacteria to what occurs in the rhizobia, then heme utilization would likely require both derepression and activation. Further studies on the function of HmuP in the various organisms that contain different coregulators will shed further light on this question.
Supplementary Material
ACKNOWLEDGMENTS
We thank Elena Fabiano and Vanesa Amarelle for useful discussions and Marty Roop for pSRKK.
This work was supported by NIH grant R01 GM067966 to M.R.O.
Footnotes
Published ahead of print 13 April 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amarelle V, et al. 2010. A new small regulatory protein, HmuP, modulates haemin acquisition in Sinorhizobium meliloti. Microbiology 156:1873–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson ES, et al. 2011. The iron-responsive regulator Irr is required for wild-type expression of the gene encoding the heme transporter BhuA in Brucella abortus 2308. J. Bacteriol. 193:5359–5364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anzaldi LL, Skaar EP. 2010. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect. Immun. 78:4977–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battisti JM, et al. 2007. Transcriptional regulation of the heme binding protein gene family of Bartonella quintana is accomplished by a novel promoter element and iron response regulator. Infect. Immun. 75:4373–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chao TC, Buhrmester J, Hansmeier N, Puhler A, Weidner S. 2005. Role of the regulatory gene rirA in the transcriptional response of Sinorhizobium meliloti to iron limitation. Appl. Environ. Microbiol. 71:5969–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuiv PO, Keogh D, Clarke P, O'Connell M. 2008. The hmuUV genes of Sinorhizobium meliloti 2011 encode the permease and ATPase components of an ABC transport system for the utilization of both haem and the hydroxamate siderophores, ferrichrome and ferrioxamine B. Mol. Microbiol. 70:1261–1273 [DOI] [PubMed] [Google Scholar]
- 8. Frustaci JM, Sangwan I, O'Brian MR. 1991. Aerobic growth and respiration of a δ-aminolevulinic acid synthase (hemA) mutant of Bradyrhizobium japonicum. J. Bacteriol. 173:1145–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Genco CA, Dixon DW. 2001. Emerging strategies in microbial haem capture. Mol. Microbiol. 39:1–11 [DOI] [PubMed] [Google Scholar]
- 10. Hamza I, Chauhan S, Hassett R, O'Brian MR. 1998. The bacterial Irr protein is required for coordination of heme biosynthesis with iron availability. J. Biol. Chem. 273:21669–21674 [DOI] [PubMed] [Google Scholar]
- 11. Hibbing ME, Fuqua C. 2011. Antiparallel and interlinked control of cellular iron levels by the Irr and RirA regulators of Agrobacterium tumefaciens. J. Bacteriol. 193:3461–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hohle TH, O'Brian MR. 2009. The mntH gene encodes the major Mn2+ transporter in Bradyrhizobium japonicum and is regulated by manganese via the Fur protein. Mol. Microbiol. 72:399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hohle TH, O'Brian MR. 2010. Transcriptional control of the Bradyrhizobium japonicum irr gene requires repression by Fur and antirepression by Irr. J. Biol. Chem. 285:26074–26080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan SR, Gaines J, Roop RM, II, Farrand SK. 2008. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl. Environ. Microbiol. 74:5053–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kirby AE, Metzger DJ, Murphy ER, Connell TD. 2001. Heme utilization in Bordetella avium is regulated by RhuI, a heme-responsive extracytoplasmic function sigma factor. Infect. Immun. 69:6951–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lenz O, Schwartz E, Dernedde J, Eitinger M, Friedrich B. 1994. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J. Bacteriol. 176:4385–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Letoffe S, Heuck G, Delepelaire P, Lange N, Wandersman C. 2009. Bacteria capture iron from heme by keeping tetrapyrrol skeleton intact. Proc. Natl. Acad. Sci. U. S. A. 106:11719–11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nienaber A, Hennecke H, Fischer HM. 2001. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol. Microbiol. 41:787–800 [DOI] [PubMed] [Google Scholar]
- 19. Noya F, Arias A, Fabiano E. 1997. Heme compounds as iron sources for nonpathogenic Rhizobium bacteria. J. Bacteriol. 179:3076–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Brian MR, Fabiano E. 2010. Mechanisms and regulation of iron homeostasis in the Rhizobia, p 37–63 Cornelis P, Andrews SC. (ed), Iron uptake and homeostasis in microorganisms, Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 21. Puri S, O'Brian MR. 2006. The hmuQ and hmuD genes from Bradyrhizobium japonicum encode heme-degrading enzymes. J. Bacteriol. 188:6476–6482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Richardson DR, Ponka P. 1997. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta 1331:1–40 [DOI] [PubMed] [Google Scholar]
- 23. Rudolph G, et al. 2006. The iron control element, acting in positive and negative control of iron-regulated Bradyrhizobium japonicum genes, is a target for the Irr protein. J. Bacteriol. 188:733–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sangwan I, Small SK, O'Brian MR. 2008. The Bradyrhizobium japonicum Irr protein is a transcriptional repressor with high-affinity DNA-binding activity. J. Bacteriol. 190:5172–5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Small SK, Puri S, Sangwan I, O'Brian MR. 2009. Positive control of ferric siderophore receptor gene expression by the Irr protein in Bradyrhizobium japonicum. J. Bacteriol. 191:1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stojiljkovic I, Hantke K. 1992. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB-dependent systems in gram-negative bacteria. EMBO J. 11:4359–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Todd JD, Sawers G, Rodionov DA, Johnston AW. 2006. The Rhizobium leguminosarum regulator IrrA affects the transcription of a wide range of genes in response to Fe availability. Mol. Genet. Genomics 275:564–577 [DOI] [PubMed] [Google Scholar]
- 28. Todd JD, et al. 2002. RirA, an iron-responsive regulator in the symbiotic bacterium Rhizobium leguminosarum. Microbiology 148:4059–4071 [DOI] [PubMed] [Google Scholar]
- 29. Vanderpool CK, Armstrong SK. 2003. Heme-responsive transcriptional activation of Bordetella bhu genes. J. Bacteriol. 185:909–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Viguier C, Cuiv PO, Clarke P, O'Connell M. 2005. RirA is the iron response regulator of the rhizobactin 1021 biosynthesis and transport genes in Sinorhizobium meliloti 2011. FEMS Microbiol. Lett. 246:235–242 [DOI] [PubMed] [Google Scholar]
- 31. Wandersman C, Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611–647 [DOI] [PubMed] [Google Scholar]
- 32. Wexler M, et al. 2001. The Rhizobium leguminosarum tonB gene is required for the uptake of siderophore and haem as sources of iron. Mol. Microbiol. 41:801–816 [DOI] [PubMed] [Google Scholar]
- 33. Yang J, Panek HR, O'Brian MR. 2006. Oxidative stress promotes degradation of the Irr protein to regulate haem biosynthesis in Bradyrhizobium japonicum. Mol. Microbiol. 60:209–218 [DOI] [PubMed] [Google Scholar]
- 34. Yang J, et al. 2006. Bradyrhizobium japonicum senses iron through the status of haem to regulate iron homeostasis and metabolism. Mol. Microbiol. 60:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.