MvfRC87, a 242-residue C-terminal segment of the LysR-type transcriptional regulator MvfR, was produced in Escherichia coli, purified and crystallized.
Keywords: MvfR, LysR-type transcriptional regulators, Pseudomonas aeruginosa
Abstract
The LysR-type transcriptional regulator MvfR plays a critical role in Pseudomonas aeruginosa pathogenicity via the transcriptional regulation of multiple quorum-sensing-regulated virulence factors. The protein also controls pathogenic type VI secretion loci. MvfRC87, a 242-residue C-terminal segment of MvfR, was produced in Escherichia coli, purified and crystallized. X-ray diffraction data were collected using synchrotron radiation and crystallographic parameters were determined.
1. Introduction
Pseudomonas aeruginosa is an opportunistic human pathogen which belongs to the category of Gram-negative bacteria and causes infections in immunocompromised and cystic fibrosis patients; it is one of the most common hospital pathogens (Xiao, Déziel et al., 2006 ▶). Because of its ability to rapidly develop resistance to a variety of antibiotics, P. aeruginosa represents a major risk factor for nosocomial infections; the study of factors associated with its virulence is thus of major importance to public health. The pathogen utilizes a quorum-sensing system (Fuqua et al., 2001 ▶) in the regulation of its gene expression in response to cell density (Déziel et al., 2004 ▶).
The MvfR protein (NCBI reference code NC_008463) is a LysR-type transcriptional regulator. LysR-type transcriptional regulators are the largest family of prokaryotic transcription factors and regulate a diverse set of genes involved in virulence, metabolism, quorum sensing and motility. They are composed of a DNA-binding domain and a cofactor-binding domain (Maddocks & Oyston, 2008 ▶). Several structures of proteins of the LysR family are known; the first structure to be reported was that of the cofactor-binding fragment of the CysB protein from Klebsiella aerogenes (Tyrrell et al., 1997 ▶), which revealed several similarities to structures of periplasmic substrate-binding proteins, although the subunit arrangement is different.
MvfR plays a critical role in the virulence of P. aeruginosa, not only by modulating the expression of multiple quorum-sensing-regulated virulence factors (Xiao et al., 2006 ▶; Déziel et al., 2004 ▶) but also by positively controlling the type VI secretion (T6S) loci HSI-II and HSI-III and negatively controlling the HSI-I locus of P. aeruginosa (Lesic et al., 2009 ▶).
P. aeruginosa controls many virulence factors using the las and rhl quorum-sensing (QS) systems, in which the LasR and RhlR proteins function as transcriptional activators of downstream virulence genes (Gallagher et al., 2002 ▶). A further cellular signal called PQS is linked to virulence, among others. This signal is associated with the mvfr gene, which positively regulates the pqsABCDE operon through binding of the MvfR protein to the pqsA promoter. This binding is enhanced in the presence of the cofactor PQS (Xiao, Déziel et al., 2006 ▶; Xiao, He et al., 2006 ▶; Hazan et al., 2010 ▶).
After the identification of the MvfR virulence pathway (Cao et al., 2001 ▶; Déziel et al., 2004 ▶), many fragments of the MvfR protein were produced, several of which were insoluble. Most of the MvfR constructs that we have investigated were insoluble and thus not suitable for crystallographic studies. The insoluble constructs include the full-length MvfR protein, two N-terminal fragments comprising the first 303 and the first 187 residues, as well as a C-terminal fragment comprising the last 185 residues. Crystals suitable for data collection were obtained using MvfRC87, which is a soluble and stable C-terminal fragment of 242 residues with a molecular weight of 27.2 kDa. MvfRC87 lacks the 87 N-terminal residues of the full-length protein, which include a predicted helix–turn–helix DNA-binding motif; however, it includes the cofactor-binding segment of MvfR.
2. Materials and methods
2.1. Protein expression and purification
The gene fragment coding for MvfRC87 was cloned, inserted into pET26b (ColE1 plasmids) vector carrying a C-terminal His6 tag and transformed into Escherichia coli strain BL21. No additional nonprotein residues were included in the construct. A sufficient amount of soluble protein was obtained after induction using the following conditions. Cells were grown in LB medium containing 40 µg ml−1 kanamycin until an OD600 of 0.6 was reached. The culture was induced with 1 mM IPTG for 3 h. Approximately 3.24 g cell paste was resuspended in 50 ml lysis buffer consisting of 50 mM Tris pH 8.0, 300 mM NaCl, 10 mM imidazole, 10 mM β-mercaptoethanol and homogenized. After the addition of protease inhibitors (20 µg ml−1 leupeptin, 1 mM PMSF, 150 µg ml−1 benzamidine), the solution was sonicated for 6 min. The precipitate was subsequently removed by centrifugation at 12 000 rev min−1 and 277 K for 45 min. Purification was carried out using His-tag affinity chromatography at 277 K with an 8 ml Ni–NTA column (Qiagen) pre-equilibrated in lysis buffer and initially washed stepwise with 10, 40 and 100 mM imidazole. The imidazole concentration was subsequently increased and the protein started eluting at 150 mM imidazole. Fractions containing the protein were dialyzed against storage buffer consisting of 20 mM Tris pH 8, 200 mM NaCl, 10 mM β-mercaptoethanol and were concentrated to approximately 8 mg ml−1 for subsequent crystallization experiments. Size-exclusion chromatography experiments suggested a dimeric form for MvfRC87.
2.2. Crystallization
Crystallization conditions for MvfRC87 were screened using the hanging-drop vapour-diffusion method in 24-well Linbro cell-culture plates. The drops were made up of 2 µl protein solution mixed with an equal volume of reservoir solution and were equilibrated against 1000 µl reservoir solution at 291 K. Initial crystallization screening was performed using commercially available crystallization kits including Grid Screen MPD, Grid Screen Ammonium Sulfate and Grid Screen PEG 6000 (Hampton Research) as well as Structure Screens I and II (Molecular Dimensions Ltd). Initial crystals were obtained from Structure Screen II using a reservoir solution consisting of 1.5 M NaCl, 10%(v/v) ethanol. Crystal optimization was performed by variation of the above conditions. The best results were obtained with 1.4–1.5 M NaCl, 10–12%(v/v) ethanol. A final crystal dimension of approximately 0.2 mm was reached in 5–6 d (Fig. 1 ▶).
Figure 1.
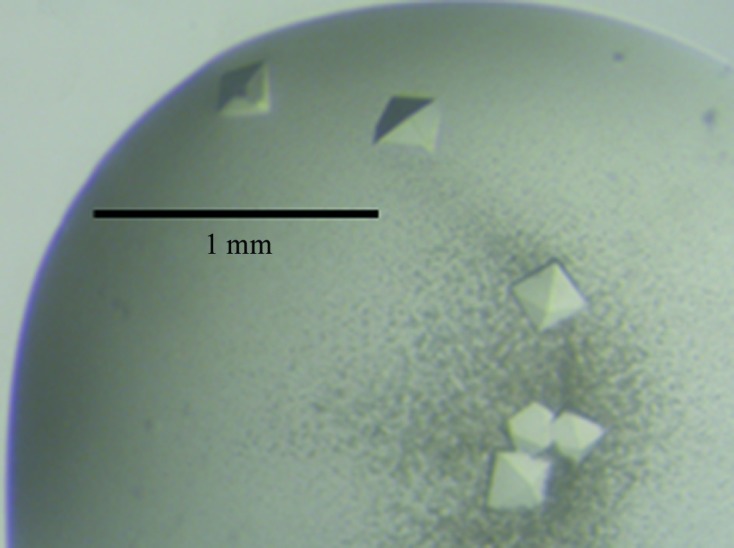
Crystals of the MvfRC87 protein. The largest crystals are approximately 0.2 mm along the longest diagonal.
2.3. Data collection and processing
X-ray diffraction data were collected from a single crystal using synchrotron radiation on the EMBL X11 beamline at the DORIS storage ring, DESY, Hamburg. The crystal was flash-cooled to 100 K in a nitrogen-gas stream using an Oxford Cryosystems device with 40% PEG 400 as a cryoprotectant, which was added to the mother liquor. 250 images of 1° rotation each were collected. The diffraction data were recorded on a MAR CCD detector with a diameter of 165 mm. X-ray diffraction data were indexed, integrated and scaled using MOSFLM and SCALEPACK from the HKL program suite (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
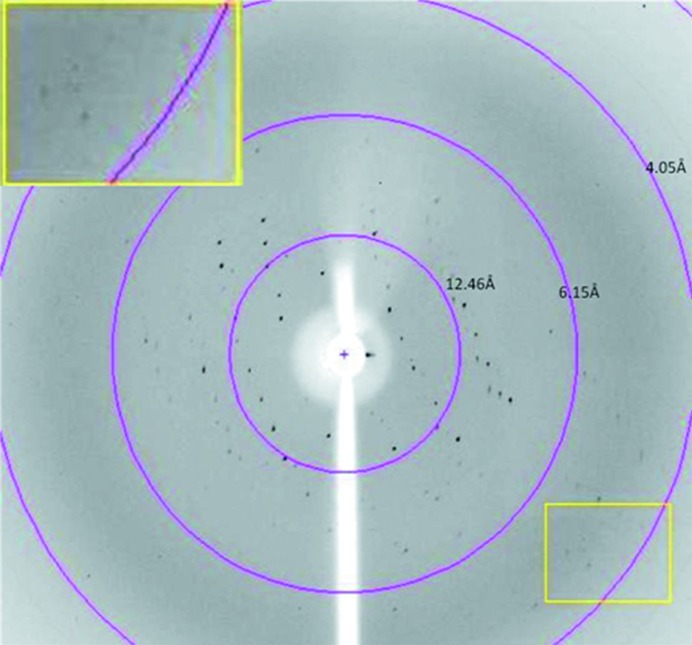
The MvfRC87 crystals obtained diffracted to a resolution of about 5 Å (Fig. 2 ▶), thus allowing only preliminary crystallographic characterization. The diffraction data were consistent with the tetragonal space group P41 or P43, with unit-cell parameters a = b = 75.63, c = 114.9 Å. Data-collection and processing statistics are given in Table 1 ▶. Assuming the presence of two molecules in the asymmetric unit, the Matthews coefficient was 2.95 Å3 Da−1, corresponding to a solvent content of 58.4%.
Figure 2.
X-ray diffraction pattern of an MvfRC87 crystal. Inset, detail of the diffraction pattern in the region close to the resolution limit of the crystal.
Table 1. Data-collection and processing statistics and crystallographic parameters.
Values in parentheses are for the highest resolution shell.
| Space group | P41 or P43 |
| Unit-cell parameters (Å) | a = b = 75.63, c = 114.9 |
| Wavelength (Å) | 0.95 |
| Resolution (Å) | 75.59–5.00 (5.27–5.00) |
| Observed reflections | 10543 (1529) |
| Unique reflections | 2851 (407) |
| Multiplicity | 3.7 (3.8) |
| Data completeness (%) | 99.9 (100) |
| Rmerge† | 0.235 (0.467) |
| Average I/σ(I) | 2.8 (1.7) |
| Mosaicity (°) | 0.55 |
R
merge = 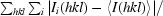
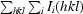 , where I
i(hkl) is the intensity of the ith observation of reflection hkl, 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl,
, where I
i(hkl) is the intensity of the ith observation of reflection hkl, 〈I(hkl)〉 is the weighted average intensity for all observations i of reflection hkl,  is the sum over all reflections and
is the sum over all reflections and 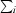 is the sum over i measurements of reflection hkl.
is the sum over i measurements of reflection hkl.
The assumption of two molecules in the asymmetric unit was consistent with size-exclusion chromatography experiments, which suggested a dimeric form for MvfRC87. This in turn was also consistent with the results of calculation of a self-rotation function, which revealed a local dyad axis located in the ab plane of the crystal and making a 45° angle with the a and b crystal axes.
The amino-acid sequences of LysR-family members of known structure exhibit an identity of approximately 20% to MvfRC87. The structures of these proteins could be used as search models for molecular replacement after improvement of the diffraction quality. Alternatively, structure determination using the multiwavelength anomalous diffraction (MAD) method could also be attempted as we have crystallized a selenomethionine-substituted variant of MvfRC87. Efforts towards improving the diffraction quality of the crystals will include screening of crystallization conditions at lower temperatures (273–277 K) and seeding techniques.
Acknowledgments
We thank the European Molecular Biology Laboratory, Hamburg Outstation and the European Union for support through the EU-I3 access grant from the EU Research Infrastructure Action under the FP6 ‘Structuring the European Research Area Programme’, contract No. RII3/CT/2004/5060008.
References
- Cao, H., Krishnan, G., Goumnerov, B., Tsongalis, J., Tompkins, R. & Rahme, L. G. (2001). Proc. Natl Acad. Sci. USA, 98, 14613–14618. [DOI] [PMC free article] [PubMed]
- Déziel, E., Lépine, F., He, J., Mindrinos, M. N., Tompkins, R. & Rahme, L. G. (2004). Proc. Natl Acad. Sci. USA, 101, 1339–1344. [DOI] [PMC free article] [PubMed]
- Fuqua, C., Parsek, M. R. & Greenberg, E. P. (2001). Annu. Rev. Genet. 35, 439–468. [DOI] [PubMed]
- Gallagher, L. A., McKnight, S. L., Kuznetsova, M. S., Pesci, E. C. & Manoil, C. (2002). J. Bacteriol. 184, 6472–6480. [DOI] [PMC free article] [PubMed]
- Hazan, R., He, J., Xiao, G., Dekimpe, V., Apidianakis, Y., Lesic, B., Astrakas, C., Déziel, E., Lépine, F. & Rahme, L. G. (2010). PLoS Pathog. 6, e1000810. [DOI] [PMC free article] [PubMed]
- Lesic, B., Starkey, M., He, J., Hazan, R. & Rahme, L. G. (2009). Microbiology, 155, 2845–2855. [DOI] [PMC free article] [PubMed]
- Maddocks, S. E. & Oyston, P. C. (2008). Microbiology, 154, 3609–3623. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Tyrrell, R., Verschueren, K. H., Dodson, E. J., Murshudov, G. N., Addy, C. & Wilkinson, A. J. (1997). Structure, 5, 1017–1032. [DOI] [PubMed]
- Xiao, G., Déziel, E., He, J., Lépine, F., Lesic, B., Castonguay, M.-H., Milot, S., Tampakaki, A. P., Stachel, S. E. & Rahme, L. G. (2006). Mol. Microbiol. 62, 1689–1699. [DOI] [PubMed]
- Xiao, G., He, J. & Rahme, L. G. (2006). Microbiology, 152, 1679–1686. [DOI] [PubMed]