Abstract
The authors prospectively examined whether caffeine intake was associated with lower risk of Parkinson disease (PD) in both men and women among 304,980 participants in the National Institutes of Health-AARP Diet and Health Study and whether smoking affected this relation. Multivariate odds ratios and 95% confidence intervals were derived from logistic regression models. Higher caffeine intake as assessed in 1995–1996 was monotonically associated with lower PD risk (diagnosed in 2000–2006) in both men and women. After adjustment for age, race, and physical activity, the odds ratio comparing the highest quintile of caffeine intake with the lowest was 0.75 (95% confidence interval: 0.60, 0.94; Ptrend = 0.005) for men and 0.60 (95% confidence interval: 0.39, 0.91; Ptrend = 0.005) for women. Further adjustment for duration of smoking and analyses carried out among never smokers showed similar results. A joint analysis with smoking suggested that smoking and caffeine may act independently in relation to PD risk. Finally, the authors conducted a meta-analysis of prospective studies and confirmed that caffeine intake was inversely associated with PD risk in both men and women. These findings suggest no gender difference in the relation between caffeine and PD.
Keywords: caffeine, coffee, Parkinson disease, prospective studies, smoking
Lifestyle habits such as coffee drinking (1, 2) and cigarette smoking (1, 3, 4) have been repeatedly linked to a lower risk of Parkinson disease (PD) in many epidemiologic studies. The inverse association of coffee consumption and caffeine intake with PD has been shown in approximately 20 epidemiologic studies, including several prospective cohort studies (5–9). Further epidemiologic and experimental evidence suggests that caffeine may be the active component that underlies this relation (5, 7, 10). It has been hypothesized that caffeine and its major metabolites may protect dopaminergic neurons by antagonizing adenosine A2A receptor (10).
Some questions remain, however, concerning the epidemiologic evidence for a link between caffeine and PD. While the relation is fairly robust among men, it is inconsistent among women (5, 6). Further, in women, a potential interaction with postmenopausal hormone use has been observed (11, 12). Adding complexity, coffee drinkers are more likely to smoke than nondrinkers (13, 14). Previous studies might not have evaluated the potential influence of cigarette smoking on the caffeine-PD relation because of small sample sizes or inadequate data on smoking.
Previously, using data from the National Institutes of Health (NIH)-AARP Diet and Health Study, we reported that smoking duration, rather than intensity, might underlie the strong inverse association between smoking and PD (4). In the current study, we evaluated caffeine intake in relation to PD, focusing on the relation in women and the potential influence of smoking on this relation.
MATERIALS AND METHODS
Study population and PD case identification
The NIH-AARP Diet and Health Study was established in 1995–1996 by the National Cancer Institute to investigate the roles of diet and lifestyle in cancer etiology (15). The cohort comprised 566,401 AARP (formerly known as the American Association of Retired Persons) members aged 50–71 years from 6 US states and 2 metropolitan areas who answered a baseline survey on diet and lifestyle (15). From 2004 to 2006, a follow-up survey was conducted among surviving participants in the cohort to update lifestyle exposures and to ascertain the occurrence of major chronic diseases, including PD.
A total of 318,260 participants (187,499 men and 130,761 women) responded to the follow-up survey and were thus eligible for the current study. Nonresponders to the follow-up survey were contacted by mail up to 4 times. Compared with follow-up survey participants, those not included in the survey were slightly older (mean age = 61.9 years vs. 61.5 years), reported higher caffeine intake (mean intake = 371 mg/day vs. 360 mg/day), and were more likely to be men (61.3% vs. 58.9%); however, they were less likely to be Caucasians (89.7% vs. 92.5%) and never smokers (30.6% vs. 37.9%) and were less physically active (never or rarely exercised: 22.1% vs. 15.8%). Among women, nonparticipants were less likely to be postmenopausal hormone users (45.5% vs. 51.4%). The follow-up questionnaire asked participants whether they had been diagnosed by a physician as having PD and the year of diagnosis, in the following categories: before 1985, 1985–1994, 1995–1999, or during or after 2000. A total of 2,432 participants reported a PD diagnosis on the follow-up questionnaire.
Because caffeine intake was assessed in 1995–1996 and we were concerned that PD patients might have altered their coffee consumption, even prior to PD diagnosis, we excluded 1,094 potential cases diagnosed before 2000 from the analyses. This left us with a total of 1,338 cases who reported having received a PD diagnosis during or after 2000. We further excluded 209 self-reported cases whose diagnosis was later denied either by the patients themselves or by their treating physicians, 24 cases who had missing information on caffeine intake, and 5 cases with missing data on PD status or errors in reporting. Of participants who did not report a PD diagnosis, we excluded 3,995 with missing data on caffeine intake and 7,953 with missing data on PD status. After these exclusions, we had a total of 1,100 PD cases that had been diagnosed during or after 2000 and 303,880 participants without PD for inclusion in the primary analyses. For PD cases, caffeine intake was assessed at least 4 years before PD diagnosis.
Between 2007 and 2010, we contacted surviving PD patients to confirm the self-reported PD diagnosis. Detailed procedures were published previously (4). Briefly, we asked the patients with self-reported PD to confirm their reports and to permit us to contact their treating physicians. We then asked the treating physicians, mostly neurologists, to complete a diagnostic questionnaire and to send us a copy of the patient’s medical records pertaining to PD diagnosis. The medical records were subsequently reviewed by a movement disorder specialist on the research team (X. H.). A case was confirmed if the diagnosis was confirmed by the treating physician or if the medical record included a final diagnosis of PD or evidence of 2 or more cardinal signs, with one being rest tremor or bradykinesia, a progressive course, responsiveness to dopaminergic treatments, or the absence of features suggesting an alternative diagnosis. Of the 1,069 responses received from physicians, 940 (87.9%) PD diagnoses were confirmed and 129 (12.1%) diagnoses were denied because of uncertainty (n = 62) or misdiagnosis (n = 67). The confirmation rates were similar across years of diagnosis: 83.3% for cases diagnosed before 1985, 92.8% for cases diagnosed in 1985–1994, 88% for cases diagnosed in 1995–1999, and 87.2% for cases diagnosed during or after 2000.
Exposure assessment
Data on caffeine intake (mg/day) were derived from the cohort’s baseline survey, which included the grid-based Diet History Questionnaire, developed by National Cancer Institute investigators (16). This questionnaire asked for the typical frequencies of consumption and portion sizes of 124 food items ingested over the past 12 months, including caffeine-containing drinks such as coffee, hot tea, iced tea, and soft drinks. Ten consumption frequencies were allowed, ranging from never to more than 6 cups per day. For each type of drink, we further asked consumers whether they drank caffeine-free beverages or caffeine-containing beverages more than half the time. In addition, the questionnaire also included food items (e.g., candy, cakes, and cookies) that contain a small amount of caffeine. Daily caffeine intake was determined as part of the nutrient calculation using the 1994–1996 US Department of Agriculture’s Continuing Survey of Food Intakes by Individuals. Information on smoking was also collected from the cohort’s baseline survey, administered in 1995–1996. On the questionnaire, participants were asked whether they had ever smoked more than 100 cigarettes during their lifetime. Ever smokers were asked about the typical number of cigarettes smoked per day and their current smoking status, and former smokers were asked about the number of years since they had last smoked. Using both baseline and follow-up data, a variable on duration of smoking was constructed for past smokers.
In addition to caffeine intake and smoking, the baseline survey collected demographic information, including data on age, gender, race, and physical activity. For physical activity, we asked how often participants performed activities at work or at home that lasted at least 20 minutes and increased their breathing or heart rate or caused sweating. For female participants, the survey asked about their menopausal status at baseline and whether they had used hormone replacement therapy and the duration of use (in years).
Statistical analysis
We estimated multivariate odds ratios and 95% confidence intervals from unconditional logistic regression models. Caffeine intake was the exposure of interest and was defined as quintiles in the primary analysis. The analysis was first conducted with all participants and then separately for men and women. Covariates included baseline age (in 5-year groups), gender, race (white vs. nonwhite), and frequency of physical activity (never/rarely, 1–3 times per month, or 1–2, 3–4, or ≥5 times per week). Similar analyses were also conducted for coffee consumption (none or number of cups per day: <1, 1, 2–3, or ≥3), overall and separately for participants drinking caffeinated or decaffeinated coffee more than half the time. To examine the potential impact of smoking on the relation between caffeine and PD, we conducted analyses with and without adjustment for smoking (never smoker, past smoker who smoked for 1–9, 10–29, or ≥30 years, or current smoker) and among never smokers. To examine the statistical significance of a linear trend in caffeine intake on PD risk, we included a continuous variable defined by the median value of each caffeine intake quintile in the regression model. Among women, we further conducted analysis stratified by postmenopausal hormone use and tested for potential interaction by including an interaction term in the regression model. In the joint analysis with smoking, we defined caffeine intake as low (first quintile), medium (second and third quintiles), or high (fourth and fifth quintiles) and smoking status as never smoker, past smoker who had smoked for 1–29 years, or past smoker who had smoked for ≥30 years/current smoker. We tested the statistical significance of interactions by adding a term for the product of 2 exposures in the regression models.
To the best of our knowledge, 9 prospective studies (5–9, 11, 12, 17, 18) have examined the relation between coffee/caffeine intake and risk of PD, including one that used PD mortality data (12). Therefore, we conducted a meta-analysis of these studies plus the current study. Because PD patients might modify their behaviors even prior to PD diagnosis, we chose to focus on prospective studies to reduce the potential impact of reverse causation. We conducted a MEDLINE search of prospective studies on coffee/caffeine and PD that had been published in English through May 2011. A combination of Medical Subject Headings and text terms was used to perform the literature search. Eligible studies were original prospective epidemiologic studies (including nested case-control studies) in which investigators reported a measure of association, with 95% confidence intervals, for the association between coffee/caffeine intake and PD. For multiple publications derived from the same cohort (5, 11), we chose to include only the most recent data (11). Pooled odds ratios and 95% confidence intervals for PD risk were calculated using random-effects models (19). Heterogeneity across studies was assessed using Cochran’s Q and I2 statistics (20). Gender-specific risk estimates comparing the highest coffee/caffeine categories with the lowest were extracted from each study whenever possible. We used meta-regression to examine gender as a potential source of heterogeneity. The meta-analysis was conducted using STATA, version 10.1 (StataCorp LP, College Station, Texas). All other statistical analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina). Significance tests were 2-tailed with α = 0.05.
Standard protocol approval, registration, and patient consent
Participants consented to the study by returning survey questionnaires. The study protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences and the Special Studies Institutional Review Board of the National Cancer Institute.
RESULTS
Table 1 shows population characteristics according to quintiles of participants’ baseline caffeine intake. Participants with higher caffeine intakes were more likely to be men, non-Hispanic Caucasians, and less physically active. Caffeine intake was strongly associated with smoking: Participants in the highest caffeine intake quintile were more likely to be current smokers at baseline than those in the lowest quintile. Women in the highest caffeine intake quintile were less likely to report postmenopausal hormone use than those in lower quintiles.
Table 1.
Baseline Characteristics of Study Participants According to Quintile of Caffeine Intake, National Institutes of Health-AARP Diet and Health Study, 1995–2006
| Quintile of Caffeine Intake | ||||||||||
| 1 (<17.4 mg/day) (n = 60,980) | 2 (17.4–<129.2 mg/day) (n = 61,010) | 3 (129.2–<510.9 mg/day) (n = 60,985) | 4 (510.9–<589.8 mg/day) (n = 61,009) | 5 (≥589.8 mg/day) (n = 60,996) | ||||||
| % | Median (IQRa) or Mean (SD) | % | Median (IQR) or Mean (SD) | % | Median (IQR) or Mean (SD) | % | Mean (SD) or Median (IQR) | % | Median (IQR) or Mean (SD) | |
| Median caffeine intake, mg/day | 7.8 (8.4) | 45.3 (50.8) | 232.9 (90.8) | 538.2 (30.6) | 946.4 (357.6) | |||||
| Mean age, years | 61.9 (5.3) | 61.5 (5.3) | 61.5 (5.4) | 61.6 (5.3) | 60.6 (5.3) | |||||
| Male gender | 51.8 | 59.6 | 52.2 | 61.6 | 67.9 | |||||
| Race | ||||||||||
| Non-Hispanic white | 90.6 | 92.1 | 90.5 | 94.6 | 95.3 | |||||
| Other | 8.3 | 6.9 | 8.3 | 4.6 | 3.7 | |||||
| Missing data | 1.2 | 1.0 | 1.2 | 0.9 | 1.0 | |||||
| Smoking status | ||||||||||
| Never smoker | 47.6 | 42.1 | 42.8 | 32.7 | 26.2 | |||||
| Past smoker | ||||||||||
| Smoked for 1–9 years | 12.4 | 11.8 | 11.8 | 12.5 | 11.0 | |||||
| Smoked for 10–29 years | 20.2 | 22.2 | 21.2 | 24.8 | 23.0 | |||||
| Smoked for ≥30 years | 10.2 | 12.0 | 11.8 | 14.9 | 15.8 | |||||
| Missing data on duration of past smoking | 3.9 | 3.8 | 3.8 | 3.6 | 3.4 | |||||
| Current smoker | 4.5 | 6.8 | 7.4 | 10.3 | 19.3 | |||||
| Missing data | 1.2 | 1.2 | 1.2 | 1.2 | 1.3 | |||||
| Frequency of physical activity | ||||||||||
| Never or rarely | 13.8 | 15.1 | 16.8 | 15.0 | 17.2 | |||||
| 1–3 times/month | 11.5 | 13.3 | 14.0 | 13.9 | 14.8 | |||||
| 1–2 times/week | 19.8 | 22.3 | 22.0 | 22.9 | 23.2 | |||||
| 3–4 times/week | 30.3 | 28.6 | 27.1 | 28.4 | 25.8 | |||||
| ≥5 times/week | 23.7 | 19.9 | 19.3 | 19.2 | 18.4 | |||||
| Missing data | 0.9 | 0.8 | 0.9 | 0.7 | 0.7 | |||||
| Hormone use (women only)b | ||||||||||
| Premenopausal | 1.9 | 2.3 | 1.9 | 2.0 | 2.0 | |||||
| Postmenopausal | ||||||||||
| Never user | 40.6 | 39.6 | 41.8 | 40.2 | 45.7 | |||||
| User | 52.3 | 52.9 | 51.2 | 53.1 | 47.8 | |||||
| Inconsistent user | 4.3 | 4.3 | 4.2 | 4.0 | 3.7 | |||||
| Missing data | 1.0 | 0.9 | 0.9 | 0.7 | 0.7 | |||||
Abbreviations: IQR, interquartile range; SD, standard deviation.
25th–75th percentiles.
Numbers of female participants—quintile 1: 29,369; quintile 2: 24,637; quintile 3: 29,171; quintile 4: 23,429; quintile 5: 19,606.
Higher coffee consumption was associated with a lower PD risk in a dose-response manner, but after adjustment for smoking the association was apparently limited to drinkers of caffeinated coffee (see Web Table 1 (http://aje.oxfordjournals.org/)). Therefore, we conducted detailed analyses based on caffeine intake. Consumption of other caffeine-containing beverages (soft drinks, hot tea, and iced tea) was not associated with the risk of PD (Web Table 2).
Higher caffeine intake in 1995–1996 was associated with lower PD risk in both men and women (Table 2). Adjustment for smoking duration did not materially change the results. Results were similar among never smokers, where presumably there was no confounding by cigarette smoking (men—odds ratio for quintile 5 vs. quintile 1 (ORQ5vs.Q1) = 0.70, 95% confidence interval (CI): 0.47, 1.04; women—ORQ5vs.Q1 = 0.74, 95% CI: 0.42, 1.29). In women, additional adjustment for postmenopausal hormone use barely changed the association between caffeine intake and PD (Table 2). Subgroup analysis by hormone use among postmenopausal women suggested that caffeine intake was associated with lower PD risk only among hormone users, not among never users (Table 3). However, the statistical test for interaction was not statistically significant. To examine the potential influence of duration of hormone use, we further conducted stratified analyses among hormone users by years of use reported at baseline. The results were similar for 1–9 years of use (ORQ5vs.Q1 = 0.55, 95% CI: 0.25, 1.23) and 10 or more years of use (ORQ5vs.Q1 = 0.48, 95% CI: 0.18, 1.31).
Table 2.
Odds Ratios for Parkinson Disease According to Quintile of Baseline Caffeine Intake, National Institutes of Health-AARP Diet and Health Study, 1995–2006
| Quintile of Caffeine Intake | Overall | Men | Women | |||||||||
| No. With PD | No. Without PD | OR | 95% CI | No. With PD | No. Without PD | OR | 95% CI | No. With PD | No. Without PD | OR | 95% CI | |
| Basic modela | ||||||||||||
| 1 | 251 | 60,729 | 1.00 | Referent | 172 | 31,439 | 1.00 | Referent | 79 | 29,290 | 1.00 | Referent |
| 2 | 251 | 60,759 | 0.98 | 0.82, 1.17 | 185 | 36,188 | 0.98 | 0.80, 1.21 | 66 | 24,571 | 1.00 | 0.72, 1.39 |
| 3 | 205 | 60,780 | 0.85 | 0.70, 1.02 | 141 | 31,673 | 0.86 | 0.69, 1.08 | 64 | 29,107 | 0.81 | 0.58, 1.13 |
| 4 | 215 | 60,794 | 0.82 | 0.68, 0.98 | 169 | 37,411 | 0.85 | 0.69, 1.06 | 46 | 23,383 | 0.73 | 0.51, 1.05 |
| 5 | 178 | 60,818 | 0.71 | 0.58, 0.86 | 148 | 41,242 | 0.75 | 0.60, 0.94 | 30 | 19,576 | 0.60 | 0.39, 0.91 |
| Ptrend | <0.001 | 0.005 | 0.005 | |||||||||
| Further adjustment for smoking status | ||||||||||||
| 1 | 251 | 60,729 | 1.00 | Referent | 172 | 31,439 | 1.00 | Referent | 79 | 29,290 | 1.00 | Referent |
| 2 | 251 | 60,759 | 1.00 | 0.84, 1.20 | 185 | 36,188 | 1.00 | 0.81, 1.23 | 66 | 24,571 | 1.02 | 0.74, 1.42 |
| 3 | 205 | 60,780 | 0.86 | 0.71, 1.04 | 141 | 31,673 | 0.87 | 0.70, 1.09 | 64 | 29,107 | 0.83 | 0.60, 1.16 |
| 4 | 215 | 60,794 | 0.86 | 0.72, 1.04 | 169 | 37,411 | 0.89 | 0.72, 1.11 | 46 | 23,383 | 0.78 | 0.54, 1.13 |
| 5 | 178 | 60,818 | 0.78 | 0.64, 0.95 | 148 | 41,242 | 0.81 | 0.65, 1.02 | 30 | 19,576 | 0.67 | 0.44, 1.03 |
| Ptrend | 0.004 | 0.04 | 0.03 | |||||||||
| Further adjustment for hormone use | ||||||||||||
| 1 | 79 | 29,290 | 1.00 | Referent | ||||||||
| 2 | 66 | 24,571 | 1.02 | 0.74, 1.42 | ||||||||
| 3 | 64 | 29,107 | 0.83 | 0.60, 1.16 | ||||||||
| 4 | 46 | 23,383 | 0.78 | 0.54, 1.13 | ||||||||
| 5 | 30 | 19,576 | 0.67 | 0.44, 1.04 | ||||||||
| Ptrend | 0.03 | |||||||||||
Abbreviations: CI, confidence interval; OR, odds ratio; PD, Parkinson disease.
In the basic model, results were adjusted for age at baseline, race, physical activity, and gender when appropriate.
Table 3.
Relation Between Caffeine Intake and Parkinson Disease According to Hormone Use* Among Postmenopausal Women, National Institutes of Health-AARP Diet and Health Study, 1995–2006
| Quintile of Caffeine Intake | Never Users of Hormones | Ever Users of Hormones | ||||||
| No. With PD | No. Without PD | ORa | 95% CI | No. With PD | No. Without PD | ORa | 95% CI | |
| 1 | 28 | 11,903 | 1.00 | Referent | 48 | 15,302 | 1.00 | Referent |
| 2 | 27 | 9,724 | 1.24 | 0.73, 2.10 | 38 | 13,000 | 0.93 | 0.60, 1.43 |
| 3 | 31 | 12,177 | 1.10 | 0.65, 1.84 | 30 | 14,902 | 0.66 | 0.42, 1.05 |
| 4 | 21 | 9,405 | 1.00 | 0.56, 1.77 | 23 | 12,420 | 0.64 | 0.39, 1.05 |
| 5 | 17 | 8,949 | 0.95 | 0.51, 1.77 | 13 | 9,365 | 0.53 | 0.28, 0.98 |
| P for trend | 0.57 | 0.02 | ||||||
Abbreviations: CI, confidence interval; OR, odds ratio; PD, Parkinson disease.
P for interaction = 0.16.
Adjusted for age at baseline, race, duration of smoking, and physical activity.
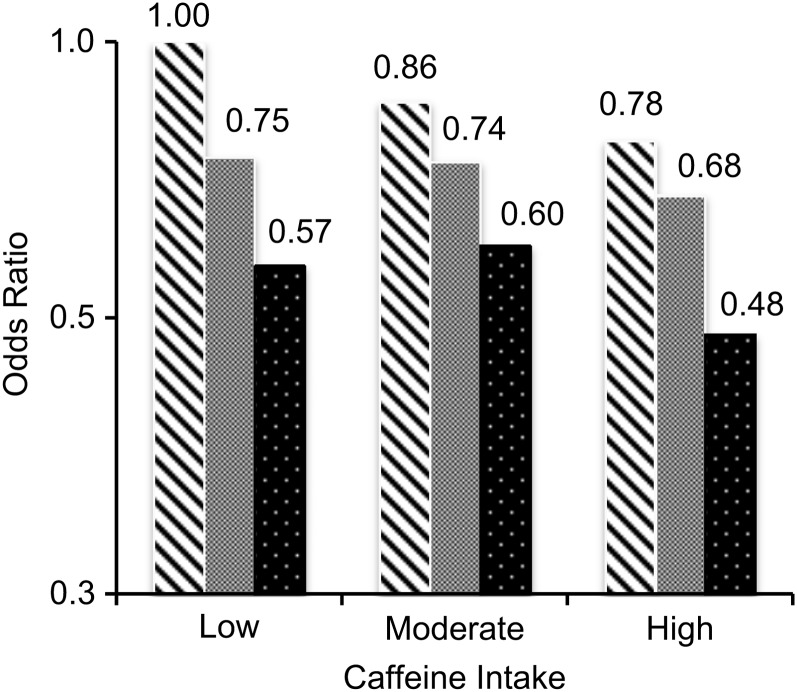
Duration of smoking was strongly associated with lower PD risk; further adjustment for caffeine intake barely changed the risk estimates for smoking (Web Table 3). Joint analysis of smoking duration and caffeine intake showed that smoking was associated with lower PD risk within each level of caffeine intake (Figure 1; for all subgroups, Ptrend ≤ 0.01). In contrast, higher caffeine intake was significantly associated with lower PD risk among never smokers (Ptrend = 0.04), but the monotonic trend was less clear among ever smokers. Nevertheless, compared with never smokers with low caffeine intake, long-term smokers with high caffeine intake had the lowest risk of PD. The statistical test for a potential interaction between smoking and caffeine intake was far from statistically significant (P = 0.57).
Figure 1.
Odds ratios for Parkinson disease according to joint categories of baseline caffeine intake and smoking status, National Institutes of Health-AARP Diet and Health Study, 1995–2006. Caffeine intake was defined as low (first quintile), moderate (second and third quintiles), or high (fourth and fifth quintiles). Smoking status was defined as never smoker (striped bars), past smoker who had smoked for 1–29 years (gray bars), or past smoker who had smoked for ≥30 years/current smoker (dotted bars). Specific odds ratios appear above the bars.
A meta-analysis of prospective studies (Web Table 4) showed associations for higher caffeine intake with lower PD risk in both men and women (Figure 2). Moderate degrees of heterogeneity were observed across the pooled studies (I2 = 45.7%, 95% CI: 0, 68; Q statistic: P = 0.02) and across studies of men (I2 = 58.9%, 95% CI: 0, 80; Q statistic: P = 0.02) and women (I2 = 51.6%, 95% CI: 0, 76; Q statistic: P = 0.04). Meta-regression analysis, however, showed no gender influence on the pooled odds ratio (P = 0.65). Visual inspections of the funnel plot revealed little asymmetry (Web Figure 1), and no significant publication bias was detected from results of the statistical tests (Begg test: P = 0.15; Egger test: P = 0.20).
Figure 2.
Results from a meta-analysis of prospective studies on coffee/caffeine intake and risk of Parkinson disease by gender. Odds ratios (ORs) and 95% confidence intervals (CIs) are for the highest coffee/caffeine intake categories versus the lowest. Squares indicate study-specific ORs; error bars indicate 95% CIs; and diamonds indicate results of pooled analyses. “Subtotal” represents the pooled OR within each subcategory, based on the random-effects model. “Overall” shows the pooled OR for all studies, based on the random-effects model. (HRT, hormone replacement therapy).
DISCUSSION
In this large prospective study, we found that higher caffeine intake was associated with lower PD risk in both men and women, a result which was confirmed in a meta-analysis of previous prospective studies. Further, our exploratory analyses did not identify a strong interaction between caffeine and smoking in the risk of PD.
The current study was prospective in design and was larger than most of the previous studies. In general, prospective studies are more suitable for research on the role of diet and lifestyle in the etiology of chronic diseases because they are less prone to recall bias and reverse causality. In addition to cognitive difficulties for prevalent cases, some early symptoms of PD (e.g., loss of smell, sleep disturbances, mild tremor, etc.) may cause patients to modify their coffee-drinking habits even prior to disease diagnosis. This may make explanations for an inverse association of coffee drinking with PD less straightforward for retrospective case-control studies than for prospective studies. To further reduce the impact of this potential bias, we included in our analysis only PD cases that had been identified at least 4–5 years after the baseline survey was completed.
With only about 10 years of follow-up, we cannot exclude the possibility that there may have been reverse causality if PD patients had decreased their coffee consumption many years before diagnosis as a result of the underlying disease process. Nonetheless, our finding was consistent with the results of the Honolulu-Asia Aging Study, a prospective cohort study with 30 years of follow-up (7). It has also been hypothesized that PD patients often have a novelty-avoidance personality that prevents them from drinking coffee in the first place. While it is very difficult to address this concern in epidemiologic studies (21), experimental studies show that caffeine protects dopaminergic neurons, probably by acting as an adenosine A2A receptor antagonist (10).
Many studies have shown that higher caffeine intake is associated with lower PD risk in men; however, data for women have been sparse and inconsistent. For example, in the Nurses’ Health Study, a prospective study containing all women (131 cases), Ascherio et al. (5) reported a U-shaped association between caffeine intake and PD risk. On the other hand, a prospective Finnish analysis showed a monotonic but statistically nonsignificant association of coffee consumption with PD risk in women (98 cases) (6). In the largest previous analysis in women, a case-control study of 392 cases, coffee consumption was associated with lower PD risk only in the highest drinking category, with no evidence for a dose-response (22).
Inconsistencies in women across previous studies may be due, at least in part, to small sample sizes and different study designs, but it is also possible that the caffeine-PD association could be gender-specific. This has led Ascherio et al. (11, 12) to explore potential interactions between estrogen use and caffeine intake in PD. They found that, among women who did not use hormone therapy, higher caffeine intake was associated with a lower PD risk (11) or lower PD mortality (12); however, among estrogen users, there was an indication of higher PD risk or mortality for heavy coffee drinkers; interactions in these studies were statistically significant. We observed the opposite in our study: Higher caffeine intake was clearly associated with lower PD risk among hormone users but not among never users, although the interaction was not statistically significant. Apparent differences across these 3 studies are intriguing but difficult to explain. To the best of our knowledge, these are the only 3 epidemiologic studies that examined potential interactions between estrogen use and caffeine intake in PD risk. Our study could not exclude the possibility of chance, since the interaction term was not statistically significant. In addition to epidemiologic studies, 1 experimental study also showed preliminary evidence that estrogen may abolish neuroprotection by caffeine among ovariectomized female mice (23). Nevertheless, a potential role of estrogen in PD etiology has yet to be defined, and the evidence to date on estrogen and PD has been inconsistent in both human studies (24, 25) and animal experiments (26, 27). Further, recent research suggests that the health implications of hormone use among women may depend on composition of the hormone preparation, time of initiation, duration of use, and type of administration (28). The role of hormone use in PD etiology and its potential influence on the caffeine-PD relation should be further explored with larger sample sizes in the future.
Coffee drinkers are much more likely to smoke cigarettes than nondrinkers, and if they smoke, they often smoke more (13, 14). Given the strong inverse association of smoking with PD, it is crucial to carefully control for potential confounding by smoking in investigations of coffee/caffeine intake and PD. Further, cigarette smoking accelerates the metabolism and clearance of caffeine in humans (29), and with the same amount of caffeine intake, nonsmokers have 2- to 3-fold higher plasma caffeine concentrations than smokers (30). It is therefore important to control for and evaluate the potential influence of cigarette smoking on the relation between caffeine and PD. To the best of our knowledge, only 2 studies have explored the joint effect of smoking and caffeine on PD risk (5, 22). By dichotomizing both exposures, Powers et al. (22) reported a significant decrease in PD risk only among smokers with high coffee consumption, as compared with never smokers with low coffee consumption; no other joint exposure group had an association with PD risk. Ascherio et al. (5) reported an association between higher caffeine intake and lower PD risk among both male smokers and male nonsmokers. As we noted above, in that particular study (5), caffeine intake was not associated with PD risk among women in a linear manner. Our analysis on the joint effect had a relatively large sample size and carefully defined exposures. Our data are compatible with the possibility that caffeine intake may act independently of smoking in the risk of PD.
Our study had several limitations. First, in such a large population, we had to rely on self-reports to identify PD cases, which will inevitably have introduced reporting and diagnostic errors. However, we were able to confirm approximately 88% of the cases in patients from whom we obtained medical information from treating neurologists. We further removed from the analysis persons with identified error reports and misdiagnoses. Second, consumption of coffee, tea, and soft drinks was assessed as part of a dietary history questionnaire, and measurement errors were likely. Likewise, misclassification of data on important covariates such as smoking was also possible, and we could not exclude the possibility of residual confounding. However, we did conduct an analysis among never smokers, which showed an association between caffeine and lower PD risk. In addition to measurement errors, exposure assessment was conducted only for the year prior to the baseline survey, and no lifetime coffee consumption data were collected. Therefore, we did not directly assess the relation between long-term caffeine intake and PD risk. To our knowledge, this study is one of the largest studies on caffeine intake and PD that has been conducted; but even with this large sample size, our statistical power for interaction testing was limited. Finally, our analysis was conducted only among participants in the follow-up survey. These participants were significantly different from nonparticipants in terms of population characteristics that may affect PD risk, and therefore we cannot exclude the possibility of selection bias.
In summary, our study showed that higher caffeine intake was associated with lower PD risk in both men and women. The possibility that hormone use may influence the caffeine-PD relation among women should be carefully evaluated in future studies.
Supplementary Material
Acknowledgments
Author affiliations: Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina (Rui Liu, Honglei Chen); Westat Inc., Research Triangle Park, North Carolina (Xuguang Guo); Nutritional Epidemiology Branch, National Cancer Institute, Rockville, Maryland (Yikyung Park, Rashmi Sinha, Neal D. Freedman); Occupational and Environmental Epidemiology Branch, National Cancer Institute, Rockville, Maryland (Aaron Blair); Department of Neurology, Perelman School of Medicine, Pennsylvania State University-Milton S. Hershey Medical Center, Hershey, Pennsylvania (Xuemei Huang); and AARP, Washington, DC (Albert R. Hollenbeck).
This study was supported by the Intramural Research Program of the National Institutes of Health (National Institute of Environmental Health Sciences grant Z01-ES-101986 and National Cancer Institute grant Z01 CP010196-02).
The authors thank Dr. David M. Umbach of the National Institute of Environmental Health Sciences (Research Triangle Park, North Carolina) for statistical advice and assistance.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- NIH
National Institutes of Health
- ORQ5vs.Q1
odds ratio for quintile 5 vs. quintile 1
- PD
Parkinson disease
References
- 1.Hernán MA, Takkouche B, Caamaño-Isorna F, et al. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 2.Costa J, Lunet N, Santos C, et al. Caffeine exposure and the risk of Parkinson’s disease: a systematic review and meta-analysis of observational studies. J Alzheimers Dis. 2010;20(suppl 1):S221–S238. doi: 10.3233/JAD-2010-091525. [DOI] [PubMed] [Google Scholar]
- 3.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64(7):990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 6.Hu G, Bidel S, Jousilahti P, et al. Coffee and tea consumption and the risk of Parkinson’s disease. Mov Disord. 2007;22(15):2242–2248. doi: 10.1002/mds.21706. [DOI] [PubMed] [Google Scholar]
- 7.Ross GW, Abbott RD, Petrovitch H, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283(20):2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 8.Sääksjärvi K, Knekt P, Rissanen H, et al. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur J Clin Nutr. 2008;62(7):908–915. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 9.Tan LC, Koh WP, Yuan JM, et al. Differential effects of black versus green tea on risk of Parkinson’s disease in the Singapore Chinese Health Study. Am J Epidemiol. 2008;167(5):553–560. doi: 10.1093/aje/kwm338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarzschild MA, Chen JF, Ascherio A. Caffeinated clues and the promise of adenosine A(2A) antagonists in PD. Neurology. 2002;58(8):1154–1160. doi: 10.1212/wnl.58.8.1154. [DOI] [PubMed] [Google Scholar]
- 11.Ascherio A, Chen H, Schwarzschild MA, et al. Caffeine, postmenopausal estrogen, and risk of Parkinson’s disease. Neurology. 2003;60(5):790–795. doi: 10.1212/01.wnl.0000046523.05125.87. [DOI] [PubMed] [Google Scholar]
- 12.Ascherio A, Weisskopf MG, O’Reilly EJ, et al. Coffee consumption, gender, and Parkinson’s disease mortality in the Cancer Prevention Study II cohort: the modifying effects of estrogen. Am J Epidemiol. 2004;160(10):977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 13.Nettleton JA, Follis JL, Schabath MB. Coffee intake, smoking, and pulmonary function in the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2009;169(12):1445–1453. doi: 10.1093/aje/kwp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Garcia E, van Dam RM, Willett WC, et al. Coffee consumption and coronary heart disease in men and women: a prospective cohort study. Circulation. 2006;113(17):2045–2053. doi: 10.1161/CIRCULATIONAHA.105.598664. [DOI] [PubMed] [Google Scholar]
- 15.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 16.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 17.Paganini-Hill A. Risk factors for Parkinson’s disease: the Leisure World Cohort Study. Neuroepidemiology. 2001;20(2):118–124. doi: 10.1159/000054770. [DOI] [PubMed] [Google Scholar]
- 18.Wirdefeldt K, Gatz M, Pawitan Y, et al. Risk and protective factors for Parkinson’s disease: a study in Swedish twins. Ann Neurol. 2005;57(1):27–33. doi: 10.1002/ana.20307. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Evans AH, Lawrence AD, Potts J, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77(3):317–321. doi: 10.1136/jnnp.2005.065417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powers KM, Kay DM, Factor SA, et al. Combined effects of smoking, coffee, and NSAIDs on Parkinson’s disease risk. Mov Disord. 2008;23(1):88–95. doi: 10.1002/mds.21782. [DOI] [PubMed] [Google Scholar]
- 23.Xu K, Xu Y, Brown-Jermyn D, et al. Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J Neurosci. 2006;26(2):535–541. doi: 10.1523/JNEUROSCI.3008-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popat RA, Van Den Eeden SK, Tanner CM, et al. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology. 2005;65(3):383–390. doi: 10.1212/01.wnl.0000171344.87802.94. [DOI] [PubMed] [Google Scholar]
- 25.Simon KC, Chen H, Gao X, et al. Reproductive factors, exogenous estrogen use, and risk of Parkinson’s disease. Mov Disord. 2009;24(9):1359–1365. doi: 10.1002/mds.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front Neuroendocrinol. 2009;30(2):142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson’s disease. Clin Exp Pharmacol Physiol. 2007;34(7):555–565. doi: 10.1111/j.1440-1681.2007.04616.x. [DOI] [PubMed] [Google Scholar]
- 28.Taylor HS, Manson JE. Update in hormone therapy use in menopause. J Clin Endocrinol Metab. 2011;96(2):255–264. doi: 10.1210/jc.2010-0536. [DOI] [PubMed] [Google Scholar]
- 29.Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53–62. doi: 10.1016/j.ccell.2005.11.007. xii. [DOI] [PubMed] [Google Scholar]
- 30.de Leon J, Diaz FJ, Rogers T, et al. A pilot study of plasma caffeine concentrations in a US sample of smoker and nonsmoker volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):165–171. doi: 10.1016/s0278-5846(02)00348-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.