Abstract
Caffeine-induced Ca2+ transients (CICTs) in rabbit nodose ganglion neurons (NGNs) are produced by two distinct mechanisms: release from intracellular stores via ryanodine receptors and Ca2+ influx across the plasma membrane, due to activation of an unknown receptor. In isolated rat NGNs, we used single-cell microfluorimetry to measure changes in intracellular Ca2+ and to test whether TRPV1 receptors underlie the Ca2+ influx pathway. Caffeine (10 mM) evoked CICTs in all NGNs tested (n = 47) averaging 365 ± 32 nM. CICTs were partially dependent upon a Ca2+ influx pathway that ranged between 33% and 98% of the total Ca2+ transient. Application of two selective TRPV1 antagonists significantly attenuated CICTs. The peak average amplitudes of CICTs in Ca2+-free Locke solution and Ca2+-free Locke solution with IRTX or with BCTC were not significantly different from one another (n = 5 and 7, respectively). These observations suggest that caffeine can induce Ca2+ influx by activating TRPV1 channels.
Keywords: Caffeine, Ca2+ Influx, TRPV1, CICR, Nodose ganglia neurons, Vagus nerve
Introduction
Caffeine can produce an elevation in intracellular Ca2+ (Ca2+ transients), in a variety of peripheral and central neurons [1]. In rabbit primary vagal sensory neurons (nodose ganglion neurons, NGNs), caffeine evokes Ca2+ transients by activating two distinct pathways [2]. In all NGNs, caffeine induces Ca2+ transients by activating ryanodine receptors (RyR) resulting in Ca2+ efflux from the endoplasmic reticulum [2, 3]. In about 50% of the rabbit NGNs, caffeine-induced Ca2+ transients (CICTs) are also produced by an additional pathway: a Ca2+ influx pathway through the plasma membrane. This pathway remains functional when intracellular Ca2+ stores is depleted, or when Ca2+-induced Ca2+ release (calcium induced calcium release, CICR) is blocked by ryanodine; it is absent when extracellular Ca2+ is nominally zero and it is independent of store-operated Ca2+ channels [2]. To date, the nature of this influx pathway has not yet been resolved.
The vanilloid family of transient receptor potential channels (TRPVs) are a good candidate for the Ca2+ influx pathway mediated by caffeine. The TRPV1 subfamily is highly permeable to Ca2+ and is modulated by a wide range of disparate molecules [4–7]. Moreover, TRPV1 channels are present and functional in 70–85% of rat NGNs [8, 9]. In the present work, we used specific antagonists of TRPV1 channels to test whether TRPV1 might function as an influx pathway activated by caffeine in rat NGNs.
Experimental procedure
Dissociation and culture of NGNs
Male Sprague-Dawley rats (120–200 g) were killed by CO2 inhalation as approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore. The NGNs were dissociated enzymatically and mechanically as described previously [10]. Briefly, ganglia were rapidly dissected from animals and the connective tissue surrounding the ganglia was removed. Whole ganglia were then incubated in an enzyme solution containing 10 mg collagenase type 1A (Sigma Chemical Co., St Louis, MO) and 10 mg dispase II (Sigma Chemical Co.) in 10 ml of Ca2+- and Mg2+-free Hanks’ balanced salt solution (HBSS). After a 75-min incubation (37°C), NGN were dissociated by trituration with fire-polished Pasteur pipettes of decreasing tip diameters. Cells were collected by centrifugation (3 times 700g for 45 s). Enzyme solutions were replaced by L15 culture medium (Gibco, BRL, Rockville, MD) and 10% foetal bovine serum (JRH Bioscience, Lenexa, KN).
The dissociated NGN were re-suspended in culture medium and plated on poly-d-lysine (0.1 mg ml−1, Sigma Chemical Co.) coated circular 25-mm glass coverslips (Fisher, Newark, DE). After 2 h incubation at 37°C, the coverslips were placed in a room temperature incubator to prevent neurite growth. NGNs were used for experiments up to 48 h in culture.
Calcium recordings
Neurons were superfused with a Locke solution (21–24°C) with the following composition (mM): 136 NaCl, 5.6 KCl, 1.2 NaH2PO4, 14.3 NaHCO3, 1.2 MgCl2, 2.2 CaCl2, and 10.0 dextrose, equilibrated with 95% O2–5% CO2 and adjusted to pH 7.2–7.4 with NaOH. For experiments where nominally Ca2+-free Locke solution was required, CaCl2 was substituted with MgCl2.
Coverslips were placed in custom fabricated recording chamber with a narrow rectangular flow path (200 µl) and superfused via a gravity-flow system (4 ml/min). Solution changes were complete within 14 s, as determined with fluorescent tracers. Prior to recording, coverslips containing NGNs were incubated with 1 µM fura-2 AM for 60 min. The recording chamber was mounted on an inverted microscope (TE200; Nikon, Tokyo, Japan) equipped with a UV-transmitting objective (SuperFluor, 40×, N.A. 1.4, Nikon). Fura-2 was alternately excited by 340 and 380 nm light from monochrometers (Deltascan Illumination System, Photonic Technology International (PTI), South Brunswick, NJ) and fura-2 emission was detected by a photomultiplier tube (PMT, D-104 microscope photomultiplier, PTI). Felix 1.1 software (PTI) was used for control and synchronization of the monochrometers and PMT.
Data analysis
[Ca2+]i was derived using the ratio method described previously [11]. Data were analysed and plotted using SigmaPlot 2000 (SPSS, Chicago, IL). Statistics were performed with SigmaStat 2.0 (SPSS) and values are presented as mean ± SEM. To determine statistical significance, one-way ANOVAs were performed with Student–Newman–Keuls to determine significance for pair-wise comparisons. P < 0.05 indicated statistical significance.
Reagents
Most drugs were dissolved in vehicle at 1000 times the final concentration and kept frozen in aliquots. N-(4-t-Butylphenyl)-4-(3-chloropyridin-2-y1) tetrahydropyrazine-1(2H)-carboxamide (BCTC), purchased from Biomol (Plymoouth Meeting, PA), and iodoresiniferatoxin (IRTX), purchased from Tocris (Ellsville, MO), were dissolved in ethanol. All other reagents were purchased from Sigma Chemical Co. (Sigma Chemical Co., St Louis, MO).
Results
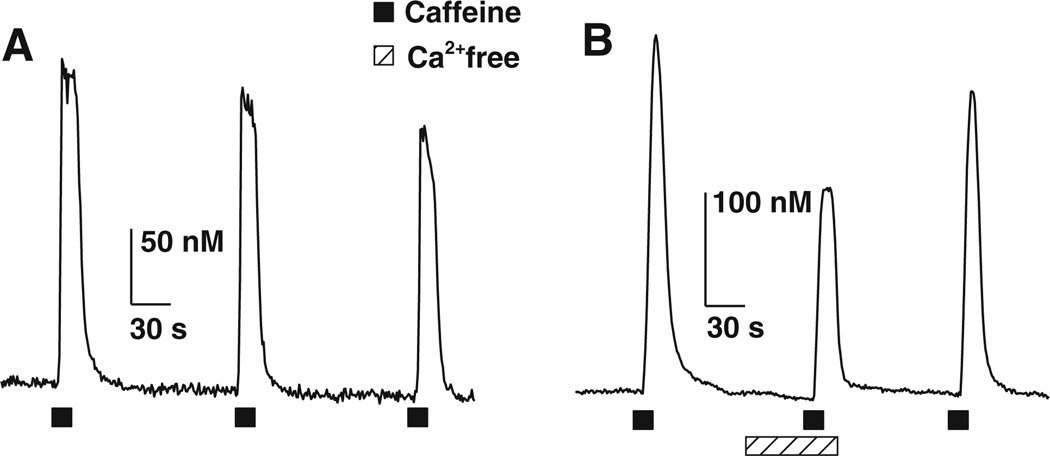
Nodose ganglion neurons from adult rats revealed a robust Ca2+ transient upon brief application of caffeine (10 mM, 15 s) in all cells tested, with a peak average amplitude of 365 ± 32 nM (n = 47). The amplitudes of the CICTs were consistent over time. In four neurons, application of caffeine (10 mM) every 250 s produced Ca2+ transients that were not significantly different (P < 0.05) from one another with average amplitudes of 218 ± 20, 221 ± 24 and 213 ± 35 nM for the first, second and third application, respectively (Fig. 1a). To determine whether extracellular Ca2+ contributed to the CICTs in rat NGNs, we stimulated NGNs with caffeine (10 mM) in normal and in Ca2+-free Locke solution. The magnitude of the CICTs was significantly attenuated in nominally Ca2+-free Locke solution: 304 ± 32 vs. 122 ± 29 nM; 54 ± 9% reduction (n = 6; Fig. 1b). After washing the NGNs with normal Locke solution, the CICT amplitudes returned to control values, 286.4 ± 28 nM. These results indicate that, like rabbit NGNs, rat NGNs also possess a caffeine-induced Ca2+ influx pathway.
Fig. 1.
Caffeine-induced Ca2+ transients (CICTs) are partially dependent upon extracellular Ca2+. a Reproducibility of CICTs. Three representative CICTs evoked by three 15-s pulses of 10 mM caffeine in normal Locke solution. The CICTs had an average peak amplitude of 218 ± 20, 221 ± 24 and 212 ± 34 nM (n = 4) for the first, second and third application of caffeine, respectively. b CICTs elicited by caffeine (10 mM) in the presence or in the absence of extracellular Ca2+. The first and third CICTs were evoked by caffeine in normal Locke solution. The middle CICT was evoked by caffeine in a Locke solution containing nominally zero Ca2+. The difference in amplitude between the averaged control CICTs and the second CICTs represents the magnitude of Ca2+ influx across the plasma membrane. The amplitude of the CICTs recorded in nominally Ca2+-free Locke solution averaged 45 ± 9% of the amplitude of control CICTs (n = 6) recorded in normal Lock solution. Solid squares depict time of caffeine application; hatched bar depicts time when the neurons were superfused with nominally Ca2+-free Locke solution
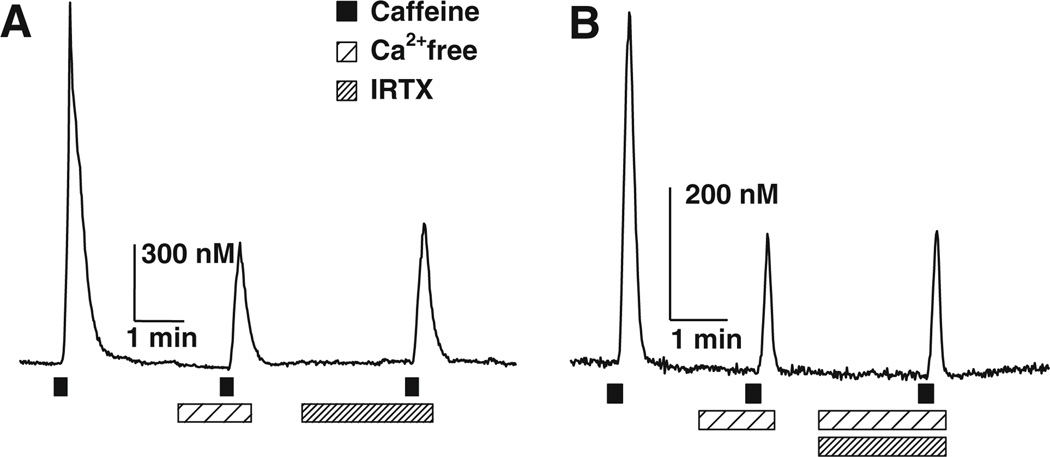
To determine if TRPV1 underlies the caffeine-induced Ca2+ influx pathway, we used specific antagonists of the TRPV1 channel, iodoresiniferatoxin (IRTX, 100 nM; EC50 ~4 nM) and N-(4-t-butylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-carboxamide (BCTC, 175 nM; EC50 ~35 nM) [12–14]. Both antagonists inhibited capsaicin-evoked Ca2+ transients 100% (n = 4 for each antagonist, data not shown). IRTX and BCTC both significantly attenuated the amplitude of the CICTs, 45 ± 8% (n = 9) and 33 ± 4% (n = 8), respectively (Fig. 2, Table 1). The inhibition of the CICT produced by these TRPV1 antagonists was not significantly different from the inhibition observed by switching to nominally zero Ca2+ Locke solution, recorded in the same cell (Fig. 2a, Table 1).
Fig. 2.
Effect of IRTX, a TRPV1 selective antagonist on the Ca2+ influx component of caffeine-induced Ca2+ transients (CICTs). a CICTs elicited by 10 mM caffeine in the presence or absence of IRTX (100 nM). IRTX significantly attenuated CICTs. The average CICT amplitude recorded in IRTX was 45 ± 8% of the control CICTs recorded in normal Ca2+ Locke solution (P = 0.001, n = 9). b CICTs elicited by 10 mM caffeine in nominally Ca2+-free Locke containing IRTX. Addition of IRTX did not significantly reduce the amplitude of CICT beyond that produced by nominally Ca+-free Locke solution alone (n = 5)
Table 1.
Effect of nominally zero Ca2+ and TRPV1 antagonists on caffeine-evoked Ca2+ transients recorded in isolated nodose ganglion neurons
| Control | IRTX | Zero Ca2+ Locke |
Zero Ca2+ Locke + IRTX |
n |
|---|---|---|---|---|
| 304 ± 32 | – | 122 ± 29 | 6 | |
| 405 ± 114 | 205 ± 53 | 220 ± 55 | 9 | |
| 398 ± 73 | – | 244 ± 53 | 231 ± 53 | 5 |
| Control | BCTC | Zero Ca2+ Locke |
Zero Ca2+ Locke + BCTC |
n |
| 572 ± 73 | 395 ± 64 | 382 ± 59 | 8 | |
| 395 ± 33 | – | 253 ± 25 | 245 ± 35 | 7 |
Each row represents a separate experimental series. TRPV1 antagonists, IRTX and BCTC, were used at 100 and 175 nM, respectively. Caffeine (10 mM) was applied for 15 s to evoke caffeine-induced Ca2+ transients (CICTs) measured with fura-2. Caffeine was applied at 1.5–3 min intervals. Drug or nominally zero Ca2+ Locke solution preceded the caffeine pulses by 0.5 and 2 min, respectively (see Figs. 1 and 2). The values are in nM. Zero Ca2+ significantly reduced the amplitude of CICT, row 1. The amplitude of the CICT in the presence of IRTX was not significantly different from that recorded in zero Ca2+ and both treatments were significantly different from control values, row 2. Addition of IRTX to zero Ca2+ Locke solution did not significantly change the amplitude of CICT. The amplitude of CICT recorded in zero Ca2+ Locke or IRTX plus zero Ca2+ Locke solution was significantly different from control values, row 3. BCTC significantly reduced the amplitude of CICT and its effect was not significantly different from that measured in zero Ca2+ Locke solution, row 4. The amplitude of the CICT in the presence of BTCT was not significantly different from that recorded in zero Ca2+ Locke solution, but both treatments were significantly different from control values, row 4. Addition of BCTC to zero Ca2+ Locke solution did not significantly reduce the amplitude of the CICTs, row 5. Statistical significance was assessed with an one-way ANOVA performed with Student–Newman–Keuls post hoc test to determine significance for pair-wise comparisons. P < 0.05 indicated statistical significance. Data in row 1 were compared with a paired Student t test; n represents the number of nodose ganglion cells studied in each row
Next, we examined whether TRPV1 antagonists were capable of blocking caffeine-induced Ca2+ influx. We compared the amplitudes of CICTs recorded in Ca2+-free Locke solution and in Ca2+-free Locke solution containing either IRTX or BCTC (Fig. 2b). The data shown in Table 1 indicate that the peak average amplitudes of CICTs in Ca2+-free Locke solution and Ca2+-free Locke solution with IRTX or with BCTC were not significantly different from one another. Finally, the percentage inhibition produced by IRTX, BCTC or zero Ca2+ (45 ± 8%, 33 ± 4% and 54 ± 9%, respectively) was not significantly different from one another. These pharmacological data suggest that caffeine-induced Ca2+ influx is due to the activation of TRPV1.
Discussion
Calcium induced calcium release is mediated by the activation of RyR and caffeine is a classic pharmacological agonist of the ryanodine receptor [15–17]. In rabbit NGNs, caffeine elicits a Ca2+ transient by causing Ca2+ release from the endoplasmic reticulum and by triggering Ca2+ influx across the plasma membrane [2]. Our results using rat NGNs indicate that caffeine can evoke an influx pathway in this species as well (Fig. 1 and “Results” section). Electrophysiological studies in rabbit NGNs demonstrated that the Ca2+ influx pathway was through a non-selective cationic channel [2]. This non-selective cationic channel has not been identified. In the present study, we provide pharmacological evidence suggesting that the non-selective cation channel may be TRPV1.
Using two chemically distinct TRPV1 antagonists, IRTX and BCTC, we demonstrated that the CICTs were significantly attenuated by 45 ± 8% and 33 ± 4%, respectively. The amplitudes of the CICTs recorded in the presence of the TRPV1 antagonists were statistically similar to those elicited in nominally Ca2+-free Locke solution. Moreover, addition of a TRPV1 antagonist to a nominally Ca2+-free Locke solution did not produce a diminution in the CICT amplitude. Thus, we surmise that TRPV1 channels are likely mediators of the caffeine-induced Ca2+ influx pathway. To our knowledge, this is the first report of a TRP channel being activated by caffeine. Whether caffeine acts directly or indirectly to gate the TRPV1 channel is not yet known.
In a separate series of experiments, we studied the effects of caffeine in HEK293 cells stably transfected with TRPV1. These cells did not show CICTs, although they responded to the TRPV1 agonist capsaicin (data not shown). These results suggest that accessory proteins or lipids resident to NGNs, but not to HEK293 cells, might make TRPV1 channels responsive to caffeine (for a recent example of accessory proteins and lipids controlling TRP channels function, see references) [7, 18]. Alternatively, another TRP channel sensitive to IRTX and BCTC could underlie caffeine-induced Ca2+ influx in NGNs. In this regard, it is noteworthy that in the CNS of TRPV1 −/− knockout mice 3H-RTX binding sites are greatly reduced but not eliminated, suggesting the existence of other vanilloid receptors [19].
We observed that 100% of the NGNs tested had CICT indicating that 100% of the cells should contain TRPV1 receptors and respond to capsaicin. Indeed, in our sample of six NGNs all responded to capsaicin. The literature reveals a lower percentage of rat NGNs responding to capsaicin. In isolated NGNs, ~60% of the cells showed capsaicin-evoked Ca2+ transients [20]. Using caged capsaicin analogs, we observed that ~85% of isolated adult NGNs produced Ca2+ transients [9]. Finally, in intact nodose ganglia 60–70% of the cells were depolarized by capsaicin [8]. The larger percentage of NGNs responding to capsaicin in our study may be due to choosing NGNs with small diameters which have been reported to show a greater response to capsaicin than larger cells [20]. Additionally, our dissociation techniques could have favoured the survival of small diameter neurons.
Acknowledgements
We would like to thank Drs. Carlos Magno Daher, Salim Kanaan and K.·S. Jagannatha Rao for general support for this project. This work was supported by NIH grant NS-22069 (D.W.).
Contributor Information
João Paulo L. Daher, Email: jpldaher@hotmail.com, Department of Pathology, School of Medicine, Fluminense Federal University, 303 Marquês do Paraná Street, Room 4, Niterói, RJ 24033-900, Brazil.
Tony D. Gover, Department of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine, Baltimore, MD, USA
Thais H. V. Moreira, Department of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine, Baltimore, MD, USA
Vânia G. S. Lopes, Department of Pathology, School of Medicine, Fluminense Federal University, 303 Marquês do Paraná Street, Room 4, Niterói, RJ 24033-900, Brazil
Daniel Weinreich, Department of Pharmacology and Experimental Therapeutics, University of Maryland School of Medicine, Baltimore, MD, USA.
References
- 1.Usachev YM, Thayer SA. Controlling the urge for a Ca2+ surge: all-or-none Ca2+ release in neurons. Bioessays. 1999;21:743–750. doi: 10.1002/(SICI)1521-1878(199909)21:9<743::AID-BIES5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 2.Hoesch RE, Weinreich D, Kao JPY. A novel Ca2+ influx pathway in mammalian primary sensory neurons is activated by caffeine. J Neurophysiol. 2001;86:190–196. doi: 10.1152/jn.2001.86.1.190. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AS, Moore KA, Bangalore R, Jafri MS, Weinreich D, Kao JP. Ca2+-induced Ca2+ release mediates Ca2+ transients evoked by single action potentials in rabbit vagal afferent neurones. J Physiol. 1997;499:315–328. doi: 10.1113/jphysiol.1997.sp021929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkable functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 5.Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002;42:873–888. doi: 10.1016/s0028-3908(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen SF, Grzegorz O, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;179:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 8.Marsh SJ, Stansfeld CE, Brown DA, Davey R, McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987;23:275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Gover TD, Muralidharan S, Auston DA, Weinreich D, Kao JP. Caged vanilloid ligands for activation of TRPV1 receptors by 1- and 2-photon excitation. Biochemistry. 2006;45:4915–4926. doi: 10.1021/bi052082f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jafri MS, Moore KA, Taylor GE, Weinreich D. Histamine H1 receptor activation blocks two classes of potassium current, IK(rest) and IAHP, to excite ferret vagal afferents. J Physiol. 1997;503:533–546. doi: 10.1111/j.1469-7793.1997.533bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 12.Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- 13.Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. In vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306:387–393. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- 14.Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, Sun Q, Rotshteyn Y, Francis J, Limberis J, Malik S, Whittemore ER, Hodges D. N-(4-t-Butylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-carboxamide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. In vitro characterization and pharmacokinetic properties. J Pharmacol Exp Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- 15.Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- 16.Fabiato A, Fabiato F. Contractions induced by calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitsapesan R, Williams AJ. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambudkar IS, Bandyopadhyay BC, Liu X, Lockwich TP, Paria B, Ong HL. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium. 2006;40:495–504. doi: 10.1016/j.ceca.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JC, Davis JB, Benham CD. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Chung E, Gu Q, Kwong K, Arden WA, Lee LY. Comparison of capsaicin-evoked calcium transients between rat nodose and jugular ganglion neurons. Auton Neurosci. 2002;97:83–88. doi: 10.1016/s1566-0702(02)00045-0. [DOI] [PubMed] [Google Scholar]