Abstract
We report the clinical, neuropsychological, genetic and radiological features of a large five-generation African-American kindred from the southern United States presenting with a progressive akinetic-rigid syndrome and severe dementia, but clinically insignificant chorea, due to mutations in JPH3. Overt disease onset was in the mid-twenties to late thirties with cognitive decline, REM sleep disturbance or psychiatric features, followed by development of a levodopa-unresponsive akinetic-rigid motor syndrome. Dystonia and myoclonus were present in some subjects. A bedridden, non-verbal severely akinetic-rigid state developed within 10 to 15 years after onset. CTG repeat expansions ranged from 47 to 53. Imaging revealed generalized cerebral atrophy with severe striatal involvement and putaminal rim hyperintensity. Analysis of our kindred indicates that JPH3 mutations should be considered in the differential diagnosis of early-onset dementia and hypokinetic-rigid syndromes in individuals of African descent. Moreover, chorea may not be overtly manifest at presentation or during significant parts of the disease course.
Keywords: Dementia, Parkinsonism, Chorea, Huntington disease, JPH3, Putaminal Rim, African-American
Introduction
Huntington’s disease (HD) is caused by a triplet repeat expansion in HTT. Mutations in HTT are not found in all individuals with symptoms and signs suggestive of HD, thereby implying the existence of other causes of this syndrome. In recent years, distinct genetic disorders mimicking HD have been identified — termed HD-like (HDL) syndromes. Among these, a CTG expansion in junctophillin 3 (JPH3) has been associated with HDL2 (OMIM 606438), originally reported in a three-generation family (pedigree W) from the southeast United States [1]. The clinical phenotype comprised early weight loss, involuntary choreic movements, dystonia, incoordination, dysarthria, pyramidal signs and psychiatric features with onset in the fourth decade. In late disease stages, about 10 to 15 years after onset, patients became bradykinetic and bedridden. The authors noted similarities to HD and it is generally thought that HDL2 resembles HD more than any other disease and these two disorders show pathological similarities [2–4].
Since the original description of HDL2, additional affected individuals and small kindreds have been reported in the literature, and the phenotypic spectrum of HDL2 has been expanded. However, JPH3 genotype-phenotype correlations remain weak and detailed descriptions of intrafamilial variability, neuropsychological profiles and radiological findings in kindreds with HDL2 is limited. Herein, we report a large five-generation autosomal-dominant kindred from the southern United States presenting with a progressive akinetic-rigid syndrome and severe dementia, but clinically insignificant chorea, due to mutations in JPH3.
Subjects and methods
We encountered a five-generation family of African ancestry in the southern United States. Family members were concentrated in Mississippi with more distant relatives located in Florida and Texas. There were no known links to pedigree W reported in the literature. All human studies were performed in accordance with institutional review board guidelines and all subjects gave written informed consent for genetic analyses, and use of their videos, audios and photographs. Family members were evaluated with the following clinical/neuropsychological rating scales: the Unified Parkinson Disease Rating Scale (UPDRS), Unified Huntington Disease Rating Scale (UHDRS) and the Mini-Mental State Examination (MMSE). The UHDRS includes motor (I), cognitive (II), behavioral (III), functional (IV), independence (V) and functional capacity scales (VI). The cognitive assessment includes the Stroop interference test along with tests of verbal fluency and symbol digit modalities.
DNA was extracted from peripheral blood leukocytes using Roche’s DNA Isolation Kit for Mammalian Blood (Indianapolis, IN, USA). DNA quantity and quality were analyzed with a NanoDrop ND-100 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Genomic DNA was PCR amplified (32 cycles: 94°C for 20 s, 60°C for 30 s, and 72°C for 30 s) using the HotStarTaq® Plus DNA polymerase from Qiagen (Valencia, CA, USA) with the following primer pair (forward: agatgccaccgcattcgg, and reverse: ggttccctgcacagaaaccatc). Each PCR reaction mix (Qiagen) contained 200 µM dNTPs, 200 nM primers, 1× PCR buffer (1.5 mM MgCl2, pH 8.7), 1× Q-Solution (Qiagen), 2.5 units polymerase, and 100 ng of template DNA in a final volume of 50 µl. PCR products were examined on agarose gels, bands were precisely cut from gels, and DNA was purified with the Qiagen QIAquick Gel Extraction Kit prior to Sanger sequencing in the forward and reverse directions using the Applied Biosystems BigDye Terminator v3.1 chemistry (Life Technologies, Carlsbad, CA, USA) on an Applied Biosystems 3130XL Genetic Analyzer.
Age of onset and CTG repeat numbers (normal and expanded alleles) were extracted from the published literature and integrated with our own data from 6 affected subjects. Univariate and correlation analyses were performed with SAS (Cary, NC, USA).
Results
The pedigree of this family is shown in Fig. 1. The clinical features of six affected family members are summarized in Tables 1 and 2.
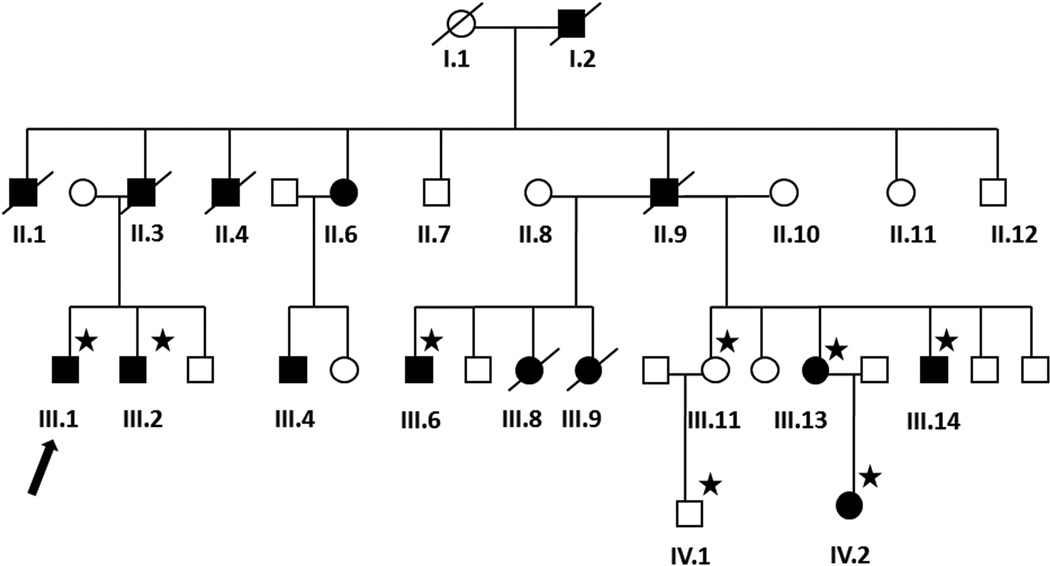
Fig. 1.
Family pedigree. Males are represented by squares, females by circles. Affected individuals are represented by filled/black symbols and asymptomatic family members by empty/white symbols. Symbols of deceased individuals are slashed. Blood for genetic analysis was available from those denoted with a star. The proband is denoted with an arrow.
Table 1.
Clinical and genetic characteristics of subjects with JPH3 CTG expansionsa
| Subject | III.1 | III.2 | III.6 | III.13 | III.14 | IV.2 |
|---|---|---|---|---|---|---|
| Video | Video 1 | - | Video 2 | - | - | Video 3 |
| Gender | M | M | M | F | M | F |
| CTG Repeat Lengths | 15/47 | 14/53 | 16/53 | 15/51 | 16/53 | 14/51 |
| Age at onset (years) | 32 | 30 | 39 | 35 | 33 | 30 |
| Age at exam (years) | 44 | 41 | 45 | 48 | 43 | 34 |
| Disease duration | 12 | 11 | 6 | 13 | 10 | 4 |
| Symptoms at onset | Dysarthia, apathy, cognitive decline | Insomnia, suicidal attempts | Cognitive decline with memory difficulty | Irritability, walking difficulty, foot drag | Cognitive decline with memory difficulty | Restlessness, cognitive decline, sleep disturbance, mild chorea |
| Weight loss | + | + | + | ++ | ++ | + |
| Psychiatric symptoms | + | ++ | + | + | ++ | + |
| Dementia | ++ | + | ++ | ++ | ++ | + |
| Primitive reflexes | -- | -- | + | + | + | -- |
| Range of gaze | N | N | N | N | N | N |
| Saccades | H | N | H | N | H | N |
| Dysarthria/Anarthria | + | ++ | ++ | ++ | + | (+) |
| Dysphagia | + | -- | + | ++ | + | -- |
| Reduced movement | ++ | + | +++ | +++ | + | (+) |
| Rigidity | ++ | + | +++ | +++ | + | (+) |
| Bradykinesia | ++ | + | +++ | +++ | + | + |
| Rest tremor | + | -- | -- | + | -- | -- |
| Action tremor | -- | + | -- | -- | + * | -- |
| Myoclonus | + | -- | -- | + | + | -- |
| Chorea | -- | (+) | -- | -- | (+) | + |
| Dystonia | -- | -- | ++ | ++ | -- | + ** |
| Reflexes | N | N | Brisk | Brisk | N | N |
| Extensor plantars | -- | -- | -- | -- | -- | -- |
| Ataxia / Dysmetria | -- | -- | (+) | -- | -- | -- |
| Gait abnormalities | + | - | ++ | +++ | + | + |
| Postural reflexes | + | - | ++ | +++ | -- | -- |
To facilitate comparisons, adapted from Table 1 in Margolis et al. [1]. H = hypometric saccades; N = normal; +++ = markedly positive/present or severe; ++ = obviously positive/present; + =mildly positive/present; (+) = very mildly or only intermittently positive/present; * = mild terminal tremor; ** = retrocollis and mild tongue deviation dystonia. Repeat numbers of unaffected family members are as follows: II.3 (14/15), IV.1 (13/14) and III.11 (13/16).
Table 2.
Clinical rating scale scores of affected HDL2 patients assessing motor and cognitive function
| Subject | III.1 | III.2 | III.6 | III.13 | III.14 | IV.2 | |
|---|---|---|---|---|---|---|---|
| UPDRS motor score | 54 | 29 | 76 | 91 | 34 | 17 | |
| UHDRS motor score (I) | 65 | 45 | 80 | 94 | 40 | 22 | |
| UHDRS chorea subscore | 3 | 7 | 0 | 4 | 3 | 5 | |
| UHDRS cognitive score (II) | NA | 158 | NA | NA | 56 | 270 | |
| Verbal fluency test | NA | 9 | NA | NA | NA | 45 | |
| Stroop test | NA | 42-62-28 | NA | NA | 26-30-NA | 64-90-22 | |
| Symbol digit modalities test | NA | 17 | NA | NA | NA | 49 | |
| UHDRS behavioral score (III) | NA | 19 | NA | NA | 0 | 20 | |
| UHDRS functional score (IV) | 7 | 11 | 0 | 0 | 12 | 25 | |
| UHDRS independence score (V) | 55 | 65 | 50 | 20 | 70 | 100 | |
| UHDRS functional capacity scale (VI) | 3 | 5 | 2 | 0 | 0 | 13 | |
| MMSE | NAa | 19 | NA | NA | 7 | 24b | |
UPDRS = United Parkinson disease rating scale motor score (possible range, 0 –108); UHDRS parts I and III, higher scores are associated with more advanced disease; UHDRS parts II, IV, V and VI, lower scores are associated with more advanced disease. UHDRS = United Huntington disease rating scale motor score (I) has a range of 0 –124; UHDRS chorea subscore = chorea-relevant motor score of the UHDRS, i.e. item 12a–g (possible range, 0–28); MMSE = Mini Mental State Examination; NA, test results could not be acquired secondary to mutism and/or severe dementia.
18 months prior, MMSE = 8; 12 months prior, MMSE = 10.
7 months prior, MMSE =27.
Case III.1
At age 32 years, this previously fit and well construction worker, developed dysarthria and cognitive decline, followed by gait difficulty. Soon he required help with cooking and dressing. His past medical history included hypercholesterolemia and gastric reflux. On examination at age 41, he was found disoriented and apathetic. Eye movements were overall relatively well preserved with normal spontaneous eye movements and full gaze range including up and down gaze. Saccades were slightly hypometric. He had facial hypomimia and his voice was hypophonic. There was prominent generalized rigidity affecting his trunk and extremities. Power, reflexes and plantar responses were unremarkable. There were no cerebellar signs. Gait was shuffling with decreased arm swing and unsteady with reduced postural reflexes and impaired tandem gait.
On follow-up at age 44 (Video 1), he had impaired attention and orientation. He scored 10 on the Mini-Mental State Examination (MMSE) with multi-domain deficits. He was unable to perform the Luria sequence. He had moderate dysarthria and a hypophonic voice. There was marked rigidity, no rest tremor, but mild postural and kinetic action tremors. Low amplitude myoclonus was noted in his fingers. Mild chorea affected the feet, but not the trunk, face or arms. His gait was unsteady with mildly reduced postural reflexes. Findings of this exam are summarized in Table 1.
Routine blood tests including ferritin, ceruloplasmin and copper studies were normal. Genetic testing was negative for HD and SCA3 repeat expansions. An electroencephalogram showed diffuse background disorganization with slowing to 6–7 Hz without epileptiform discharges. Brain computed tomography was performed at age 41, and magnetic resonance imaging (MRI) was performed at age 42 (see below). A trial of levodopa had limited benefit. He continues to take galantamine for dementia.
Case III.6
At age 39 this man developed impulsivity and cognitive decline. Around the same time his family noted that he had difficulty coordinating his hands, gait impairment and mild truncal chorea. He had a medical history of hypertension, diabetes, hypercholesterolemia and urinary tract infections; and a past medical history of marijuana and cocaine abuse.
On examination at age 40, he was slightly disoriented and had a tendency to perseverate. His face was hypomimic and his voice was hypophonic. Eye movements were grossly intact. Symmetrical rest tremor of the hands was noted. When keeping the hands outstretched, there was mild choreiform movements of the fingers. He had mild terminal tremor on finger-to-nose testing. Motor power was normal. Gait revealed decreased arm swing and unsteadiness on tandem gait.
Due to further deterioration, he had to withdraw from his job in a fish factory at age 41. At age 44 he lost independent ambulation and became anarthric. Video 2 demonstrates his examination at age 45.
Case IV.2
At age 31, she presented to her local general practitioner because of restlessness which had begun one year prior. She had a past medical history of headaches, but had otherwise felt fit and well. Examination revealed cognitive decline with impaired memory, spelling and counting and pathological clock-drawing. She had a mild action tremor of her arms and decreased arm swing when walking. Only very infrequent, low amplitude, choreiform movements of the trunk and arms were noted. The remainder of the neurological examination was normal. Brain MRI showed very mild generalized atrophy. On follow up at age 32, she reported difficulties with fine finger movements and writing which had become smaller. She also noted difficulty walking and poor coordination. She reported hyperhidrosis, mainly affecting the palms and feet. She denied delusions or hallucinations. She scored 27/30 on the MMSE. She had mild generalized chorea accompanied by Parkinsonian features such as hypomimia, bradykinesia of repetitive movements, reduced arm swing, micrographia, rigidity and small-stepped gait. At age 33, she developed vivid dreams and talked in her sleep. She also felt increasingly depressed and anxious. Therefore, treatment with clonazepam and sertraline was initiated. Video 3 demonstrates the patient at age 34.
Neuropsychological testing
Table 2 provides results of the MMSE, verbal fluency test, Stroop test and symbol digit modalities test for each subject.
Neuroimaging
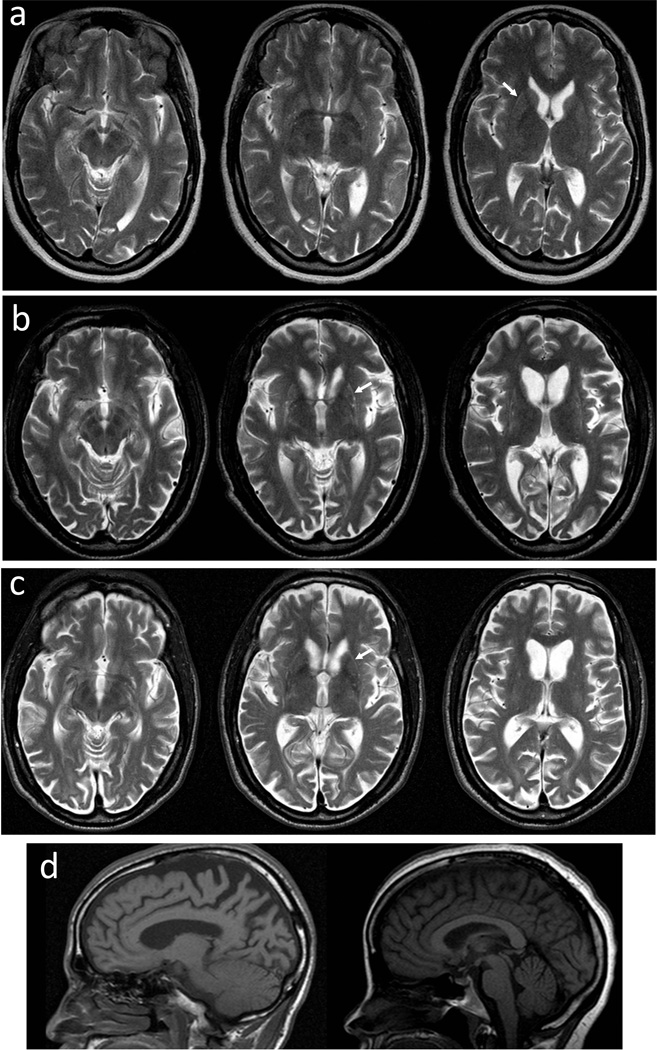
Computed tomography (CT) imaging of patient III.1 at age 41 demonstrated moderate-to-marked cerebral atrophy with volume loss in the caudate and putamen, and no evidence of parenchymal calcifications or signal changes in the white matter. MRIs were available for 3 affected individuals in the kindred (Figs. 2 and S1). In 2 subjects with more advanced clinical manifestations, MRIs revealed marked bilateral atrophy of the caudate heads and putamina in the context of generalized brain atrophy, predominantly affecting the parietal-occipital regions (Fig. S1). Putaminal rim hyperintensities of variable clarity, best appreciated on T2-weighted images, were detected in these 3 subjects (Fig. 2). The brainstems and cerebellae appeared relatively well preserved (Fig. 2d). T2*-weighted images from subject IV.2 showed some iron deposition in the globus pallidus, which was thought to be at the upper limits of normal.
Fig. 2.
Magnetic resonance images (MRIs) of subjects III.1, III.6, and IV.2. a T2-axial images from subject IV.2 two years after disease onset. b T2-axial images from subject III.1 ten years after disease onset. c T2-axial images from subject III.6 two years after disease onset. Putaminal rim hyperintensities are marked with arrows. d Sagittal MRIs show that the brainstems and cerebellae were relatively well preserved in subjects III.6 (left) and IV.2 (right).
Genetic Data
Affected members had CTG repeat expansions within the JPH3 gene, ranging from 47 to 53 (Table 1). Both clinically-unaffected family members that were tested had normal repeat lengths. Meiotic instability was observed in a maternal transmission (III.13 and IV.2, Table 1).
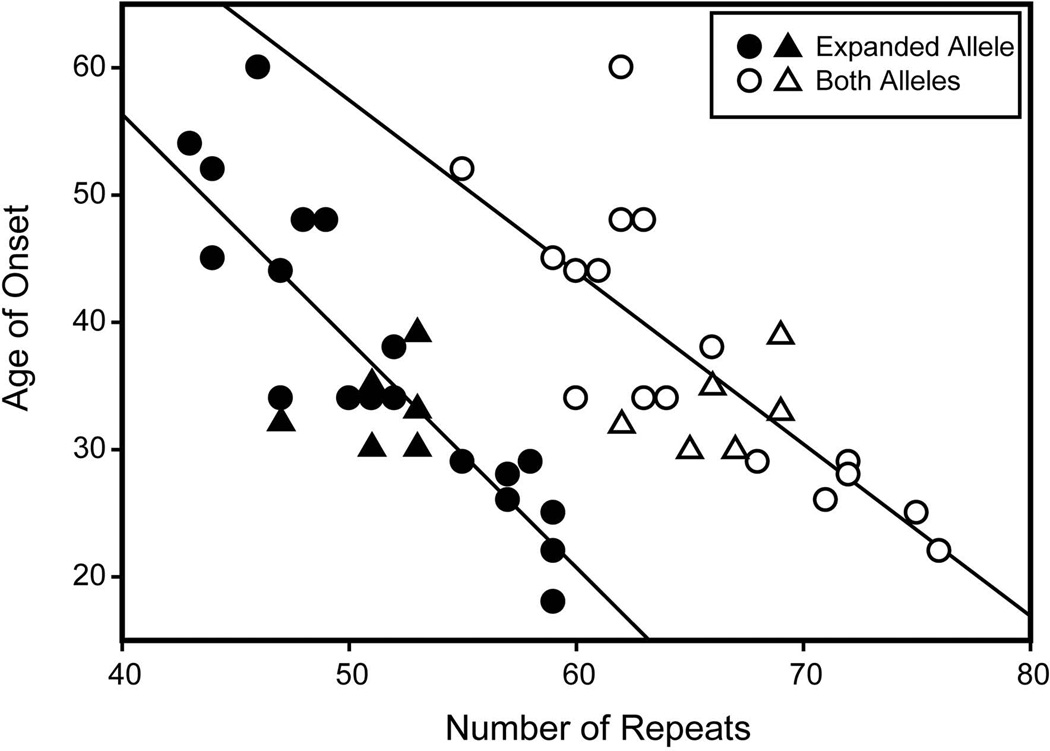
When integrated with previously published cases (Table 3), we found that age of onset was negatively correlated with the number of CTG repeats on the expanded allele (r = −0.85, p < 0.001) and both alleles (r = −0.76, p < 0.001). Overall, for the entire group of 26 subjects, mean age of onset was 36.3 ± 10.4 yrs with a range of 18 to 60 yrs. The mean number of CTG repeats on the expanded allele was 51.3 ± 4.9 with a minimum of 43 and maximum of 59.
Table 3.
Published JPH3 CTG repeat lengths and ages of disease onset
| Publication | Subject | CTG Repeat Length |
Age at Onset |
|---|---|---|---|
| Walker et al. [19] | Patient 1 (W) | 14/57 | 26 |
| Patient 2 | 15/44 | 45 | |
| Patient 3 | 13/51 | 34 | |
| Patient 4 | 14/58 | 29 | |
| Patient 5 | 15/57 | 28 | |
| Bardien et al. [7] | III-17 | 16/59 | 25 |
| III-9 | 14/49 | 48 | |
| IV-1 | 14/52 | 38 | |
| Greenstein et al. [3] | Family 1 | --/43 | 54 |
| Family 2 son | --/ 59 | 18 | |
| Family 2 father | --/52 | 34 | |
| Rudnicki et al. [4] | HDL2-2205 | 13/50 | 34 |
| HDL2-2232 | 13/47 | 44 | |
| HDL2-2244 | 11/44 | 52 | |
| HDL2-2261 | 13/55 | 29 | |
| HDL2-2267 | 13/54 | NA | |
| Santos et al. [20] | Index | 14/47 | 44 |
| Rodrigues et al. [21] | Patient 1 | 13/47 | 34 |
| Patient 2 | 17/59 | 22 | |
| Patient 3 | 16/46 | 60 | |
| Patient 4 | 14/48 | 48 |
Discussion
Here we report a large five-generation family from the southern United States with an autosomal dominant progressive neurological disorder with motor impairment and severe dementia due to CTG repeat expansions in JPH3. While previous reports of JPH3-associated neurodegeneration highlighted chorea as a key feature, giving rise to the designation as an HD-like disorder, the motor phenotype in our family was dominated by a severe akinetic-rigid syndrome, whereas chorea was relatively mild.
In our family, mean age at onset was in the fourth decade in line with previous reports of HDL2, and similar to the age range associated with classic HD. The clinical course in our patients began with cognitive decline mainly manifest as memory difficulty, REM sleep disturbance or psychiatric features including impulsivity, anxiety, depression, and substance abuse, again compatible with presenting manifestations in some patients with HD. However, in our affected family members, full blown dementia developed rapidly and affected multiple spheres including attention, orientation, memory, language, praxis and visuo-spatial skills, reflected by low scores on the MMSE, verbal fluency test, Stroop test and symbol digit modalities test.
The movement disorder phenomenology of classic HD is predominated by chorea and motor impersistence while hypokinesia mainly occurs in advanced disease stages [5]. In contrast, there was a paucity of chorea in our HDL2 patients and little or no motor impersistence. Instead, the phenotype was predominated by an akinetic-rigid syndrome without an associated Parkinsonian tremor, and modest postural instability which was seen later in the disease course. The preponderance of negative motor features exhibited by our subjects is reflected by their high UPDRS motor scores and the small UHDRS chorea subscores in relation to their total UHDRS motor scores (Table 2).
In affected family members, the disease progressed to a severely akinetic-rigid bedridden, nonverbal state with almost no spontaneous movements within 10 to 15 years after onset. Although our correlation analyses showed that larger CTG repeats tended to be associated with earlier ages of onset, we could not identify reliable associations between CTG repeat lengths and other phenotypic features of HDL2 such as chorea severity or rate of disease progression. Our patients showed no response to levodopa, similar to observations reported for the hypokinesia seen in more advanced cases of HD [6]. In line with a previous report [7], we noted the presence of dystonia and myoclonus, both of which can be seen in HD, in some family members with HDL2 [8]. Progressive weight loss, which was an early sign in the original family with HDL2 [1], occurred throughout the disease course in our kindred, and is a common feature of HD.
In HD, eye movement abnormalities are common, mainly consisting of slow hypometric saccades and catchy smooth pursuit and are already present in pre-symptomatic disease stages [9]. In contrast, in our HDL2 patients, eye movements were relatively intact with normal spontaneous eye movements and full gaze range including up and down gaze. Saccades were slightly hypometric in some affected family members but normal in others. In our patients, mildly hypometric saccadic eye movements may simply represent a nonspecific sign of a dementing illness. Cerebellar signs were absent in the affected individuals- if present, HDL4 may have come into diagnostic consideration.
In keeping with previous reports, neuroimaging showed severe striatal atrophy in addition to moderate generalized cerebral atrophy, most prominent in the parietal-occipital regions in our cases. The brainstem and cerebellum appeared well preserved on MRI in our patients, consistent with their clinical findings of grossly normal eye movements and cerebellar testing. We wish to highlight the presence of putaminal rim hyperintensity on MRI, as early as two years after disease onset. It remains uncertain whether putaminal rim hyperintensity is present only in a proportion of patients or at certain points during the disease course. Interestingly, putaminal rim has been observed in a case of SCA17, another HD-like disorder (HDL4) [10, 11]. Striatal hyperintensity [10] may also occur in DRPLA and neuroacanthocytosis, both also important differential diagnoses of HD. Putaminal rim is otherwise most typically associated with MSA. In addition, it has recently been described as a constant feature in the dystonic, combined, and Parkinsonian phases of X-linked dystonia Parkinsonism (Lubag), in which caudate atrophy was also present in the majority of patients [12]. The sign may thus be an imaging correlate of neurodegenerative cell loss with in the striatum.
HD and HDL2 are histologically similar, characterized by prominent striatal atrophy with selective loss of medium spiny neurons and astrocytic gliosis in a dorsal to ventral gradient [4] as well as presence of nuclear protein aggregates and neuronal ubiquitin-immunoreactive intranuclear inclusions [3]. Pathological differences, however, include more occipital lobe involvement in HDL2, whereas more brainstem involvement in HD and this is in line with our clinical and neuroimaging findings.
HDL2 is rare; representing only about 0.7% of patients with a clinical picture indistinguishable from HD who are negative for HTT mutations, as based on pooled data of 1,247 cases [13–15]. The frequency, however, varies between populations and HDL2 seems relatively common in black Africans [2, 16] and all cases reported to date had definite or probable African roots, consistent with haplotype studies [7]. Our family was also of black African descent. In fact, according to the U.S. Census 2010 [17], the State of Mississippi had the highest percentage of African-Americans in the United States (37%), in line with migration from the coast of West Africa, between present-day Senegal and Angola, into the Delta region in the early 19th century. A study of South African patients who presented with a phenotype compatible with HD revealed that only about one third of black patients actually carried HTT expansions; whereas one quarter of black patients and 50% of mixed-ancestry patients had HDL2-causing expansions in JPH3, emphasizing the importance of this disorder in this racial group [18].
Analysis of our Mississippi pedigree shows that expanded repeats in JPH3 should be included in the differential diagnosis of early-onset dementia and bradykinetic-rigid syndromes in individuals of black African descent. In a significant proportion of affected individuals, chorea may be absent or subtle during major periods of the disease process. Penetrance is very high and a positive family history should prompt genetic testing. Additional diagnostic clues include striatal atrophy and putaminal rim on MRI, relatively intact eye movements and hypophonic dysarthria. Age of onset shows an inverse linear association with CTG repeat length on the expanded allele [3, 4, 7, 19–21].
Supplementary Material
Fig. 3.
Correlations between JPH3 CTG repeat lengths and HDL2 age-of-onset. Previously published cases are marked with circles whereas triangles are used to identify those subjects newly described herein. Linear coefficients of determination (r2) for the expanded allele and both alleles were 0.72 and 0.57, respectively.
Acknowledgements
We thank the family for their participation; Professor Adolfo Bronstein, Imperial College London, for his opinion on the eye movements; and Dr. Amrish Mehta, Charing Cross Hospital, London, for evaluation of the MRI images.
Footnotes
Disclosure None of the authors have a conflict of interest. Dr. LeDoux serves on the speakers’ bureaus for Lundbeck, Merz, and Teva Neuroscience; serves as an advisor for Merz; serves on the Xenazine Advisory Board for Lundbeck, Inc., and the Botulinum Toxin Type A Advisory Board for Allergan; receives research support from the National Institutes of Health, Dystonia Medical Research Foundation, and Merz; and receives royalty payments for Animal Models of Movement Disorders (Elsevier).
References
- 1.Margolis RL, O'Hearn E, Rosenblatt A, Willour V, Holmes SE, Franz ML, Callahan C, Hwang HS, Troncoso JC, Ross CA. A disorder similar to Huntington's disease is associated with a novel CAG repeat expansion. Ann Neurol. 2001;50:373–380. doi: 10.1002/ana.1312. [DOI] [PubMed] [Google Scholar]
- 2.Margolis RL, Rudnicki DD, Holmes SE. Huntington's disease like-2: review and update. Acta Neurol Taiwan. 2005;14:1–8. [PubMed] [Google Scholar]
- 3.Greenstein PE, Vonsattel JP, Margolis RL, Joseph JT. Huntington's disease like-2 neuropathology. Mov Disord. 2007;22:1416–1423. doi: 10.1002/mds.21417. [DOI] [PubMed] [Google Scholar]
- 4.Rudnicki DD, Pletnikova O, Vonsattel JP, Ross CA, Margolis RL. A comparison of Huntington disease and Huntington disease-like 2 neuropathology. J Neuropathol Exp Neurol. 2008;67:366–374. doi: 10.1097/NEN.0b013e31816b4aee. [DOI] [PubMed] [Google Scholar]
- 5.Penney JB, Jr, Young AB, Shoulson I, Starosta-Rubenstein S, Snodgrass SR, Sanchez-Ramos J, Ramos-Arroyo M, Gomez F, Penchaszadeh G, Alvir J, et al. Huntington's disease in Venezuela: 7 years of follow-up on symptomatic and asymptomatic individuals. Mov Disord. 1990;5:93–99. doi: 10.1002/mds.870050202. [DOI] [PubMed] [Google Scholar]
- 6.García Ruiz PJ, Hernández J, Cantarero S, Bartolomé M, Sánchez Bernardos V, García de Yébenez J. Bradykinesia in Huntington's disease. A prospective, follow-up study. J Neurol. 2002;249:437–440. doi: 10.1007/s004150200035. [DOI] [PubMed] [Google Scholar]
- 7.Bardien S, Abrahams F, Soodyall H, van der Merwe L, Greenberg J, Brink T, Carr J. A South African mixed ancestry family with Huntington disease-like 2: clinical and genetic features. Mov Disord. 2007;22:2083–2089. doi: 10.1002/mds.21672. [DOI] [PubMed] [Google Scholar]
- 8.Becker N, Munhoz RP, Raskin S, Cesar W, Teive HA. Non-choreic movement disorders as initial manifestations of Huntington's disease. Arq Neuro-Psiquiatr. 2007;65:402–405. doi: 10.1590/s0004-282x2007000300007. [DOI] [PubMed] [Google Scholar]
- 9.Ross CA, Margolis RL. Huntington's disease. Clin Neurosci Res. 2001;1:142–152. [Google Scholar]
- 10.Loy CT, Sweeney MG, Davis MB, Wills AJ, Sawle GV, Lees AJ, Tabrizi SJ. Spinocerebellar ataxia type 17: extension of phenotype with putaminal rim hyperintensity on magnetic resonance imaging. Mov Disord. 2005;20:1521–1523. doi: 10.1002/mds.20529. [DOI] [PubMed] [Google Scholar]
- 11.Bech S, Petersen T, Nørremølle A, Gjedde A, Ehlers L, Eiberg H, Hjermind LE, Hasholt L, Lundorf E, Nielsen JE. Huntington's disease like and ataxia syndromes: identification of a family with a de novo SCA17/TBP mutation. Parkinsonism Relat Disord. 2010;16:12–15. doi: 10.1016/j.parkreldis.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Pasco PM, Ison CV, Muňoz EL, Magpusao NS, Cheng AE, Tan KT, Lo RW, Teleg RA, Dantes MB, Borres R, Maranon E, Demaisip C, Reyes MV, Lee LV. Understanding XDP through imaging, pathology, and genetics. Int J Neurosci. 2011;121(Suppl 1):12–17. doi: 10.3109/00207454.2010.526729. [DOI] [PubMed] [Google Scholar]
- 13.Stevanin G, Camuzat A, Holmes SE, Julien C, Sahloul R, Dodé C, Hahn-Barma V, Ross CA, Margolis RL, Durr A, Brice A. CAG/CTG repeat expansions at the Huntington's disease-like 2 locus are rare in Huntington's disease patients. Neurology. 2002;58:965–967. doi: 10.1212/wnl.58.6.965. [DOI] [PubMed] [Google Scholar]
- 14.Stevanin G, Fujigasaki H, Lebre AS, Camuzat A, Jeannequin C, Dode C, Takahashi J, San C, Bellance R, Brice A, Durr A. Huntington's disease-like phenotype due to trinucleotide repeat expansions in the TBP and JPH3 genes. Brain. 2003;126:1599–1603. doi: 10.1093/brain/awg155. [DOI] [PubMed] [Google Scholar]
- 15.Wild EJ, Mudanohwo EE, Sweeney MG, Schneider SA, Beck J, Bhatia KP, Rossor MN, Davis MB, Tabrizi SJ. Huntington's disease phenocopies are clinically and genetically heterogeneous. Mov Disord. 2008;23:716–720. doi: 10.1002/mds.21915. [DOI] [PubMed] [Google Scholar]
- 16.Bauer I, Gencik M, Laccone F, Peters H, Weber BH, Feder EH, Weirich H, Morris-Rosendahl DJ, Rolfs A, Gencikova A, Bauer P, Wenning GK, Epplen JT, Holmes SE, Margolis RL, Ross CA, Riess O. Trinucleotide repeat expansions in the junctophilin-3 gene are not found in Caucasian patients with a Huntington's disease-like phenotype. Ann Neurol. 2002;51:662. doi: 10.1002/ana.10184. [DOI] [PubMed] [Google Scholar]
- 17.Rastogi S, Johnson TD, Hoeffel EM, Drewery MP., Jr The Black Population: 2010. U.S. Census Bureau. 2010 available at http://www.census.gov/prod/cen2010/briefs/c2010br-06.pdf.
- 18.Krause A, Hetem C, Holmes SE, Margolis RL. HDL2 mutations are an important cause of Huntington's disease in patients with African ancestry. J Neurol Neurosurg Psychiatry. 2005;76:A16–A26. [Google Scholar]
- 19.Walker RH, Rasmussen A, Rudnicki D, Holmes SE, Alonso E, Matsuura T, Ashizawa T, Davidoff-Feldman B, Margolis RL. Huntington's disease--like 2 can present as chorea-acanthocytosis. Neurology. 2003;61:1002–1004. doi: 10.1212/01.wnl.0000085866.68470.6d. [DOI] [PubMed] [Google Scholar]
- 20.Santos C, Wanderley H, Vedolin L, Pena SD, Jardim L, Sequeiros J. Huntington disease-like 2: the first patient with apparent European ancestry. Clin Genet. 2008;73:480–485. doi: 10.1111/j.1399-0004.2008.00981.x. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues GG, Walker RH, Brice A, Cazeneuve C, Russaouen O, Teive HA, Munhoz RP, Becker N, Raskin S, Werneck LC, Junior WM, Tumas V. Huntington's disease-like 2 in Brazil--report of 4 patients. Mov Disord. 2008;23:2244–2247. doi: 10.1002/mds.22223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.