Abstract
Each cell hosts thousands of proteins that vary greatly in abundance, structure, and chemical properties. To ensure that all proteins are biologically active and properly localized, efficient quality control systems have evolved. While the structure, function, and regulation of some individual protein folding factors and proteases were resolved up to atomic resolution, others remain poorly characterized. In addition, little is known about which factors are required for viability under specific stress conditions. We therefore determined the physiological implications of 15 factors of the E. coli cell envelope by an integrated genetic approach comprising phenotypic analyses. Our data indicate that surA and tsp null mutations are a lethal combination in rich medium, that surA dsbA and surA dsbC double mutants are temperature sensitive, and that surA ptrA, surA yfgC, dsbA fkpA, degP tsp, degP ppiD, tsp ppiD, and degP dsbA double mutants are temperature sensitive in rich medium containing 0.5 M NaCl, while degP dsbA, degP yfgC, tsp ydgD, and degP tsp double mutants do not grow in the presence of SDS/EDTA. Furthermore, we show that in degP dsbA, degP tsp, and degP yfgC double mutants a subpopulation of LamB exists as unfolded monomers. In addition, dsbA null mutants expressed lower levels of the outer membrane proteins LptD, LamB, FhuA, and OmpW while FhuA levels were reduced in surA single and degP ppiD double mutants. Lower FhuA levels in degP ppiD strains depend on Tsp, since in a tsp degP ppiD triple mutant FhuA levels are restored.
INTRODUCTION
The cell envelope of the Gram-negative bacterium Escherichia coli represents an experimental model system to address the physiological implications of protein quality control involving protein diagnosis, repair, and turnover (45). Protein quality control systems are mainly comprised of unfolded protein response signal transduction pathways, molecular chaperones, folding catalysts, and proteases. Chaperones recognize exposed hydrophobicity of proteins present in a nonnative state, thereby discriminating against native proteins. Two factors, Skp and Spy, are general molecular chaperones in the E. coli periplasm. Skp function has so far been mainly attributed to the folding and assembly of outer membrane proteins (49), and Spy prevents protein aggregation and promotes protein folding (54).
Protein folding catalysts carry out related functions, but in contrast to chaperones they improve slow steps in protein folding by catalyzing disulfide (S-S) bond formation or cis-trans isomerization of proline residues. The periplasmic redox machinery is genetically and biochemically well studied. The main players belong to the Dsb family, which are involved in the formation (DsbA), reduction (DsbC), and isomerization (DsbC and DsbA) of S-S bonds (31, 41). Native substrates include dozens of proteins such as alkaline phosphatase, OmpA, heat-stable enterotoxins, LamB, periplasmic binding proteins, pilin proteins, and components of various secretion apparatuses involved in virulence. There are also four proline isomerases in the cell envelope, PpiA, SurA, PpiD, and FkpA. While an involvement in outer membrane biogenesis has been described for some proline isomerases, their exact physiological implications remain to be determined (55).
Proteases promote protein turnover by catalyzing the cleavage of peptide bonds. There are at least 24 proteolytic enzymes in the cell envelope of E. coli. While a few are well studied, many have only been identified via bioinformatics (36). For example, DegP and DegQ are members of the widely conserved HtrA family of serine proteases that are implicated in the tolerance against various folding stresses, including bacterial pathogenicity (16, 17). These functions are based on unique features such as the combination of chaperone and protease activities and the reversible switch from the resting hexameric to the active dodecameric or tetracosameric conformations (2, 29, 35, 62, 66). This switch is based on an allosteric mechanism that involves binding of misfolded proteins to the PDZ domain and the active site (34, 44, 46, 62). Interestingly, 12- and 24-meric DegP cages contain folded monomers of mislocalized porins, implicating DegP in the biogenesis of outer membrane proteins (35). There are other factors in the cell envelope that are bifunctional. For example, chaperone activity has been demonstrated for DegQ and the proline isomerase SurA, as well as for the redox factors DsbC and DsbG (7, 15, 40, 65).
Other proteases such as the serine protease Tsp and the metalloprotease PtrA (pitrilysin A) have been implicated in protein quality control. For example, a Tn5 insertion in tsp increases the sensitivity to multiple antibiotics, probably because of a defective outer membrane (64), and degQ is a multicopy suppressor of tsp null mutations (5). In addition, like other PDZ proteases, Tsp prefers unfolded proteins with hydrophobic C termini as substrates (6, 67, 72). Even though less information on PtrA is available, the degradation of misfolded maltose binding protein mutant 31 (8) and β-lactamase (3) suggest a role in protein quality control.
There are four hypothetical proteases in the cell envelope that might be involved in protein quality control. YdgD is a small periplasmic serine protease. It is expressed in tissues of a chicken infection model, and ydgD mutants exhibit increased sensitivity toward the antibiotics ampicillin and cephradine, which target cell wall biosynthesis (19, 38). The predicted metalloproteases YfgC, YcaL, and YggG are homologous proteins belonging to the widely conserved M48 family (56), prominent members of which include the mammalian OMA1 and STE24 proteases (4, 42). The yfgC promoter is regulated by sigma E, i.e., protein folding stress (23, 57), and yfgC mutants are hypersensitive toward multiple antibiotics (24, 38). The other M48 family members, YcaL and YggG, are outer membrane lipoproteins (37, 69). The yggG promoter is upregulated by heat shock (28) and by the Rcs two-component system (25), while insertions within the ycaL gene are lethal in Salmonella enterica (32) but not in E. coli.
Like all other cells, E. coli has developed compartment-specific systems to respond to the presence of misfolded proteins. In the cell envelope, two major regulons have been identified, Sigma E (RpoE) and Cpx (60). Targets of the Sigma E pathway include the degP and fkpA promoters and rpoH, encoding the cytoplasmic heat shock sigma factor, as well as rpoE and its regulators rseABC (55). Among the known protein quality control factors that are regulated by two-component Cpx pathway are the promoters of the degP, dsbA, ppiD, ppiA, and spy genes (55).
To obtain information about the physiological implications of established and hypothetical periplasmic protein quality control factors, we analyzed 15 genes by studying the synthetic phenotypes of combined knockout mutations, membrane integrity, metabolomic profiling, induction of stress response genes, and the biogenesis of outer membrane proteins.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Strains containing null mutations were obtained from the E. coli Genetic Stock Centre, which distributes the Keio collection (1). These mutations were introduced into wild-type (wt) strain BW30270 (E. coli Stock Center no. 7925) by P1 transduction (47) to generate isogenic strains containing the individual and double mutants (see Table S1 in the supplemental material). To verify the constructed strains, Western blot assays or PCR analyses were performed.
Bacterial cultures were routinely grown at 37°C in rich medium containing 10 g/liter NZ-amine A, 5 g/liter Bacto yeast extract, and 10 g/liter NaCl. When required, antibiotics were added to the following final concentrations: ampicillin, 200 μg/ml; kanamycin, 100 μg/ml; tetracycline, 5 μg/ml; and chloramphenicol, 15 μg/ml.
Growth tests.
Bacterial cultures were grown at 37°C in rich medium overnight. Cells (103) were spotted onto rich medium agar plates containing either 0.5% SDS and 0.5 mM EDTA or 500 mM NaCl. Plates were incubated at various temperatures.
Assay of membrane permeability: β-galactosidase assays.
Membrane defects were determined by assaying levels of β-galactosidase in the supernatant of the growth medium. β-Galactosidase assays were performed as described previously (20), except using the supernatant instead of cell lysate. Cells were grown in rich medium, and the wt lac operon was induced with 50 μM IPTG (isopropyl-β-d-thiogalactopyranoside) and grown further until mid-log phase. Cells (5 × 108) were harvested by centrifugation (4,000 × g, 10 min, 4°C), and 50 μl of the supernatant was mixed with 100 μl ONPG (o-nitrophenyl-β-d-galactopyranoside) (4 mg/ml). β-Galactosidase activities are expressed as Δ(OD420/t)/(OD600 × V), where OD420 and OD600 are the optical densities at 420 and 600 nm, respectively, and V refers to the volume of supernatant of the culture.
Analyses of outer membrane proteins.
Outer membrane fractions were isolated from cells grown in rich medium until mid-log phase. Cells were harvested by centrifugation, washed with 100 mM NaCl, and resuspended in 10 mM HEPES, pH 7.4. After DNase digestion, cells were broken and cell debris was removed by centrifugation (10 min, 16,000 × g). Membrane fractions were pelleted by an additional centrifugation step (45 min, 120,000 × g). The pellet was resuspended in buffer (10 mM HEPES, pH 7.4, 2% [wt/vol] lauryl sarcosine) and stirred overnight at 4°C. The outer membrane fraction was pelleted by ultracentrifugation (45 min, 120,000 × g), washed twice, and resuspended in 10 mM HEPES, pH 7.4.
For detection of unfolded LamB monomer, cells were grown until mid-log phase in liquid rich medium at 37°C and gentle lysis was performed (69a).
Phenotype microarray assays.
Phenotype microarrays were performed at Biolog, Inc., Hayward, CA, as described previously (9).
RNA extraction and real-time quantitative PCR (RT-qPCR).
For extraction of total RNA, cells were grown in M9 minimal medium until mid-log phase (47). RNA was stabilized, isolated, and DNase digested using the RNeasy kit (Qiagen) according to the manufacturer's protocol. RNA (1 μg) was used for cDNA synthesis using the First Strand cDNA synthesis kit (Fermentas). cDNA (45 ng) was quantified on Rotor-Gene 3000 (Corbett Research) by using Absolute QPCR SYBR green mix (Thermo). After an initial 15-min activation step at 94°C, 45 cycles (94°C for 15 s, 56°C for 30 s, 72°C for 30 s, 78°C for 15 s, and 84°C for 15 s) were performed. A melting curve analysis was performed at the end of cycling to ensure that there was single amplification. Transcript levels were normalized to the level of gapA, and quantitation analysis was performed as described previously (51). Assays were done with two biological and three statistical replicates, and standard deviations were calculated.
RESULTS
Identification of synthetic phenotypes of double-null mutants.
To obtain in vivo evidence for the functional importance of protein quality control factors of the E. coli cell envelope, we constructed 113 isogenic derivatives of wt strain BW30270, i.e., 15 single mutants and 98 double mutants. The single-knockout mutants were those with deletions of the ppiA, surA, ppiD, fkpA, dsbA, dsbC, and skp genes, encoding folding factors, and deletions of the degP, degQ, ptrA, tsp, ydgD, yfgC, ycaL, and yggG genes, encoding proteases. Of those, double mutant strains were constructed in the most possible combinations, except that a dsbA dsbC deletion strain was not constructed because the biology of the disulfide bond formation system is well understood (31), nor were strains with double deletions of the ppiA, surA, ppiD, and fkpA genes constructed, because previous work suggested that the phenotypes of the quadruple-null mutant were very similar to those of the individual surA null mutant (30). The constructed strains were used to test growth on rich medium plates at 28, 37, and 43°C. Phenotype analyses verified published evidence, i.e., that surA degP and surA skp double mutations are synthetic lethal combinations (58). In addition, surA tsp double mutations were found to be synthetically lethal (Table 1). In addition, dsbA surA and dsbC surA double mutants were inviable at 43°C and exhibited reduced growth at 37°C. Table 1 lists only those double mutants that have additional phenotypes compared to the corresponding single-null mutants. A complete list of all mutants generated and their phenotypes is provided in Table S2 in the supplemental material.
Table 1.
Growth of cells lacking various folding factors and proteases under different conditionsa
| Strain or relevant genotype | Growth of cells under indicated condition(s) |
||||||
|---|---|---|---|---|---|---|---|
| Temp (°C) |
0.5 mM NaCl |
SDS/EDTA |
|||||
| 28 | 37 | 43 | 37°C | 42°C | 37°C | 42°C | |
| BW30270 (wt) | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| surA | +++ | +++ | +++ | ++ | + | − | − |
| ppiD | +++ | +++ | +++ | +++ | +++ | + | + |
| fkpA | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| ppiA | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| skp | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| dsbA | +++ | +++ | +++ | ++ | + | + | + |
| dsbC | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| degP | +++ | +++ | − | +++ | +++ | + | − |
| degQ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| tsp | +++ | +++ | +++ | ++ | + | + | + |
| ptrA | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| yfgC | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| ydgD | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| ycaL | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| yggG | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| surA degP | ND | ND | ND | ND | ND | ND | ND |
| surA skp | ND | ND | ND | ND | ND | ND | ND |
| surA tsp | ND | ND | ND | ND | ND | ND | ND |
| surA ptrA | +++ | +++ | +++ | ++ | − | − | − |
| surA yfgC | +++ | +++ | +++ | ++ | − | − | − |
| surA dsbA | +++ | ++ | − | ++ | + | − | − |
| surA dsbC | +++ | ++ | − | ++ | + | − | − |
| degP yfgC | +++ | +++ | − | +++ | +++ | − | − |
| tsp ydgD | +++ | +++ | +++ | ++ | + | − | − |
| tsp ppiD | +++ | +++ | +++ | ++ | − | + | + |
| degP ppiD | +++ | +++ | − | ++ | − | + | − |
| degP tsp | +++ | +++ | − | ++ | − | − | − |
| dsbA degP | +++ | +++ | − | ++ | − | − | − |
| dsbA fkpA | +++ | +++ | +++ | ++ | − | + | + |
Sizes of cell colonies were determined following growth on (i) rich-medium agar plates, (ii) hyperosmolar rich-medium agar plates containing 500 mM NaCl, and (iii) rich-medium agar plates containing 0.5% SDS plus 0.5 mM EDTA following overnight incubation at the temperatures indicated. +++, growth comparable to that of wt strain BW30270; ++, weak growth; +, minimal growth; −, no growth; ND, not done because these mutations were lethal combinations. The phenotypes of all strains constructed are provided in Table S2 in the supplemental material.
Stress conditions.
We subsequently tested growth of the single and double mutants under conditions causing protein folding problems, including hyperosmolarity and sensitivity toward SDS/EDTA in combination with elevated temperatures. On plates containing 500 mM NaCl (Table 1), surA, dsbA, and tsp single mutants showed reduced growth at 37°C, indicating that these factors are individually important for growth under salt stress. In addition, the ppiD degP double mutant showed reduced growth at 37°C. At 42°C, surA, dsbA, and tsp single mutants displayed severely reduced growth. Of the double-null mutations, surA ptrA, surA yfgC, tsp ppiD, degP ppiD, degP tsp, dsbA degP, and dsbA fkpA were synthetic lethal combinations.
Media containing SDS and EDTA are commonly used to detect defective cell envelopes (7). Therefore, strains were grown on rich-medium plates containing 0.5% SDS and 0.5 mM EDTA at 37 and 42°C. Under these conditions, surA single mutants and their corresponding double mutants were inviable. degP mutants showed reduced growth at 37°C and no growth at 42°C. In addition, dsbA, ppiD, and tsp single mutants showed reduced growth at 37 and 42°C. Furthermore, dsbA degP, tsp degP, yfgC degP, and ydgD tsp were synthetic lethal combinations at 37°C and ydgD tsp was synthetically lethal at 42°C (Table 1). Since dsbA degP and tsp degP were also lethal combinations when cells were grown in hyperosmolar media at 42°C, these data suggest that DsbA, DegP, and Tsp contribute to survival under stress conditions.
Metabolic profiling.
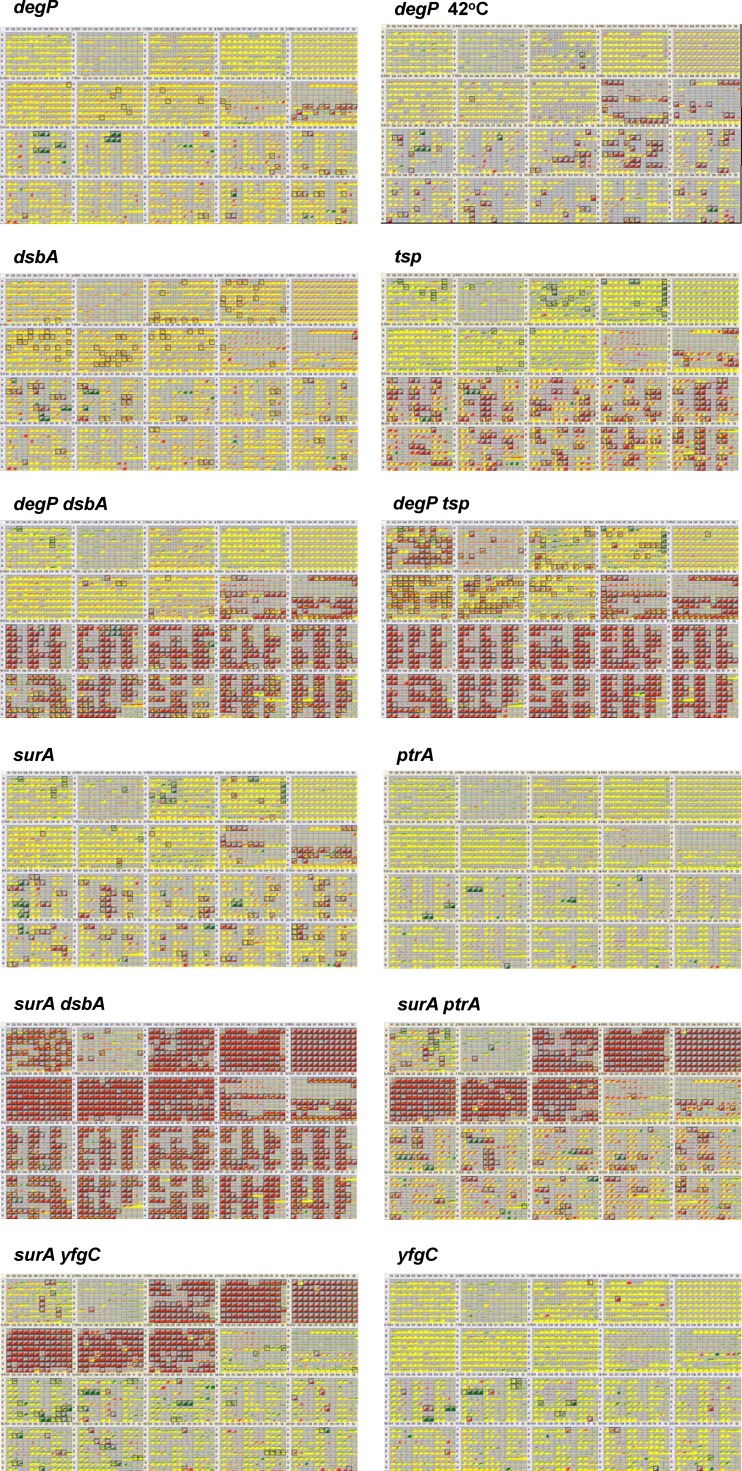
To extend the phenotypic analyses, global metabolic profiling was performed using phenotype microarrays examining 1,920 different growth conditions for each mutant strain (9). Based on the data generated so far, we selected a set of 10 single mutants (surA, fkpA, ppiD, dsbA, dsbC, degP, tsp, ydgD, yfgC, and ptrA mutants) and 12 double mutants (dsbA surA, dsbC surA, ptrA surA, yfgC surA, dsbA degP, tsp degP, yfgC degP, ppiD degP, ydgD tsp, ppiD tsp, fkpA tsp, and dsbA fkpA mutants) for phenotype microarray analyses that were performed at 37°C (Fig. 1; see also Fig. S1 in the supplemental material). Among the single mutants, the tsp mutant exhibited the most severe phenotypes. The tsp mutant was particularly sensitive to pH and chemicals (antimicrobials and dyes) and somewhat sensitive to medium osmolarity and ions. This phenotype is qualitatively similar to phenotype microarray data obtained for degP mutants grown at 42°C. However, at 37°C, a degP mutant displayed almost no significant phenotypes. The surA single mutant was hypersensitive to KCl, NaCl, and Na2SO4 as well as to folate antagonists, macrolides, and rifamycin/rifampin. The macrolide hypersensitivity is also observed in the yfgC mutant. The dsbA mutant displayed scattered phenotypes.
Fig 1.
Global metabolic profiling. Phenotype microarrays of selected knockout mutants (comparison of wt and mutant strains) grown at 37°C unless otherwise indicated. Yellow indicates metabolic activity of the mutant comparable to that of the wt strain, green indicates better metabolic activity than wt, and red indicates reduced metabolic activity compared to wt. In each panel of 20 rectangles, each rectangle represents a 96-well plate subjected to various growth conditions. Top row (from the left): the first two plates show carbon sources, followed by N, P, S, and nutrient supplements. Second row from top: the first three plates show N sources followed by osmotic compounds and ions (2 plates). In the lower two rows, the sensitivity to chemicals (detergents, antibiotics, dyes, etc.) is tested. Boxes indicate wells where the metabolic activity of the mutant is significantly different from that of the wt. The complete results of all phenotype microarray experiments are provided in Fig. S1 in the supplemental material.
Of the double mutants, the surA dsbA mutant had the strongest phenotypes, as most growth conditions were negatively affected, confirming the original observation that this double mutant is synthetically lethal on rich medium plates at 42°C and severely compromised at 37°C (Table 1). While the individual dsbA and degP mutants had only a few phenotypes, the double mutant displayed a severe hypersensitivity toward chemicals, pH, and ions. A similar although even stronger phenotype was observed for the tsp degP double-null strain. In addition to the hypersensitivity against chemicals, pH, and ions, this strain was unable to use many carbon sources and peptides as nitrogen sources. A rather unusual phenotype was observed for ptrA surA and yfgC surA double mutants. These strains did not grow in minimal media (Fig. 1), except when leucine was added to the growth medium. The reason for the Leu auxotrophy is unknown. In addition, ppiD tsp and fkpA tsp double mutants exhibited fewer phenotypes than did the tsp single mutant (see Fig. S1 in the supplemental material), suggesting that ppiD and fkpA null mutants are suppressors of the tsp null mutant under some but not all growth conditions. Together, these in vivo results confirm the physiological importance of the folding factors and proteases investigated.
Membrane permeability.
Since folding factor and protease mutations can cause defects in integral membrane protein biogenesis and are known, for example, to increase sensitivity to antibiotics due to increased membrane permeability, we tested the integrity of the cytoplasmic and outer membranes of mutants that displayed growth defects or synthetic lethality in the assays described above by measuring the release of cytoplasmic β-galactosidase into the growth medium from cells grown in rich medium at 37°C. Since β-galactosidase is a homotetramer of >450 kDa, its release from cells indicates severe membrane defects that could result in cell lysis. About 4% of the expressed β-galactosidase was released into the growth medium in the surA single mutant, while tsp, degP, and dsbA single mutants leaked 4- to 10-fold less β-galactosidase into the growth medium than did the surA mutant. Two double mutants, degP tsp and degP dsbA, leaked twice as much and as much β-galactosidase, respectively, into the medium as the surA mutant. tsp ydgD and tsp fkpA mutants leaked about one-half the amount of β-galactosidase that the surA single mutant leaked, while degP yfgC and dsbA fkpA double mutants leaked at least twice as much β-galactosidase as the corresponding individual single mutants (Table 2). Leakage of β-galactosidase into the growth medium could result from cell lysis, even though others reported membrane permeability (leakage of DNA and proteins) without loss of viability (33).
Table 2.
Leakage of β-galactosidase into the medium of cells lacking folding factorsa
| Strain or relevant genotype | β-Galactosidase activity (μmol/min) |
|---|---|
| Strain BW30270 (wt) | <1 |
| surA | 43 |
| ppiD | <1 |
| fkpA | 1 |
| dsbA | 4 |
| dsbC | <1 |
| tsp | 9 |
| degP | 5 |
| ptrA | 1 |
| yfgC | 2 |
| ydgD | 1 |
| dsbA fkpA | 10 |
| degP dsbA | 41 |
| degP yfgC | 13 |
| degP tsp | 81 |
| surA dsbC | 24 |
| tsp fkpA | 23 |
| tsp ydgD | 24 |
| tsp ppiD | 7 |
Mutant strains were grown to mid-log phase in liquid rich medium at 37°C. Activity of cytoplasmic β-galactosidase in cell culture supernatant was determined as an indicator of a membrane defect. Assays were done with two biological and three statistical replicates, and standard deviation was <40%. Total β-galactosidase activity of cells was on average 970 μmol/min.
Induction of protein stress response genes.
Since folding factors and proteases perform important tasks in protein quality control, their absence is expected to induce promoters of genes that are controlled by unfolded protein response pathways. We therefore tested the induction of the stress genes rpoE and spy, which are members of the sigma E and Cpx regulons and the Cpx and Bae regulons, respectively (50, 52) in surA dsbC, dsbA fkpA, degP dsbA, degP ppiD, degP yfgC, tsp fkpA, tsp ppiD, degP tsp, and tsp ydgD double mutants and the corresponding single mutants. These mutations were chosen because of their phenotypes in the previous assays. All other surA double mutants could not be investigated because they did not grow in minimal medium. Differential expression of the rpoE and spy genes was measured by RT-qPCR after growth of cells in minimal media at 37°C (Table 3).
Table 3.
Induction of rpoE and spy genes in folding factor mutantsa
| Strain or relevant genotype | Fold induction (mean normalized expression) of: |
|
|---|---|---|
| rpoE | spy | |
| BW30270 (wt) | 1.0 | 1.0 |
| surA | 5.0 | 2.8 |
| dsbA | 2.6 | 6.0 |
| degP | 1.1 | 0.9 |
| tsp | 1.3 | 1.4 |
| dsbC | 1.4 | 1.0 |
| fkpA | 1.2 | 1.3 |
| ppiD | 1.2 | 1.0 |
| ydgD | 0.7 | 0.9 |
| yfgC | 1.0 | 0.9 |
| surA dsbC | 3.2 | 6.0 |
| dsbA fkpA | 3.3 | 11.2 |
| degP dsbA | 3.5 | 7.7 |
| degP tsp | 2.3 | 12.9 |
| tsp fkpA | 1.1 | 2.5 |
| tsp ydgD | 2.3 | 4.3 |
| tsp ppiD | 1.8 | 4.9 |
| degP ppiD | 0.8 | 0.9 |
| degP yfgC | 1.2 | 1.1 |
Cells were grown to mid-log phase in liquid minimal medium at 37°C. RNA levels of rpoE and spy were determined by RT-qPCR. Assays were done with two biological and three statistical replicates, and standard deviations were <40%.
The strongest induction of rpoE was observed in surA mutants (factor of 5), confirming earlier observations (7). This effect can be explained by the fact that SurA is involved in the biogenesis of outer membrane proteins and mislocalized outer membrane proteins are the main inducers of the sigma E pathway (60). In addition, the single dsbA null allele (2.6-fold induction) as well as the dsbA fkpA (3.3-fold induction), degP dsbA (3.5-fold induction), and degP tsp (2.3-fold induction) double-null mutations induced rpoE expression. Interestingly, these factors are also involved in outer membrane protein biogenesis (Fig. 2 and 3). Moreover, rpoE expression was induced 2.3-fold in tsp ydgD cells, while the combination of surA and dsbC null mutations decreased rpoE promoter activation compared to that of the single surA mutant from 5- to 3.2-fold. This slightly weaker phenotype was also observed when assaying membrane permeability (Table 2).
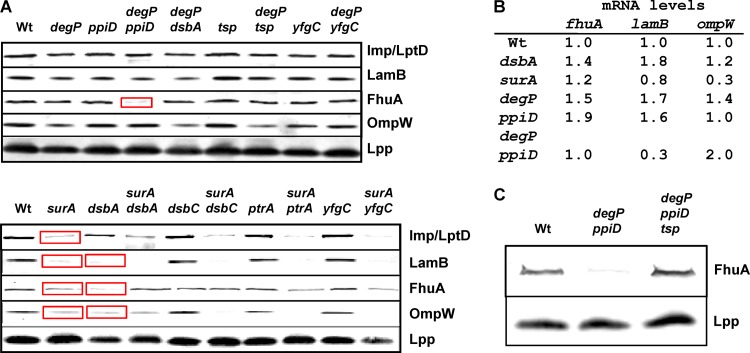
Fig 2.
Outer membrane protein biogenesis. (A) Steady-state levels of various proteins in the outer membrane fraction of indicated mutant strains. Pronounced effects are marked by red boxes. Braun's outer membrane lipoprotein (Lpp) served as a loading control. (B) mRNA levels of fhuA, lamB, and ompW were compared in wt and mutant strains by RT-qPCR as described in Table 3. Standard deviation was below 40%. For comparative and quantitative analysis, transcript levels were normalized to the level of gapA. (C) FhuA levels in various mutant strains. The comparison of FhuA levels in degP ppiD mutants with those in degP ppiD tsp mutants suggests that Tsp degrades FhuA in degP ppiD strains.
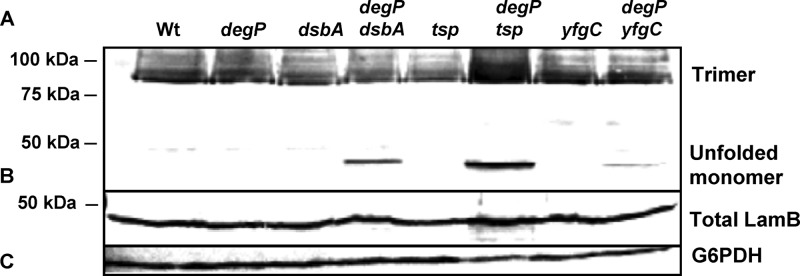
Fig 3.
LamB folding intermediates in various mutant strains. Bacteria were gently lysed and incubated at 37°C for 10 min (A) or heated at 95°C for 5 min (B). The apparent molecular mass is 80 kDa for trimeric LamB and 49 kDa for the unfolded monomer. Glucose-6-P-dehydrogenase served as a loading control (C).
The strongest induction of the spy gene was observed for degP tsp (12.9-fold induction) and for dsbA fkpA (11.2-fold induction) mutants. Other strong inducers of spy included the degP dsbA (7.7-fold induction), dsbA (6-fold induction), surA dsbC (6-fold induction), tsp ydgD (4.3-fold induction), tsp ppiD (4.9-fold induction), and tsp fkpA (2.5-fold induction) mutations, while the surA single-null mutation induced spy expression by a factor of 2.8 (Table 3).
In general, mutations that caused a strong induction of either rpoE or spy also induced the other stress gene as well. The only exception was surA dsbC, causing an activation of spy but a slight reduction of rpoE expression compared to the surA single mutation. In addition, the results of these tests correlate with the phenotypes detected for the double mutants dsbA fkpA (salt stress and membrane permeability), degP dsbA (salt and EDTA/SDS stress and membrane permeability), degP tsp (salt and EDTA/SDS stress and membrane permeability), and tsp ydgD (EDTA/SDS stress and membrane permeability) (Table 1).
Implications of folding factors and proteases in the biogenesis of outer membrane proteins.
Having so far performed global in vivo analyses, we determined the fates of individual proteins in selected single and double mutants (Fig. 2 and 3). We focused on outer membrane proteins because these proteins have been used previously to characterize, for example, surA, degP, and skp mutants (68). First, we isolated outer membrane fractions and determined the abundance of FhuA, Imp (LptD), LamB, OmpA, OmpC, OmpF, OmpW, and PhoE by Western blotting. In the following, we describe only pronounced effects (marked by red boxes in Fig. 2A). We detected that OmpW biogenesis is severely affected in surA single mutants and consequently in the relevant double mutants. OmpW is a conserved monomeric porin forming an 8-stranded β barrel (27). FhuA levels were strongly reduced in degP ppiD double mutants. FhuA, a monomeric porin forming a 22-stranded β barrel, is the receptor for ferrichrome-iron, for the antibiotic albomycin, for several bacteriophages, and for the bacterial toxin colicin M (13, 21). Furthermore, dsbA mutants produced reduced levels of FhuA and of the lipopolysaccharide (LPS) transporter Imp (LptD) (22), the maltoporin LamB (63), and OmpW. These proteins have 4, 4, 2, and 0 Cys residues, respectively, and S-S bonds were described for FhuA, Imp (LptD), and LamB (10, 12, 39). We were also able to reproduce the previously reported phenotypes of the surA mutant, i.e., lower levels of Imp (LptD), FhuA, and LamB (68, 71). These reductions were therefore also seen when the surA mutation was combined with dsbA, dsbC, ptrA, and yfgC mutations. Surprisingly, however, FhuA levels were not reduced in the surA dsbA double mutant. The reason for this effect is unknown, and it cannot be explained by the other phenotypes detected so far. Since Imp (LptD) levels are reduced in dsbA and surA mutants (Fig. 2A), these strains will have reduced LPS levels. Reduced LPS levels will interfere with the biogenesis of outer membrane proteins, the assembly of which depends on LPS such as OmpA, PhoE, and presumably FhuA (14, 18, 21). In addition, we observed other published effects, i.e., reduced levels of LamB in surA and dsbA single mutants and of OmpC and OmpF in surA mutants in outer membrane preparations (data not shown) (53, 59, 68, 71).
Since the expression levels of genes encoding outer membrane proteins can be affected in folding factor mutants (71), we determined the mRNA levels in those strains exhibiting defects in outer membrane protein biogenesis (Fig. 2B). Reduced mRNA levels were observed for lamB and ompW in surA mutants as well as for lamB in degP ppiD double mutants. Therefore, the effects of surA on the levels of LamB and OmpW and the small effect of degP ppiD on LamB levels were most likely indirect. The effects of surA on Imp/LptD and FhuA biogenesis were previously reported to be direct, as mRNA levels were not affected (71). Therefore, protein levels of LamB, FhuA, and OmpW depend on the presence of DsbA, and those of FhuA depend on the presence of DegP, PpiD, and SurA. Furthermore, since FhuA protein levels were reduced in degP ppiD double mutants, we tested whether FhuA is degraded by Tsp under these conditions. Western blotting confirmed this hypothesis, i.e., FhuA levels are increased in degP ppiD tsp triple mutants compared to the degP ppiD double mutants (Fig. 2C).
In a second set of experiments, we analyzed the levels of LamB in whole-cell lysates to detect the presence of unfolded monomers in the periplasm (Fig. 3). Significant amounts of unfolded monomers were detected in degP dsbA and degP tsp strains, while traces of unfolded monomers were observed in degP yfgC strains.
DISCUSSION
A systematic genetic approach involving 15 conserved genes was used to determine the phenotypes of single and double mutants by assaying 7 growth conditions (Table 1), membrane integrity (Table 2), and induction of unfolded protein response genes (Table 3) and by metabolomic profiling (Fig. 1). These results reveal a role for the to-date poorly characterized periplasmic proteases YfgC and YdgD in protein quality control (see the phenotypes of the corresponding mutants listed below). While our data confirm the synthetic lethal phenotypes of surA degP and surA skp mutations, surA tsp was identified as an additional lethal combination. Furthermore, 14 synthetic lethal phenotypes were detected under specific growth conditions: surA dsbA (43°C), surA dsbC (43°C), surA ptrA (0.5 M NaCl at 42°C), surA yfgC (0.5 M NaCl at 42°C), dsbA fkpA (0.5 M NaCl at 42°C), degP dsbA (0.5 M NaCl at 42°C and SDS/EDTA at 37°C), degP yfgC (SDS/EDTA at 37°C), degP tsp (0.5 M NaCl at 42°C and SDS/EDTA at 37°C), degP ppiD (0.5 M NaCl at 42°C), tsp ppiD (0.5 M NaCl at 42°C), and tsp ydgD (SDS/EDTA at 37°C and 42°C) double mutants (Table 1). Future studies are required to understand the reasons for these phenotypes. However, since dsbA fkpA, degP dsbA, degP yfgC, degP tsp, and tsp ydgD mutants release β-galactosidase into the growth medium, presumably because of cell lysis (Table 2), a membrane defect could be responsible for the synthetic lethality under the stress conditions described above.
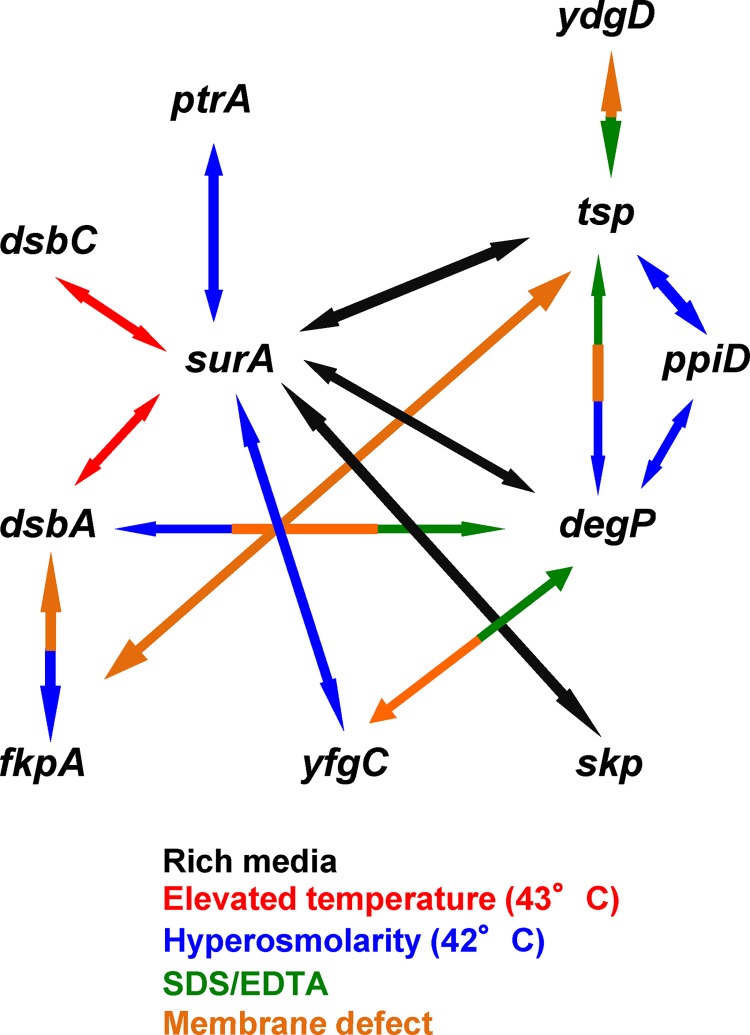
The graphic representation of the detected negative synthetic phenotypes (Fig. 4) illustrates that the combinatory effects of single mutations vary significantly depending on the growth (stress) conditions. These results suggest that under each specific stress condition tested, a specific set of protein quality control factors is critical for cell fate. Furthermore, the relative importance of individual factors can be deduced by simply counting the number of synthetic phenotypes for each gene. Based on these criteria, surA (7 negative synthetic combinations with other single mutations), degP (5 such combinations), tsp (5 combinations), and dsbA (3 combinations) appear to be more important than yfgC (2 combinations), fkpA (2 combinations), and dsbC, ptrA, skp, and ydgD (1 combination) or degQ, ppiA, ycaL, and yggG (none).
Fig 4.
Overview of synthetic phenotypes. Arrows indicate synthetic negative phenotypes of the double mutants (Tables 1 and 2). Colors represent growth conditions: black, rich medium; red, high temperature; blue, hyperosmolarity in combination with high temperature; green, SDS/EDTA; and orange, membrane defect.
Metabolomic analyses monitoring 1,920 growth conditions in a single experiment revealed the global importance of cell envelope folding factors for cell growth (Fig. 1). For example, tsp single and degP dsbA and surA dsbA double mutants are highly sensitive to chemicals, including antibiotics and dyes, confirming earlier reports for tsp (64). Also, the surA yfgC and surA ptrA double mutants are leucine auxotrophs, a phenomenon that remains to be understood but might be related to the previously described partial isoleucine and valine auxotrophy of cpx mutants (43).
Since a phenotype depends on the altered function of a gene product, we initiated a search for individual substrates of folding factors and proteases (Fig. 2 and 3). We concentrated on outer membrane proteins, as this class of cell envelope proteins has been previously investigated (11, 48, 61). In agreement with published evidence, we detected a strong phenotype of the surA single mutation, i.e., decreased levels of LamB, OmpC, and OmpF (53, 59, 71). In addition, our data reveal that a subpopulation of LamB exists as unfolded monomers in degP dsbA, degP tsp, and degP yfgC double mutants. These unfolded monomers would normally enter the degradation pathway. However, in the protease mutant strains the unfolded monomers are stabilized but remain assembly incompetent.
Furthermore, the dsbA null mutant expressed lower levels of several outer membrane proteins containing Cys residues, including LptD, LamB, and FhuA. Surprisingly, however, OmpW was also affected, even though it does not contain Cys residues. It has been reported previously that other Cys-less proteins such as the porin PhoE and the periplasmic protein MdoG are present at lower levels in dsbA mutants (26). Several explanations for this effect must be considered because these data do not allow us to distinguish between direct and indirect effects. A direct effect could be caused by a chaperone activity of DsbA, which was shown to assist in the refolding of chemically denatured proteins lacking Cys residues (73). Since the model of chaperone activity for DsbA is not widely accepted, alternative models suggest, for example, that defects in the cell envelope of dsbA strains prevent a set of proteins from folding correctly in the periplasm (26, 70). An indirect effect of the dsbA mutation on OmpW levels might be the interference of LPS biogenesis that is caused by a reduction of Imp (LptD) levels. Even though the involvement of LPS in the biogenesis of OmpW is unknown, this scenario seems not unlikely. In any case, because OmpW levels are also reduced in degP tsp double-null strains, our data suggest that the small monomeric porin OmpW does not fold well in the periplasm and might thus represent a suitable model for studying the assisted folding of outer membrane proteins.
It will be interesting to determine in future experiments the underlying molecular mechanisms of the detected lethal phenotypes of single mutants and the synthetic lethality of double mutants that were detected under specific growth conditions. These experiments should be designed to clarify whether lethality results from the loss of function of specific substrates of protein quality control factors, from the accumulation and aggregation of misfolded polypeptides or protein fragments, from alterations in lipid composition or destabilization of the murein layer, or from negative effects on the protein translocation systems for periplasmic and outer membrane proteins or lipopolysaccharides.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the National BioResource Project (NIG, Japan) for E. coli knockout strains, to Jon Beckwith, Volkmar Braun, Xaunxian Peng, Tom Silhavy, and Jan Tommassen for providing antisera, and to Barry Bochner and Melisa Merdanovic for discussions.
M.E. was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 13 April 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bai XC, et al. 2011. Characterization of the structure and function of Escherichia coli DegQ as a representative of the DegQ-like proteases of bacterial HtrA family proteins. Structure 19:1328–1337 [DOI] [PubMed] [Google Scholar]
- 3. Baneyx F, Georgiou G. 1991. Construction and characterization of Escherichia coli strains deficient in multiple secreted proteases: protease III degrades high-molecular-weight substrates in vivo. J. Bacteriol. 173:2696–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrowman J, Michaelis S. 2009. ZMPSTE24, an integral membrane zinc metalloprotease with a connection to progeroid disorders. Biol. Chem. 390:761–773 [DOI] [PubMed] [Google Scholar]
- 5. Bass S, Gu Q, Christen A. 1996. Multicopy suppressors of prc mutant Escherichia coli include two HtrA (DegP) protease homologs (HhoAB), DksA, and a truncated R1pA. J. Bacteriol. 178:1154–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beebe KD, et al. 2000. Substrate recognition through a PDZ domain in tail-specific protease. Biochemistry 39:3149–3155 [DOI] [PubMed] [Google Scholar]
- 7. Behrens S, Maier R, de Cock H, Schmid FX, Gross CA. 2001. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 20:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Betton JM, Sassoon N, Hofnung M, Laurent M. 1998. Degradation versus aggregation of misfolded maltose-binding protein in the periplasm of Escherichia coli. J. Biol. Chem. 273:8897–8902 [DOI] [PubMed] [Google Scholar]
- 9. Bochner BR. 2009. Global phenotypic characterization of bacteria. FEMS Microbiol. Rev. 33:191–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bos C, Braun V. 1997. Specific in vivo thiol-labeling of the FhuA outer membrane ferrichrome transport protein of Escherichia coli K-12: evidence for a disulfide bridge in the predicted gating loop. FEMS Microbiol. Lett. 153:311–319 [DOI] [PubMed] [Google Scholar]
- 11. Bos MP, Robert V, Tommassen J. 2007. Biogenesis of the gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61:191–214 [DOI] [PubMed] [Google Scholar]
- 12. Braun M, Silhavy TJ. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol. Microbiol. 45:1289–1302 [DOI] [PubMed] [Google Scholar]
- 13. Braun V. 2009. FhuA (TonA), the career of a protein. J. Bacteriol. 191:3431–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bulieris PV, Behrens S, Holst O, Kleinschmidt JH. 2003. Folding and insertion of the outer membrane protein OmpA is assisted by the chaperone Skp and by lipopolysaccharide. J. Biol. Chem. 278:9092–9099 [DOI] [PubMed] [Google Scholar]
- 15. Chen J, et al. 1999. Chaperone activity of DsbC. J. Biol. Chem. 274:19601–19605 [DOI] [PubMed] [Google Scholar]
- 16. Clausen T, Kaiser M, Huber R, Ehrmann M. 2011. HTRA proteases: regulated proteolysis in protein quality control. Nat. Rev. Mol. Cell Biol. 12:152–162 [DOI] [PubMed] [Google Scholar]
- 17. Clausen T, Southan C, Ehrmann M. 2002. The HtrA family of proteases. Implications for protein composition and cell fate. Mol. Cell 10:443–455 [DOI] [PubMed] [Google Scholar]
- 18. de Cock H, Brandenburg K, Wiese A, Holst O, Seydel U. 1999. Non-lamellar structure and negative charges of lipopolysaccharides required for efficient folding of outer membrane protein PhoE of Escherichia coli. J. Biol. Chem. 274:5114–5119 [DOI] [PubMed] [Google Scholar]
- 19. Dozois CM, Daigle F, Curtiss R., III 2003. Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc. Natl. Acad. Sci. U. S. A. 100:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ehrmann M, Boos W, Ormseth E, Schweizer H, Larson TJ. 1987. Divergent transcription of the sn-glycerol-3-phosphate active transport (glpT) and anaerobic sn-glycerol-3-phosphate dehydrogenase (glpA glpC glpB) genes of Escherichia coli K-12. J. Bacteriol. 169:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferguson A, Hofmann E, Coulton J, Diederichs K, Welte W. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215–2220 [DOI] [PubMed] [Google Scholar]
- 22. Freinkman E, Chng SS, Kahne D. 2011. The complex that inserts lipopolysaccharide into the bacterial outer membrane forms a two-protein plug-and-barrel. Proc. Natl. Acad. Sci. U. S. A. 108:2486–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerken H, Leiser OP, Bennion D, Misra R. 2010. Involvement and necessity of the Cpx regulon in the event of aberrant beta-barrel outer membrane protein assembly. Mol. Microbiol. 75:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Girgis HS, Hottes AK, Tavazoie S. 2009. Genetic architecture of intrinsic antibiotic susceptibility. PLoS One 4:e5629 doi:10.1371/journal.pone.0005629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagiwara D, et al. 2003. Genome-wide analyses revealing a signaling network of the RcsC-YojN-RcsB phosphorelay system in Escherichia coli. J. Bacteriol. 185:5735–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hiniker A, Bardwell JC. 2004. In vivo substrate specificity of periplasmic disulfide oxidoreductases. J. Biol. Chem. 279:12967–12973 [DOI] [PubMed] [Google Scholar]
- 27. Hong H, Patel DR, Tamm LK, van den Berg B. 2006. The outer membrane protein OmpW forms an eight-stranded beta-barrel with a hydrophobic channel. J. Biol. Chem. 281:7568–7577 [DOI] [PubMed] [Google Scholar]
- 28. Huang Y, et al. 2008. Expression and regulation of the yggG gene of Escherichia coli. Curr. Microbiol. 56:14–20 [DOI] [PubMed] [Google Scholar]
- 29. Jiang J, et al. 2008. Activation of DegP chaperone-protease via formation of large cage-like oligomers upon binding to substrate proteins. Proc. Natl. Acad. Sci. U. S. A. 105:11939–11944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Justice SS, et al. 2005. Periplasmic peptidyl prolyl cis-trans isomerases are not essential for viability, but SurA is required for pilus biogenesis in Escherichia coli. J. Bacteriol. 187:7680–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kadokura H, Beckwith J. 2010. Mechanisms of oxidative protein folding in the bacterial cell envelope. Antioxid. Redox Signal. 13:1231–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knuth K, Niesalla H, Hueck CJ, Fuchs TM. 2004. Large-scale identification of essential Salmonella genes by trapping lethal insertions. Mol. Microbiol. 51:1729–1744 [DOI] [PubMed] [Google Scholar]
- 33. Komanapalli IR, Lau BH. 1996. Ozone-induced damage of Escherichia coli K-12. Appl. Microbiol. Biotechnol. 46:610–614 [DOI] [PubMed] [Google Scholar]
- 34. Krojer T, Sawa J, Huber R, Clausen T. 2010. HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat. Struct. Mol. Biol. 17:844–852 [DOI] [PubMed] [Google Scholar]
- 35. Krojer T, et al. 2008. Structural basis for the regulated protease and chaperone function of DegP. Nature 453:885–890 [DOI] [PubMed] [Google Scholar]
- 36. Kucz N, Meltzer M, Ehrmann M. 2006. Periplasmic proteases, p 150–170 In Ehrmann M. (ed), The periplasm. ASM Press, Washington, DC [Google Scholar]
- 37. Lee EY, et al. 2007. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7:3143–3153 [DOI] [PubMed] [Google Scholar]
- 38. Liu A, et al. 2010. Antibiotic sensitivity profiles determined with an Escherichia coli gene knockout collection: generating an antibiotic bar code. Antimicrob. Agents Chemother. 54:1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luckey M, Ling R, Dose A, Malloy B. 1991. Role of a disulfide bond in the thermal stability of the LamB protein trimer in Escherichia coli outer membrane. J. Biol. Chem. 266:1866–1871 [PubMed] [Google Scholar]
- 40. Malet H, et al. 2012. Binding of substrate proteins inside the molecular cage of the chaperone-protease DegQ. Nat. Struct. Mol. Biol. 19:152–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mamathambika BS, Bardwell JC. 2008. Disulfide-linked protein folding pathways. Annu. Rev. Cell Dev. Biol. 24:211–235 [DOI] [PubMed] [Google Scholar]
- 42. McBride H, Soubannier V. 2010. Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr. Biol. 20:R274–R276 [DOI] [PubMed] [Google Scholar]
- 43. McEwen J, Silverman P. 1980. Mutations in genes cpxA and cpxB of Escherichia coli K-12 cause a defect in isoleucine and valine syntheses. J. Bacteriol. 144:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meltzer M, et al. 2008. Allosteric activation of HtrA protease DegP by stress signals during bacterial protein quality control. Angew. Chem. Int. Ed. Engl. 47:1332–1334 [DOI] [PubMed] [Google Scholar]
- 45. Merdanovic M, Clausen T, Kaiser M, Huber R, Ehrmann M. 2011. Protein quality control in the bacterial periplasm. Annu. Rev. Microbiol. 65:149–168 [DOI] [PubMed] [Google Scholar]
- 46. Merdanovic M, et al. 2010. Determinants of structural and functional plasticity of a widely conserved protease chaperone complex. Nat. Struct. Mol. Biol. 17:837–843 [DOI] [PubMed] [Google Scholar]
- 47. Miller J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 48. Mogensen J, Otzen D. 2005. Interactions between folding factors and bacterial outer membrane proteins. Mol. Microbiol. 57:326–346 [DOI] [PubMed] [Google Scholar]
- 49. Muller M, Koch HG, Beck K, Schafer U. 2001. Protein traffic in bacteria: multiple routes from the ribosome to and across the membrane. Prog. Nucleic Acid Res. Mol. Biol. 66:107–157 [DOI] [PubMed] [Google Scholar]
- 50. Mutalik VK, Nonaka G, Ades SE, Rhodius VA, Gross CA. 2009. Promoter strength properties of the complete sigma E regulon of Escherichia coli and Salmonella enterica. J. Bacteriol. 191:7279–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45 doi:10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Price NL, Raivio TL. 2009. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191:1798–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pugsley AP. 1993. A mutation in the dsbA gene coding for periplasmic disulfide oxidoreductase reduces transcription of the Escherichia coli ompF gene. Mol. Gen. Genet. 237:407–411 [DOI] [PubMed] [Google Scholar]
- 54. Quan S, et al. 2011. Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nat. Struct. Mol. Biol. 18:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Raivio TL, Silhavy TJ. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591–624 [DOI] [PubMed] [Google Scholar]
- 56. Rawlings ND, Barrett AJ, Bateman A. 2010. MEROPS: the peptidase database. Nucleic Acids Res. 38:D227–D233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rhodius V, Suh W, Nonaka G, West J, Gross C. 2006. Conserved and variable functions of the sigmaE stress response in related genomes. PLoS Biol. 4:e2 doi:10.1371/journal.pbio.0040002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rizzitello AE, Harper JR, Silhavy TJ. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J. Bacteriol. 183:6794–6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rouviere PE, Gross CA. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170–3182 [DOI] [PubMed] [Google Scholar]
- 60. Rowley G, Spector M, Kormanec J, Roberts M. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4:383–394 [DOI] [PubMed] [Google Scholar]
- 61. Ruiz N, Kahne D, Silhavy T. 2006. Advances in understanding bacterial outer-membrane biogenesis. Nat. Rev. Microbiol. 4:57–66 [DOI] [PubMed] [Google Scholar]
- 62. Sawa J, et al. 2011. Molecular adaptation of the DegQ protease to exert protein quality control in the bacterial cell envelope. J. Biol. Chem. 286:30680–30690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schirmer T, Keller T, Wang Y, Rosenbusch J. 1995. Structural basis for sugar translocation through maltoporin channels at 3.1 A resolution. Science 267:512–514 [DOI] [PubMed] [Google Scholar]
- 64. Seoane A, Sabbaj A, McMurry LM, Levy SB. 1992. Multiple antibiotic susceptibility associated with inactivation of the prc gene. J. Bacteriol. 174:7844–7847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shao F, Bader MW, Jakob U, Bardwell JC. 2000. DsbG, a protein disulfide isomerase with chaperone activity. J. Biol. Chem. 275:13349–13352 [DOI] [PubMed] [Google Scholar]
- 66. Shen QT, et al. 2009. Bowl-shaped oligomeric structures on membranes as DegP's new functional forms in protein quality control. Proc. Natl. Acad. Sci. U. S. A. 106:4858–4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Silber KR, Keiler KC, Sauer RT. 1992. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc. Natl. Acad. Sci. U. S. A. 89:295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sklar J, Wu T, Kahne D, Silhavy T. 2007. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 21:2473–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tokuda H, Matsuyama S-I, Tanaka-Masuda K. 2006. Structure, function, and transport of lipoproteins in Escherichia coli, p 67–79 In Ehrmann M. (ed), The periplasm. ASM Press, Washington, DC [Google Scholar]
- 69a. Ureta AR, Endres RG, Wingreen NS, Silhavy TJ. 2007. Kinetic analysis of the assembly of the outer membrane protein LamB in Escherichia coli mutants each lacking a secretion or targeting factor in a different cellular compartment. J. Bacteriol. 189:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vertommen D, et al. 2008. The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner. Mol. Microbiol. 67:336–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vertommen D, Ruiz N, Leverrier P, Silhavy TJ, Collet JF. 2009. Characterization of the role of the Escherichia coli periplasmic chaperone SurA using differential proteomics. Proteomics 9:2432–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weski J, et al. 2012. Chemical biology approaches reveal conserved features of a C-terminal processing PDZ protease. Chembiochem 13:402–408 [DOI] [PubMed] [Google Scholar]
- 73. Zheng WD, Quan H, Song JL, Yang SL, Wang CC. 1997. Does DsbA have chaperone-like activity? Arch. Biochem. Biophys. 337:326–331 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.