Abstract
We have previously reported that peri-transplant conditioning leads to successful induction of renal allograft tolerance via the mixed chimerism approach in nonhuman primates (NHP) and humans. However, this strategy requires treatments beginning six days prior to transplantation, which limits its relevance only to living donor transplant recipients. To extend the clinical applicability of this approach, we developed a novel regimen “delayed tolerance,” with which the recipient initially undergoes organ transplantation with conventional immunosuppression, followed by conditioning and donor bone marrow transplantation (DBMT) at a later date. This approach might be likened to “planting flowers in a battle field.” That is, the recipient’s immunologic environment after organ transplantation is like a battlefield filled with hostile innate and adaptive immune-responses directed against donor antigeneic specificities. Implanting fragile donor hematopoietic progenitors into this environment and encouraging them to bloom in this vicious field requires special treatments.
In our NHP studies recently published in The American Journal of Transplantation, we showed that such “delayed tolerance,” in fact, can be induced in NHP through the mixed chimerism approach, if specific modifications to overcome/avoid donor-specific memory T cell responses are provided. These modifications include adequate depletion of CD8 memory T cells and timing of donor bone marrow administration to minimize levels of pro-inflammatory cytokines. This article addendum will provide a short summary of the original paper with our additional insights and interpretations.
Keywords: bone marrow transplantation, chimerism, kidney transplantation, memory T cell, tolerance
Introduction
Based on our rodent studies on mixed chimerism,1,2 we initially developed a clinically relevant non-myeloablative preparative regimen that permits the induction of mixed chimerism and renal allograft tolerance when combined with simultaneous donor bone marrow transplantation (DBMT) in MHC fully-mismatched cynomolgus monkeys.3-5 This approach has been successfully extended to HLA matched6 or mismatched7 clinical kidney transplantation. In murine models, the primary mechanism of tolerance induction through mixed chimerism was shown to be via thymic deletion. That is, donor derived dendritic cells (DC) migrate to the recipient thymus, where they induce negative selection of donor reactive T cell clones.1,8 Therefore, induction of stable mixed chimerism appeared to be a prerequisite for stable allograft tolerance through this strategy.2 However, the mixed chimerism induced in primates with our non-myeloablative regimen has always been transient in nature, but nevertheless, essential to induce renal allograft tolerance in this model. This led us to conclude that the mechanisms associated with induction of tolerance in primates include peripheral as well as central thymic deletion pathways.
Our original protocol requires treatment of subjects beginning six days prior to organ transplantation,3,5,7 which limits its applicability to living donor transplant recipients. Therefore, our next major goal has been to develop a strategy that is applicable to deceased donor organ transplantation. We initially evaluated regimens in which conditioning was begun within 24 hours of kidney transplantation (KTx). However, simple compression of the previously effective six-day therapeutic protocol into a 24-hour period failed to induce chimerism and also led to unacceptable toxicity (Fig. 1D). We thus developed a novel “delayed tolerance” approach, with which the recipient initially undergoes organ transplantation with conventional immunosuppression, followed by conditioning and donor bone marrow transplantation (DBMT) at a later date. This approach would potentially extend the applicability of our regimen to not only current recipients of deceased donor transplantation but also to any recipient of a previously transplanted allograft from either a living or deceased donor, if DBM is available. However, the “delayed tolerance” strategy has the theoretical disadvantage that donor-specific memory T cells (Tmem) might have been elicited despite administration of potent immunosuppressive agents during the interval between transplantation and attempted tolerance induction. Therefore, we have extensively monitored Tmem subsets and alloreactive Tmem responses in these studies.
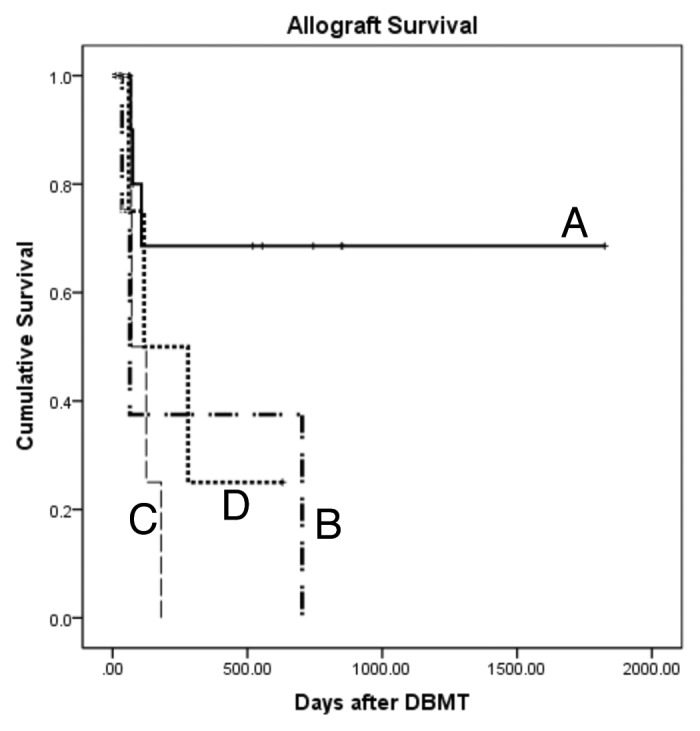
Figure 1. Renal allograft survival in the delayed tolerance (deaths due to infectious complication censored). Simple compression of the previously effective 6-d therapeutic protocol into a 24-h period (D) failed to induce chimerism and also led to unacceptable toxicity. The conditioning regimen that was successful in simultaneous kidney and bone marrow transplantation (SKBMT) failed to induce long-term allograft survival in the delayed tolerance protocol at 4 mo (B). When anti-CD8 mAb was added to the original regimen, approximately 70% of recipients achieved long-term survival (A). However, this modified regimen with anti-CD8 mAb was not successful in recipients of the delayed tolerance protocol at 1 mo (C).
Memory T Cell Responses Following Kidney Transplantation with Conventional Immunosuppression
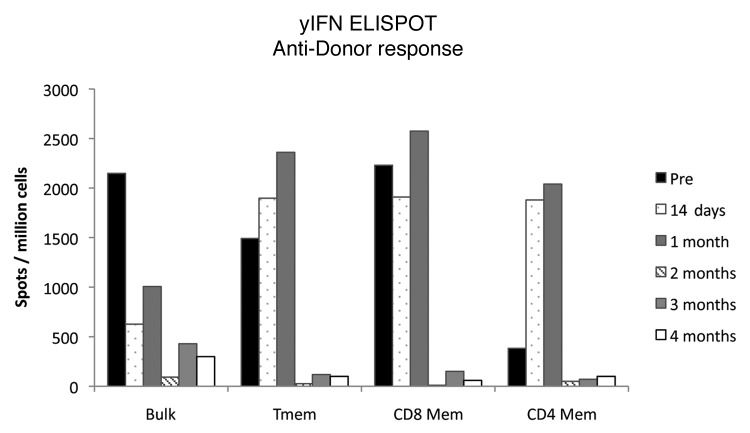
Primates including monkeys subjected to these experiments typically exhibit rigorous heterologous Tmem responses even before KTx.9 In addition to naïve T cell responses, these preexisting Tmem that heterologously respond to alloantigens may further impair induction of chimerism and allograft tolerance. We thus monitored recipient Tmem responses by measuring γIFN or IL-2 production by ELISPOT. Somewhat unexpectedly, the initially high alloreactive Tmem responses appeared to decline after KTx in a time-dependent fashion. As shown in Figure 2, γIFN and IL-2 Tmem responses progressively fell after KTx and became almost undetectable by four months. Since third party Tmem responses were relatively preserved, this was not simply due to the global effects of immunosuppression. Development of such donor-specific Tmem hyporesponsiveness has also been reported in clinical KTx10,11 and is speculated to result from the interaction between recipient lymphocytes and tolerogenic graft parenchymal cells.12 An alternative explanation is memory T cell exhaustion by antigen exposure.13 The important point is that, if these ELISPOT results truly reflect the in vivo status of Tmem responses, induction of chimerism might be even easier when DBMT is delayed.
Figure 2. γIFN Tmem responses measure by ELISPOT after KTx. Post KTx anti-donor responses were measured by ELISPOT in various populations, Bulk(PBMCs), Tmem(CD16-CD95+), CD8 Mem(CD16-CD8+CD95+) and CD4 Mem(CD16-CD4+CD95+). Tmem responses declined in a time dependent fashion.
The Initial Conditioning Regimen that was Successful for Simultaneous Kidney and DBM Transplantation Failed to Induce Chimerism in the Delayed Tolerance Approach
In the delayed tolerance, recipients initially underwent KTx alone and were treated with conventional immunosuppression (tacrolimus, MMF and steroids). Four months later, the recipients received our standard conditioning regimen (low dose total body irradiation, local thymic irradiation, ATG and anti-CD40L mAb). With this regimen, recipients of simultaneous kidney and DBM transplantation (SKBMT) consistently developed multilineage chimerism and most achieved long-term survival without immunosuppression.5 In contrast, no recipients conditioned at four months with the same therapeutic regimen developed multilineage chimerism (Fig. 3C) and all rejected their previously well-functioning kidney allografts soon after discontinuation of immunosuppression (Fig. 1B). Rapid homeostatic recovery of CD8 Tmem was observed (Fig. 3A) following conditioning in these recipients. This homeostatic recovery was faster than that observed in SKBMT (data not shown) leading us to conclude that CD8 Tmems had been insidiously activated by the kidney allograft but that this had not been detectable by ELISPOT monitoring of γIFN and IL-2.
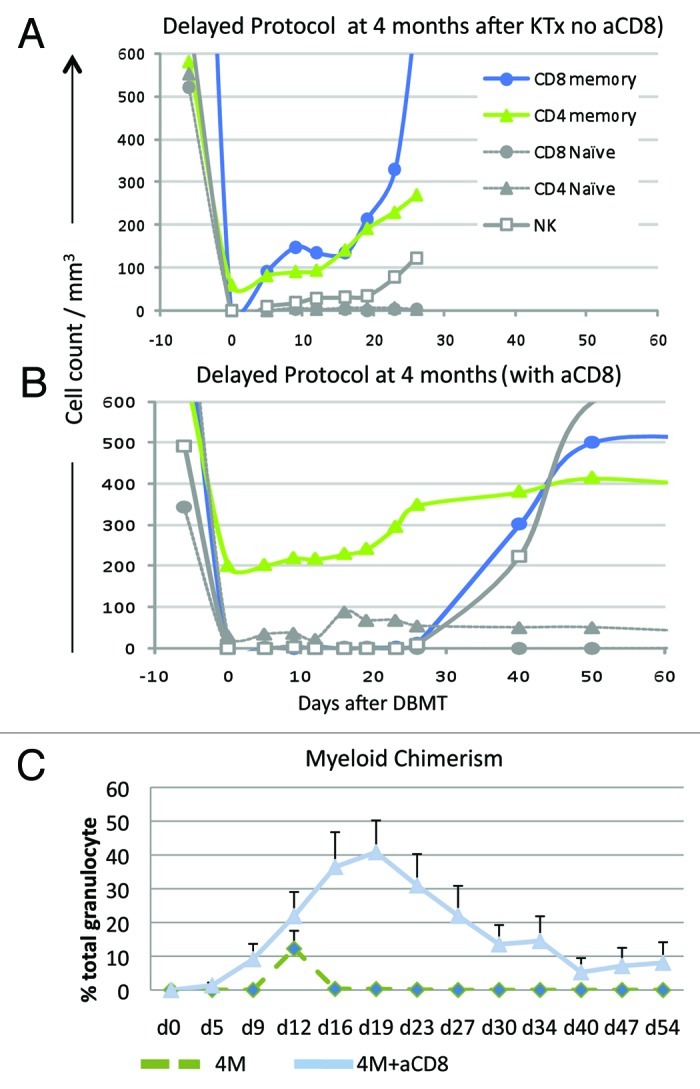
Figure 3. Lymphocyte subset and chimerism induction in the Delayed Protocol. (A) In recipients treated with the conditioning regimen without anti-CD8 mAb, rapid homeostatic recovery of CD8+ Tmem was observed after day 5. CD4+ T cells were not completely deleted with a nadir around 100/mm3. (B) By adding humanized anti-CD8 mAb to the conditioning regimen, CD8 Tmems were effectively suppressed until day 30. CD4 Tmem levels were even higher after administration of the modified regimen. (C) All recipients who received the delayed protocol at 4 mo without anti-CD8 mAb (4M) failed to develop chimerism. By adding humanized anti-CD8 mAb to the conditioning regimen, recipients consistently developed multilineage chimerism despite the presence of residual CD4 Tmem.
CD8 Depletion Facilitates the Development of Donor Cell Chimerism
Since the faster homeostatic recovery of CD8 Tmem seemed to prevent induction of chimerism, we added anti-CD8 mAb to the conditioning regimen. This modified regimen significantly delayed homeostatic recovery of CD8 Tmem (Fig. 3B) and most recipients (11/13) successfully developed mixed chimerism (Fig. 3C). If death from infectious complications is censored, approximately 70% of recipients survived long-term following withdrawal of all immunosuppression (Fig. 1A). These observations suggest that, although not detected by ELISPOT, CD8 Tmem had been activated during the four months following KTx despite the administration of immunosuppression potent enough to prevent rejection of the kidney.
More recently, we have evaluated replacing anti-CD8 mAb in the conditioning regimen with LFA-3/IgG1 (LFA3Ig) anticipating that the agent will be more readily available for clinical use. LFA3Ig modulates the function of CD2 (+) and depletes efficiently primate CD95+CD28- Effector Tmem in vivo. This molecule mediates cognate interactions between cells expressing human CD2 and CD16 to activate cells, increase extracellular signal-regulated kinase phosphorylation, upregulate cell surface expression of the activation marker CD25, and induce release of Granzyme B.14-17 Three recipients treated with the modified regimen with LFA3Ig but with no anti-CD8mAb successfully developed chimerism and achieved long-term survival (manuscript in preparation).
Inflammation is Detrimental to Tolerance Induction
Since our results suggested that Tmem activation occurs after KTx, we speculated that a shorter interval between organ transplantation and DBMT might limit this response and increase the likelihood of inducing allograft tolerance. Therefore, we evaluated DBMT at one month after KTx in an attempt to identify the optimal timing of DBMT. As we anticipated, chimerism induction in recipients who received DBMT earlier after KTx was more successful. All seven recipients who received DBMT at one month developed excellent chimerism (data not shown). The fact that two of 13 recipients who received DBMT at four months failed to develop any detectable chimerism, suggested that Tmem activation may indeed be lower earlier after KTx. However, to our surprise, no recipients of DBMT at one month achieved renal allograft tolerance (Fig. 1C) despite consistently successful induction of chimerism. The state of the inflammatory milieu during the peritransplant period has been shown to impact the molecular phenotype and function of alloreactive T cells.18,19 We therefore hypothesized that higher proinflammatory responses during the earlier post-transplant period adversely affected tolerance induction. RT-PCR analyses of the peripheral blood mononuclear cells revealed that mRNA levels of proinflammatory cytokines in the recipients who received DBMT at one month were significantly higher than those who received DBMT at four months. LUMINEX assays also showed higher IL-6 and IL-17 levels in the one month group. These results suggest that the presence of higher proinflammatory cytokines is detrimental to tolerance induction.
Conclusion
Tolerance induction several months after organ transplantation (delayed tolerance) is feasible via the mixed chimerism approach with additional modifications to mitigate Tmem responses that have been induced by the transplanted allograft. Timing of delayed DBMT also appeared to be critical for successful induction of allograft tolerance, which is affected by higher inflammatory responses during the early post-transplant period.
Footnotes
Previously published online: www.landesbioscience.com/journals/chimerism/article/20096
References
- 1.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–98. [PubMed] [Google Scholar]
- 2.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–62. [PubMed] [Google Scholar]
- 4.Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767–75. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–8. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, et al. Combined histocompatibility leukocyte antigen-matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68:480–4. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–61. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan A, Tomita Y, Sykes M. Thymic dependence of loss of tolerance in mixed allogeneic bone marrow chimeras after depletion of donor antigen. Peripheral mechanisms do not contribute to maintenance of tolerance. Transplantation. 1996;62:380–7. doi: 10.1097/00007890-199608150-00014. [DOI] [PubMed] [Google Scholar]
- 9.Nadazdin O, Boskovic S, Murakami T, O’Connor DH, Wiseman RW, Karl JA, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10:1375–84. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Lechler RI. The role of the allograft in the induction of donor-specific T cell hyporesponsiveness. Transplantation. 2001;72:480–5. doi: 10.1097/00007890-200108150-00020. [DOI] [PubMed] [Google Scholar]
- 11.Gebauer BS, Hricik DE, Atallah A, Bryan K, Riley J, Tary-Lehmann M, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant. 2002;2:857–66. doi: 10.1034/j.1600-6143.2002.20908.x. [DOI] [PubMed] [Google Scholar]
- 12.Marelli-Berg FM, Lechler RI. Antigen presentation by parenchymal cells: a route to peripheral tolerance? Immunol Rev. 1999;172:297–314. doi: 10.1111/j.1600-065X.1999.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 13.Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci U S A. 2010;107:20453–8. doi: 10.1073/pnas.1008437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper JC, Morgan G, Harding S, Subramanyam M, Majeau GR, Moulder K, et al. Alefacept selectively promotes NK cell-mediated deletion of CD45R0+ human T cells. Eur J Immunol. 2003;33:666–75. doi: 10.1002/eji.200323586. [DOI] [PubMed] [Google Scholar]
- 15.da Silva AJ, Brickelmaier M, Majeau GR, Li Z, Su L, Hsu YM, et al. Alefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and CD2/CD16-dependent apoptosis of CD2(+) cells. J Immunol. 2002;168:4462–71. doi: 10.4049/jimmunol.168.9.4462. [DOI] [PubMed] [Google Scholar]
- 16.Gordon KB, Vaishnaw AK, O’Gorman J, Haney J, Menter A, Alefacept Clinical Study Group Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563–70. doi: 10.1001/archderm.139.12.1563. [DOI] [PubMed] [Google Scholar]
- 17.Krueger GG. Selective targeting of T cell subsets: focus on alefacept—a remittive therapy for psoriasis. Expert Opin Biol Ther. 2002;2:431–41. doi: 10.1517/14712598.2.4.431. [DOI] [PubMed] [Google Scholar]
- 18.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 19.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]