TEXT
His-Asp phosphorylation pathways (also known as two-component systems) enable bacteria (and primitive eukaryotic cells and/or eukaryotic organelles) to make important adaptive decisions in response to fluctuations in intra- or extracellular chemical and/or physical conditions. These sensory systems are composed of a dimeric sensor protein, with an input domain and histidine kinase domain (HK), and a response regulator (RR). The HK first autophosphorylates on a conserved histidine residue (His) and subsequently loses the phosphate to an aspartic acid residue (Asp) on the RR receiver domain. While most RRs additionally carry an output domain that is activated by phosphorylation of the aforementioned Asp in the receiver domain, other RRs are single-domain proteins, where phosphorylation modulates their interaction with other proteins (4). His-Asp systems have been appropriated for remarkably diverse adaptive responses in bacterial physiology, ranging from cell cycle progression, morphogenesis, and virulence to adaptation to specific and/or general stress conditions.
Most knowledge on general stress response signaling stems from research in two model systems: the Gram-negative gammaproteobacterium Escherichia coli and the Gram-positive member of the Firmicutes Bacillus subtilis. Recently, the sensory pathway of the alphaproteobacterial general stress response has been investigated and the components identified, primarily in the cell cycle model system Caulobacter crescentus and its symbiotic relatives. Here, the PhyK HK and the PhyR RR are responsible for the activation of an alternative EcfG-like sigma factor (σT) through a partner-switching mechanism with the σT antagonist NepR (1, 2, 5, 7). Activation of PhyK is triggered by osmotic and oxidative stress and requires a specific Cys residue in the periplasmic domain of PhyK, although the precise mechanism by which PhyK senses the stress remains to be elucidated (11). After accepting a phosphoryl group from PhyK, phosphorylated PhyR (PhyR∼P) acts as a sigma factor decoy that snatches NepR from the NepR-σT complex, thus releasing σT and enabling the formation of a σT-RNA polymerase holoenzyme (EσT) that can then activate transcription of the σT regulon (1, 2, 5, 7). Remarkably, the attraction of NepR for PhyR∼P arises from its infatuation with the σ-like shape (fold) in the PhyR output domain. Whereas PhyR and NepR homologs have also been identified in other alphaproteobacteria, such as Sinorhizobium meliloti and Bradyrhizobium japonicum, so far a PhyK HK has been characterized only in C. crescentus (11).
Complex HK-RR relationships exist in several His-Asp sensory systems. While HK-RRs usually form solitary pairs, they can also lie vertically to form a regulatory cascade composed of sequentially acting HK-RRs. In an act of infidelity, an HK is bedded horizontally to phosphorylate or dephosphorylate an alternative RR. For example, when E. coli is grown in standard aerobic conditions, the expression of the outer membrane porins OmpC and OmpF is regulated by the osmolarity of the medium through the RR OmpR, which usually pairs with EnvZ. However, under anaerobic conditions, expression of OmpC and OmpF is also modulated by the ArcB HK, an anaeroresponsive sensor that can also phosphorylate OmpR, in addition to its usual partner ArcA (12). Recently, similar relationships have surfaced for the E. coli NarX-NarL and NarQ-NarP pairs in response to nitrate and nitrite, with NarQ exhibiting similar phosphotransferase activity toward both NarP and NarL, while NarX remains faithful to NarL (13). In B. subtilis, the PhoP-PhoR pair, which responds to phosphate limitation, and the essential YycF-YycG couple, which plays an important role in cell division and cell membrane and cell wall homeostasis, cross these boundaries. In phosphate limitation-induced stationary phase, the sensor kinase PhoR is able to phosphorylate the YycF response regulator, even in the presence of YycG (8). Finally, the HK component of the BceRS sensory system can hook up with the RR protein of the YvcPQ bacitracin resistance system in B. subtilis (17).
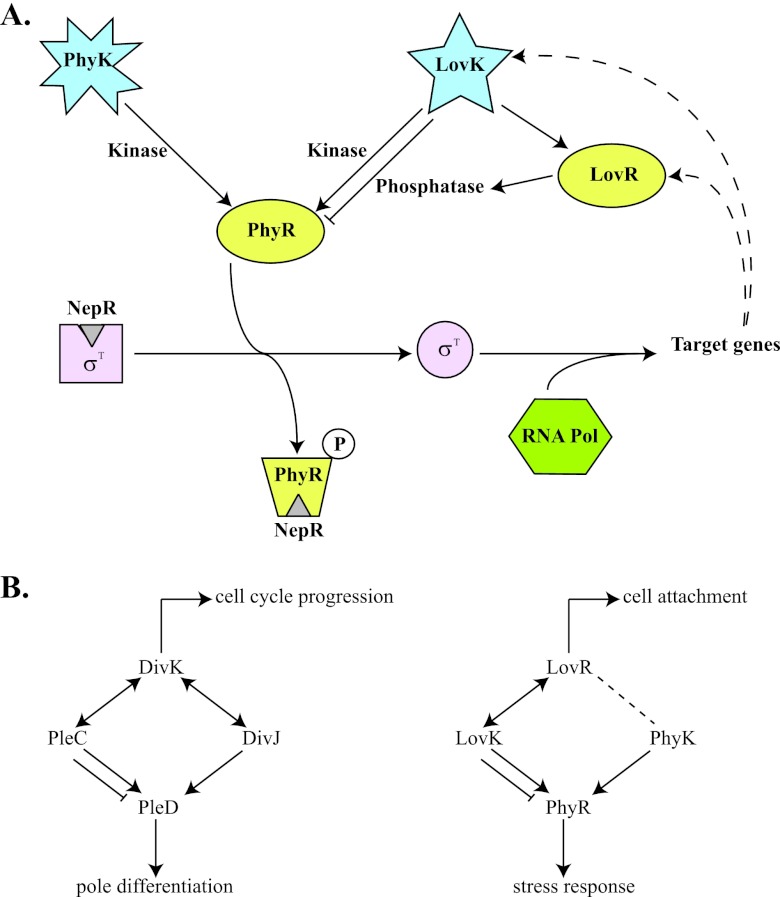
In this issue of Journal of Bacteriology, Foreman et al. (1a) show that the Caulobacter crescentus LovK HK (featuring a LOV [light-oxygen-voltage sensory domain]) can functionally substitute for the general stress HK PhyK (the primary HK for PhyR), at least when its preferred phosphotransfer partner, LovR, a single-domain RR, is absent. This leads to a model in which LovR dictates whether LovK acts as a phosphatase or a kinase for PhyR in wild-type cells. An increase in LovR concentration drains the phosphate from LovK, which in turn dephosphorylates PhyR, enhancing the attenuation of σT-dependent transcription through NepR. Foreman et al. also show that transcription of lovK and lovR is under the control of the EσT (and PhyK-PhyR) and, therefore, that activation of this pathway determines an increase in the levels of LovK and LovR, which in turn attenuates PhyK-PhyR-σT signaling. However, under conditions in which LovR is absent (or present at low levels), LovK seems to transfer phosphate from its HK domain to PhyR, thus increasing the expression of σT-dependent genes independently from PhyK (Fig. 1A).
Fig 1.
(A) PhyR-NepR-EcfG-like sigma factor regulatory pathway in C. crescentus. Under nonstress conditions, the anti-sigma factor NepR binds to σT, preventing its association with RNA polymerase. Upon phosphorylation of PhyR by PhyK (activated by stress conditions) or LovK (under conditions that determine low levels of LovR), PhyR∼P binds to NepR so that σT is released and can recruit the RNA polymerase complex for the expression of target genes (dashed lines indicate that lovK and lovR are among the genes activated by σT). When present at high levels, LovR can act as a sink for phosphate and turn LovK into a phosphatase that would keep PhyR unphosphorylated, which would then result in a decrease of expression for σT-dependent genes. (B) Parallel between the DivK-DivJ-PleC-PleD and LovR-LovK-PhyK-PhyR systems. In both cases, a single-domain RR (DivK and LovR), two His kinase-phosphatases (DivJ-PleC and LovK-PhyK), and an RR with output domain that regulate downstream events (PleD and PhyR) are involved. DivK can stimulate the autokinase activity of both DivJ and PleC and determine whether PleC acts as a kinase or a phosphatase. LovR determines if LovK acts as kinase or phosphatase on PhyR, but it is not known whether LovR interacts with PhyK as well. PleD is a substrate for both PleC and DivJ, whereas PhyR is a substrate for both LovK and PhyK. The single-domain RRs not only are a sink for phosphate but can also regulate other processes (cell cycle progression in the case of DivK and cell attachment in the case of LovR).
This relationship between a single-domain RR, two HK phosphatases and an RR with receiver and output domains is reminiscent of what happens between the members of the DivK-DivJ-PleC-PleD developmental phosphosignaling system in the same organism (Fig. 1B). In this case, DivK is a single-domain RR able to stimulate the autokinase activity of both DivJ and PleC, and it is essential to switch PleC from its phosphatase to its autokinase activity during differentiation (14). PleC is an active DivK phosphatase at the new cell pole, whereas DivK and PleD compete for phosphorylation by DivJ (with DivK being the preferred substrate, at least in in vitro assays) at the old pole. However, there is a short window during polar remodeling when DivK, DivJ, PleC, and PleD colocalize at the same pole. At this moment, DivJ actively phosphorylates DivK, which in turn activates the autokinase activity of PleC and as a consequence determines the phosphorylation of PleD (14). In the case of the Lov-Phy pathway, it is possible that under certain (stress) conditions PhyK is not activated, but the levels of LovR are sufficiently low to switch LovK activity from phosphatase to kinase. In this context, it would be interesting to investigate if PhyK is able to phosphorylate LovR as well, or whether LovR has any effect on the activity of LovK and/or PhyK, as this would further modulate the phosphate fluxes between Lov and Phy proteins.
In C. crescentus, the LovK sensor kinase has been shown to respond to blue light (its ATP hydrolysis activity and transautophosphorylation increase upon illumination) and to regulate cell attachment (16). The LOV domain of LovK binds a flavin mononucleotide (FMN) cofactor that forms a transient covalent adduct with a conserved Cys residue upon absorption of blue light (18). The formation of this adduct is favored when the FMN cofactor is in its oxidized state, and the reduction potential of the C. crescentus LovK FMN cofactor is −258 mV, close to the redox potential of the cytoplasm of Gram-negative cells in exponential phase (15). Although the effect of the oxidation state of LOV domains on their ability to respond to light has not been investigated yet, it could be hypothesized that the redox state of the cell can influence the activation of LovK proteins, therefore integrating light and redox stimuli.
Cross talk between the LovK-LovR and PhyK-PhyR systems also underscores the complexity and interconnectivity of the regulation of bacterial responses to different environmental and stress stimuli, which in C. crescentus eventually modulate the expression of about 40 transcription units through the activation of σT (11). In the case of aquatic bacteria like C. crescentus, blue light can be an important signal, as these wavelengths penetrate deep in water, and depth is related to the availability of nutrients. As both the LovK-LovR and PhyK-PhyR-NepR-σT modules are found within the alphaproteobacterial lineage (10), it is certainly conceivable that a similar regulatory topology is operational in this group of related bacteria that occupy very distinct biological niches. In support of this, a kinase with a Pfam:HisKA_2 or a Pfam:HWE domain like PhyK from C. crescentus is found at the same locus encoding phyR, nepR, and σT homologs in symbiotic alphaproteobacteria like S. meliloti, Sinorhizobium fredii NGR234, and Sinorhizobium medicae. The Pfam:HisKA_2 and Pfam:HWE kinase domains are similar to each other but different from the majority of sensor histidine kinases and are typical of sensor histidine kinases involved in the phosphorylation of PhyR (9). Both PhyR and PhyK homologs are conserved but also almost exclusively present (in the case of PhyK) in alphaproteobacteria. Furthermore, another level of complexity that could allow the integration of multiple signals is the presence, at least in some symbionts (S. meliloti, S. fredii NGR234, and S. medicae), of two PhyR and two NepR homologs. However, in species that live in other environments, the outcome for the activation of the LovK-LovR system can be different, like the regulation of virulence in the case of Brucella abortus, where it has been shown that light stimulates infection of host cells (mouse macrophages) through LovK (19).
This is the first time that the LovK-LovR phosphosignaling system has been directly implicated in the regulation of general stress response. Interestingly, in B. subtilis, the LOV domain protein YtvA modulates the general stress response that is mounted by the alternative sigma factor σB (3). Although σB does not belong to the EcfG sigma factor family, a partner-switching mechanism regulated at the level of phosphorylation (and dephosphorylation) on Ser and Thr residues underlies the activation of σB in B. subtilis (reviewed in reference 6), similarly to what happens for σT in C. crescentus. The biochemical activity of YtvA in the stressosome that controls the σB activation pathway through Ser/Thr kinases and phosphatases in B. subtilis remains to be determined, while in C. crescentus a LOV domain has been coopted to control the release of σT via a His-Asp phosphoflux.
Several elements of the pathways that control the general stress response in alphaproteobacteria still have to be identified in order to have a global description of this network. Nevertheless, the available data suggest that similar components and mechanisms (such as two-component systems, including different sensor domains as well as response regulators with or without output modules, and alternative sigma factors together with their anti- and anti-anti-sigma factors) can be integrated in different ways in signaling cascades that eventually drive a general response to a whole set of environmental and stress stimuli.
ACKNOWLEDGMENTS
Funding support is from the Swiss National Science Foundation (31003A_127287), the Human Frontiers Science Program (RGP0051/2010), the Fondation Leenaards, and the University of Geneva.
Footnotes
Published ahead of print 23 March 2012
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1. Bastiat B, Sauviac L, Bruand C. 2010. Dual control of Sinorhizobium meliloti RpoE2 sigma factor activity by two PhyR-type two-component response regulators. J. Bacteriol. 192:2255–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a. Foreman R, Fiebig A, Crosson S. 2012. The LovK-LovR two-component system is a regulator of the general stress pathway in Caulobacter crescentus. J. Bacteriol. 194:3038–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Francez-Charlot A, et al. 2009. Sigma factor mimicry involved in regulation of general stress response. Proc. Natl. Acad. Sci. U. S. A. 106:3467–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaidenko TA, Kim TJ, Weigel AL, Brody MS, Price CW. 2006. The blue-light receptor YtvA acts in the environmental stress signaling pathway of Bacillus subtilis. J. Bacteriol. 188:6387–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao R, Stock AM. 2009. Biological insights from structures of two-component proteins. Annu. Rev. Microbiol. 63:133–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gourion B, et al. 2009. The PhyR-sigma(EcfG) signalling cascade is involved in stress response and symbiotic efficiency in Bradyrhizobium japonicum. Mol. Microbiol. 73:291–305 [DOI] [PubMed] [Google Scholar]
- 6. Hecker M, Pane-Farre J, Volker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236 [DOI] [PubMed] [Google Scholar]
- 7. Herrou J, Foreman R, Fiebig A, Crosson S. 2010. A structural model of anti-anti-sigma inhibition by a two-component receiver domain: the PhyR stress response regulator. Mol. Microbiol. 78:290–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howell A, Dubrac S, Noone D, Varughese KI, Devine K. 2006. Interactions between the YycFG and PhoPR two-component systems in Bacillus subtilis: the PhoR kinase phosphorylates the non-cognate YycF response regulator upon phosphate limitation. Mol. Microbiol. 59:1199–1215 [DOI] [PubMed] [Google Scholar]
- 9. Karniol B, Vierstra RD. 2004. The HWE histidine kinases, a new family of bacterial two-component sensor kinases with potentially diverse roles in environmental signaling. J. Bacteriol. 186:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krauss U, et al. 2009. Distribution and phylogeny of light-oxygen-voltage-blue-light-signaling proteins in the three kingdoms of life. J. Bacteriol. 191:7234–7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lourenço RF, Kohler C, Gomes SL. 2011. A two-component system, an anti-sigma factor and two paralogous ECF sigma factors are involved in the control of general stress response in Caulobacter crescentus. Mol. Microbiol. 80:1598–1612 [DOI] [PubMed] [Google Scholar]
- 12. Matsubara M, Kitaoka SI, Takeda SI, Mizuno T. 2000. Tuning of the porin expression under anaerobic growth conditions by his-to-Asp cross-phosphorelay through both the EnvZ-osmosensor and ArcB-anaerosensor in Escherichia coli. Genes Cells 5:555–569 [DOI] [PubMed] [Google Scholar]
- 13. Noriega CE, Lin HY, Chen LL, Williams SB, Stewart V. 2010. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol. Microbiol. 75:394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paul R, et al. 2008. Allosteric regulation of histidine kinases by their cognate response regulator determines cell fate. Cell 133:452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Purcell EB, McDonald CA, Palfey BA, Crosson S. 2010. An analysis of the solution structure and signaling mechanism of LovK, a sensor histidine kinase integrating light and redox signals. Biochemistry 49:6761–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Purcell EB, Siegal-Gaskins D, Rawling DC, Fiebig A, Crosson S. 2007. A photosensory two-component system regulates bacterial cell attachment. Proc. Natl. Acad. Sci. U. S. A. 104:18241–18246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rietkotter E, Hoyer D, Mascher T. 2008. Bacitracin sensing in Bacillus subtilis. Mol. Microbiol. 68:768–785 [DOI] [PubMed] [Google Scholar]
- 18. Salomon M, Christie JM, Knieb E, Lempert U, Briggs WR. 2000. Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39:9401–9410 [DOI] [PubMed] [Google Scholar]
- 19. Swartz TE, et al. 2007. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science 317:1090–1093 [DOI] [PubMed] [Google Scholar]